Summary
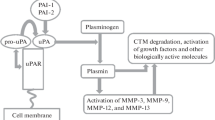
The cellular receptor for urokinase-type plasminogen activator (uPAR) in glioblastoma cell lines has been identified and found to be similar to the uPAR expressed by other tumor cell lines. Increased levels of uPAR have been found in primary malignant brain tumor tissues, especially highly malignant glioblastoma, and, to a lesser degree, in malignant astrocytomas, suggesting that this receptor might be involved in efficient activation of pro-uPA and confinement of uPA activity on the cell surface of invading brain tumors. The cell surface uPARs in gliomas could constitute an optimum environment for the generation and activity of plasmin, which is known to play a crucial role in the dissolution of the extracellular matrix during tumor cell invasion.In situ hybridization studies have shown that uPAR mRNA is expressed abundantly in tumor cells and is consistently present at the invasive edges of malignant gliomas. These results imply that uPAR is involved in plasmincatalyzed proteolysis during glioma invasion and that interference with the uPA∶uPAR interactions could constitute a novel approach for developing therapeutic strategies to counteract invasion of brain tumors.
Similar content being viewed by others
References
Duffy MJ: Plasminogen activators and cancer. Blood Coagulation and Fibrinolysis. 1: 681–687, 1990
Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64: 327–336, 1991
Sloane BF, Moin K, Krepela E, Rozhin J: Cathepsin B and its endogenous inhibitors: the role in tumor malignancy. Cancer Metastasis Rev 9: 333–352, 1990
Pöllänen J, Stephens RW, Vaheri A: Directed plasminogen activation at the surface of normal and malignant cells. Adv Cancer Res 57: 273–328, 1991
Reith A, Rucklidge GJ: Invasion of brain tissue by primary glioma: evidence for the involvement of urokinase-type plasminogen activator as an activator of type IV collagenase. Biochem Biophys Res Commun 186: 348–353, 1992
Danø K, Andreasen PA, Grøndahl-Hansen J, Kristensen P, Nielsen LS, Skriver L: Plasminogen activators, tissue degradation and cancer. Adv Cancer Res 44: 140–239, 1985
Duffy MJ: The role of proteolytic enzymes in cancer invasion and metastasis. Clin Exp Metastasis 10: 145–155, 1992
Pöllänen J, Hedman K, Nielsen LS, Danø K, Vaheri A: Ultrastructural localization of plasma membrane associated urokinase type plasminogen activator at focal contacts. J Cell Biol 106: 87–95, 1988
Cohen RL, Xi X-P, Crowley W, Lucas BK, Levinson D, Shuman A: Effects of urokinase receptor occupancy on plasmin generation and proteolysis of basement membrane by human tumor cells. Blood 78: 479–487, 1991
Quax PHA, Pedersen N, Masucci MT, Weening-Werhoeff EJD, Danø K, Verheijen J, Blasi F: Complementation of urokinase and its receptor in extracellular matrix degradation. Cell Regul 2: 793–803, 1991
Scully MF: Plasminogen activator-dependent pericellular proteolysis. Brit J Haematol 79: 537–543, 1991
Roldan AL, Cubellis MV, Masucci MT, Behrendt N, Lund LR, Danø K, Appella E, Blasi F: Cloning and expression of the receptor for human urokinase plasminogen activator: a central molecule in cell-surface, plasmin-dependent proteolysis. EMBO J 9: 467–474, 1990
Børglum AD, Byskov A, Ragno P, Roldan AL, Tripputi P, Cassani G, Danø K, Blasi F, Bolund L, Kruse TA: Assignment of the urokinase type plasminogen activator receptor gene (PLAUR) to chromosome 19q13.1-q13.2. Am J Hum Genet 50: 492–497, 1992
Moller LB: Structure and function of the urokinase receptor. Blood Coagul-Fibrinolysis 4: 293–303, 1993
Behrendt N, Ploug M, Patthy L, Houen G, Blasi F, Danø K: The ligan binding domain of the cell surface receptor for urokinase-type plasminogen activator. J Biol Chem 266: 7842–7847, 1991
Ploug M, Rønne E, Behrendt N, Jensen AL, Blasi F, Danø K: Cellular receptor for urokinase plasminogen activator-carboxyl terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem 266: 1926–1933, 1991
Lund LR, Romer J, Ronne E, Ellis V, Blasi F, Danø K: Urokinase receptor biosynthesis mRNA level and gene transcription are increased by transforming growth factor in human A549 lung carcinoma cells. EMBO J 10: 3399–3407, 1991
Ellis V, Pyke C, Eriksen J, Solberg H, Danø K: The urokinase receptor: involvement in cell surface proteolysis and cancer invasion. Ann N Y Acad Sci 667: 13–31, 1992
Pyke C, Eriksen J, Solberg H, Nielsen BS, Kristensen P, Lund R, Danø K: An alternatively spliced variant of mRNA for the human receptor for urokinase plasminogen activator. FEBS Lett 326: 69–74, 1993
Solberg H, Løber D, Eriksen J, Ploug M, Rønne E, Behrendt N, Danø K, Høyer-Hansen G: Identification and characterization of the murine cell surface receptor for the urokinase-type plasminogen activator. Eur J Biochem 205: 451–458, 1992
Rabbani SA, Rajwans N, Achbarou A, Murthy KK, Goltzman D: Isolation and characterization of multiple isoforms of the rat urokinase receptor in osteoblasts. FEBS Lett 338: 69–74, 1994
Kristensen P, Kriksen J, Blasi F, Danø K: Two alternatively spliced mouse urokinase receptor mRNAs with different histological localization in the gastrointestinal tract. J Cell Biol 115: 1763–1771, 1991
Huber R, Carrell RW: Implications of the three-dimensional structure of α1-antitrypsin for structure and function of serpins. Biochemistry 28: 8951–8966, 1989
Ellis V, Wun T-C, Behrendt N, Rønne E, Danø K: Inhibition of receptor bound urokinase by plasminogen-activator inhibitors. J Biol Chem 265: 9904–9908, 1990
Preissner KT, Jenne D: Structure of vitronectin and its biological role in hemostasis. Thromb Haemost 66: 123–132, 1991
Ciambrone GJ, McKeown-Longho J: Plasminogen activator inhibitor type 1 stabilizes vitronectin dependent adhesion in HT-1080 cells. J Cell Biol 111: 2183–2195, 1990
Pöllänen J, Vaheri A, Tapiovaara H, Riley E, Bertram K, Woodrow G, Stephens RW: Prourokinase activation on the surface of human rhabdomyosarcoma cells: localization and inactivation of newly formed urokinase type plasminogen activator by recombinant class 2 plasminogen activator inhibitor. Proc Natl Acad Sci USA 87: 2230–2234, 1990
Schwartz BS: Differential inhibition of soluble and cell surface receptor bound single chain urokinase by plasminogen activator inhibitor type 2. J Biol Chem 269: 8319–8323, 1994
Olson D, Pöllänen J, Høyer-Hansen G, Rønne E, Sakaguchi K, Wun TC, Apella E, Danø K, Blasi F: Internalization of the urokinase plasminogen activator inhibitor type 1 complex is mediated by the urokinase receptor. J Biol Chem 267: 9129–9133, 1992
Andreasen PA, Sottrup-Jensen L, Kjøller L, Nykjaer A, Moestrup SK, Petersen CM, Gliemann J: Receptor-mediated endocytosis of plasminogen activators and activator/inhibitor complexes. FEBS Lett 338: 239–245, 1994
Nykjaer A, Petersen CM, Møller B, Jensen PH, Moestrup SK, Holtet TL, Etzerodt M, Thøgersen HC, Munch M, Andrease PA, Gliemann J: Purified α2macroglobulin receptor/LDL receptor related protein binds urokinase plasminogen activator inhibitor type I complex. Evidence that the α2macroglobulin receptor mediates cellular degradation of urokinase receptor bound complexes. J Biol Chem 267: 14543–14546, 1992
Ossowski L, Russo-Payne H, Wilson EL: Inhibition of urokinase type plasminogen activator by antibodies; the effect on dissemination of a human tumor in the nude mouse. Cancer Res 51: 274–281, 1991
Ellis V, Behrendt N, Danø K: Plasminogen activation by receptor bound urokinase; a kinetic study with both cell associated and isolated receptors. J Biol Chem 266: 12752–12758, 1991
Stephens RW, Pöllänen T, Tapiovaara H, Leung K-C, Sim P-S, Salonen E-M, Rønne E, Behrendt N, Danø K, Vaheri A: Activation of prourokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol 108: 1987–1995, 1989
Ellis V, Scully MF, Kakkar W: Plasminogen activation initiated by single chain urokinase-type plasminogen activator: potentiation by U937 monocytes. J Biol Chem 264: 2185–2188, 1989
Rønne E, Behrendt N, Ellis V, Ploug M, Danø K, Høyer-Hansen G: Cell induced potentiation of the plasminogen activation system is abolished by a monoclonal antibody that recognizes the NH2-terminal domain of the urokinase receptor. FEBS Lett 288: 233–236, 1991
Kobayashi H, Schmitt M, Goretzki L, Chucholowski N, Calvete J, Kremer M, Günzler WA, Jänicke F, Graeff H: Cathepsin B efficiently activates the soluble and tumor cell receptor-bound form of the proenzyme urokinase-type plasminogen activator (pro-uPA). J Biol Chem 266: 5147–5152, 1991
Andreasen PA, Lund LR, Riccio A, Stacey SN: Plasminogen activator inhibitors: hormonally regulated serpins. Mol Cell Endocrinol 68: 1–19, 1990
Vaheri A, Tapiovaara H, Bizik J, Sirén V, Myöhänen H, Reisberg T, Lymboussakis A, Palolahti, Stephens R: Basic research and its clinical applications. In: Rabes H, Peters PE, Munk K (eds) Metastasis. Karger 1992, vol. 44 pp 127–140
Hollas W, Blasi F, Boyd D: Role of urokinase receptor in facilitating extracellular matrix invasion by cultured colon cancer. Cancer Res 51: 3690–3695, 1991
Ossowski L, Clunie G, Masucci M-T, Blasi F:In vivo paracrine interaction between urokinase and its receptor: effect on tumor invasion. J Cell Biol 115: 1107–1112, 1991
Gross JL, Behrens DL, Mullins DE, Kornblith PL, Dexter DL: Plasminogen activator and inhibitor activity in human glioma cells and modulation by sodium butyrate. Cancer Res 48: 291–296, 1988
Quax PHA, van Muijen GNP, Weening-Verhoeff EJD, Lund LR, Danø K, Ruiter DJ, Verheijen JH: Metastatic behavior of human melanoma cell lines in nude mice correlates with urokinase-type plasminogen activator, its tupe-1 inhibitor and urokinase-mediated matrix degradation. J Cell Biol 115: 191–199, 1991
Estricher A, Mühlhauser J, Carpentier J-L, Orci L, Vassalli J-D: The receptor for urokinase-type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol 111: 783–792, 1990
Pöllänen J, Hedman K, Nielsen LS, Danø K, Vaheri A: Ultrastructural localization plasma-membrane associated urokinase plasminogen activation. J Cell Biol 106: 87–95, 1988
Sawaya R, Highsmith R: Plasminogen activator activity and molecular weight patterns in human brain tumors. J Neurosurg 68: 73–79, 1988
Landau BJ, Kwaan HC, Verrusio EN, Brem SS: Elevated levels of urokinase-type plasminogen activator and plasminogen activator inhibitor type-1 in malignant human brain tumors. Cancer Res 54: 1105–1108, 1994
Picone R, Kajtaniak EL, Nielsen LS, Behrendt N, Mastronicola MR, Cubellis MV, Stoppelli MP, Pedersen S, Danø K, Blasi F: Regulation of urokinase receptors in monocyte-like U937 cells by phorbol ester phorbol myristate acetate. J Cell Biol 108: 693–702, 1989
Bruckner A, Filderman AE, Kirchheimer JC, Binder BR, Remold HG: Endogenous receptor bound urokinase mediates invasion of the human lung carcinoma cell lines A549 and Calu-1. Cancer Res 52: 3043–3047, 1992
Behrendt N, Rønne E, Danø K: Binding of the urokinase-type plasminogen activator to its cell surface receptor is inhibited by low doses of suramin. J Biol Chem 268: 5985–5989, 1993
Mohanam S, Sawaya R, McCutcheon I, Ali-Osman F, Boyd D, Rao JS: Modulation ofin vitro invasion of human glioblastoma cells by urokinase-type plasminogen activator receptor antibody. Cancer Res 53: 4143–4147, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mohanam, S., Sawaya, R.E., Yamamoto, M. et al. Proteolysis and invasiveness of brain tumors: Role of urokinase-type plasminogen activator receptor. J Neuro-Oncol 22, 153–160 (1994). https://doi.org/10.1007/BF01052890
Issue Date:
DOI: https://doi.org/10.1007/BF01052890