Summary
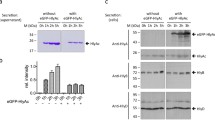
We have identified the polypeptides encoded by the haemolysin export genes from a haemolytic determinant 2001 carried by pLG570. This was previously cloned from an E. coli strain, serotype 04 isolated from a human urinary tract infection. Subclones from the recombinant plasmid pLG570 carrying hlyD analysed in vitro and in minicells showed that this gene is transcribed from an independent promoter and encodes a 53 Kd polypeptide. In contrast, detectable levels of the gene products encoded by hlyB were only observed when transcription presumably emanated from a vector promoter. This gene was found to encode at least two polypeptides apparently expressed from alternative translational start sites within a single reading frame. In minicells the major product was a 66 Kd polypeptide whilst after expression in nitro the major product was a 46 Kd polypeptide. Transposon mutagenesis leading to the synthesis of the expected truncated polypeptides was used to confirm the identity of the hlyD and the two hlyB products. Preliminary results suggest that the majority of the 53 Kd polypeptide is located in the inner membrane when cell envelopes from minicells and maxicells were fractionated using sarkosyl, although residual amounts of the 53 Kd polypeptide were also found in the outer membrane.
Similar content being viewed by others
References
Chang ACY, Cohen SN (1978) Construction and characterisation of amplifiable multicopy DNA cloning vehicles derived from P15A cryptic miniplasmid. J Bacteriol 134:1141–1156
Churchward GG, Holland IB (1976) Envelope synthesis during the cell cycle in Escherichia coli B/r. J Mol Biol 105:245–261
Felmlee T, Pellett S, Lee E-Y, Welch RA (1985 a) Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol 163:88–93
Felmlee T, Pellett S, Welch RA (1985b) Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol 163:94–105
Filip C, Fletcher G, Wulff JL, Earhart C (1973) Solubilisation of the cytoplasmic membrane of Escherichia coli by an ionic detergent sodium lauryl sarcosinate. J Bacteriol 115:717–722
Goebel W, Hedgpeth J (1982) Cloning and functional characterisation of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol 151:1290–1298
Gonzalez-Carrero MI, Zabala JC, de la Cruz F, Oritz JM (1985) Purification of α-hemolysin from an overproducing E. coli strain. Mol Gen Genet 199:106–110
Hartlein M, Schiessl S, Wagner W, Rdest U, Kreft J, Goebel W (1983) Transport of hemolysin by Escherichia coli. J Cell Biochem 22:87–97
Jackson M (1984) PhD Thesis, University of Leicester
Jones C, Holland IB (1985) Role of SulB(FtsZ) protein in division inhibition during the SOS response in E. coli: FtsZ stabilises the inhibitor SulA, in maxicells. Proc Natl Acad Sci USA 82:6045–6049
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Mackman N, Holland IB (1984a) Secretion of a 107 K dalton polypeptide into the medium from a haemolytic E. coli K12 strain. Mol Gen Genet 193:312–315
Mackman N, Holland IB (1984b) Functional characterisation of a cloned haemolysin determinant from E. coli of human origin, encoding information for the secretion of a 107 K polypeptide. Mol Gen Genet 196:123–134
Mackman N, Nicaud J-M, Gray L, Holland IB (1985) Genetical and functional organisation of the Escherichia coli haemolysin determinant 2001. Mol Gen Genet (in press)
Muller D, Hughes C, Goebel W (1983) Relationship between plasmid and chromosomal hemolysin determinants of Escherichia coli. J Bacteriol 153:846–851
Nicaud J-M, Mackman N, Gray L, Holland IB (1985a) Regulation of haemolysin synthesis in E. coli determined by Hly genes of human origin. Mol Gen Genet 199:111–116
Nicaud J-M, Mackman N, Gray L, Holland IB (1985b) Characterisation of HlyC and mechanism of activation and secretion of haemolysin from E. coli 2001. FEBS Lett 187:339–344
Nicaud J-M, Mackman N, Holland IB (1985c) Amplification of synthesis and secretion of haemolysin using a run-away plasmid in E. coli. J Biotech (in press)
Osborn MJ, Gander JE, Parisi E (1972) Mechanism of assembly of the outer membrane of Salmonella typhimurium: site of synthesis of lipopolysaccharide. J Biol Chem 247:3973–3986
Plastow GS, Pratt JM, Holland IB (1981) The ferrichrome receptor protein (tonA) of Escherichia coli is synthesised as a precursor in vitro FEBS Lett 131:262–264
Pratt JM, Boulnois GJ, Darby V, Orr E, Wahle E, Holland IB (1981) Identification of gene products programmed by restriction endonuclease DNA fragments using an E. coli in vitro system. Nucl Acid Res 9:4459–4474
Reeve J (1979) In: Wu R (ed) Methods in enzymology. Academic Press Inc, London and New York, 68:493–503
Sancar A, Hack AM, Rupp WD (1979) Simple method for identification of plasmid encoded proteins. J Bacteriol 137:692–693
Spratt BG (1977) Properties of the penicillin-binding proteins of Escherichia coli K12. Eur J Biochem 72:341–352
Smith HW (1963) The haemolysins of Escherichia coli. J Pathol Bacteriol 85:197–211
Stark J, Shuster C (1983) The structure of cloned hemolysin DNA from plasmid pHly185. Plasmid 10:45–54
Wagner W, Vogel M, Goebel W (1983) Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol 154:200–210
Welch RA, Hall R, Falkow S (1983) Molecular cloning and physical characterisation of a chromosomal hemolysin from Escherichia coli. Infect Immunol 42:178–186
Author information
Authors and Affiliations
Additional information
Communicated by J. W. Lengeler
Rights and permissions
About this article
Cite this article
Mackman, N., Nicaud, J.M., Gray, L. et al. Identification of polypeptides required for the export of haemolysin 2001 from E. coli . Molec Gen Genet 201, 529–536 (1985). https://doi.org/10.1007/BF00331351
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00331351