Abstract
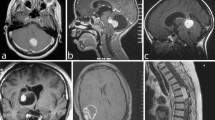
Diffusely infiltrating low-grade astrocytomas (WHO grade II) have an intrinsic tendency for progression to anaplastic astrocytoma (WHO grade III) and glioblastoma (WHO grade IV). This change is due to the sequential acquisition of genetic alterations, several of which have recently been identified. In low-grade astrocytomas, p53 mutations with or without loss of heterozygosity on chromosome 17p are the principal detectable change. Anaplastic astrocytomas contain p53 mutations at an overall incidence of 34% and, in addition, loss of heterozygosity on chromosome 19q and frequent homozygous deletion of the p16 tumor suppressor (MTS-1) gene. The most malignant astrocytic neoplasms, the glioblastoma, further shows loss of chromosome 10 and amplification of the epidermal growth factor receptor (EGF-R) gene at overall incidences of 66% and 34%, respectively. The type and distribution of p53 mutations in astrocytic brain tumours are not suggestive of specific environmental carcinogens operative in their aetiology. Analysis of 91 families with p53 germline mutations reported to date show that tumours of the nervous system account to 12% of all neoplasms. Of a total of 57 brain tumours reported, 30 were classified histologically and of these, 73% were of astrocytic origin. The observation that somatic p53 mutations in sporadic brain tumours are largely restricted to those of astrocytic origin and that astrocytomas also prevail among CNS neoplasms associated with p53 germline mutation strongly suggests, that p53 mutations are capable of initiating neoplastic transformation in astrocytes of the human nervous system.
Similar content being viewed by others
References
Bigner BH, Vogelstein B (1990) Cytogenetic and molecular genetics of malignant gliomas and medulloblastoma. Brain Pathol 1:12–18
Bigner SH, Burger PC, Wong AJ, Werner MH, Hamilton SR, Muhlabier LH, Vogelstein B, Bigner DD (1988) Gene amplification in malignant human gliomas: clinical and histopathologic aspects. J Neuropathol Exp Neurol 47:191–205
Daumas DC, Scheithauer B, O'Fallon J, Kelly P (1988) Grading of astrocytomas. A simple and reproducible method. Cancer 62:2152–2165
Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP (1991) Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res 15:2164–2172
Frankel RH, Bayona W, Koslow M, Newcomb EW (1992) p53 Mutations in human malignant gliomas: comparison of loss of heterozygosity with mutation frequency. Cancer Res 52:1427–1433
Fults D, Brockmeyer D, Tullous MW, Pedone CA, Cawthon RM (1992) p53 Mutation and loss of heterozygosity on chromosomes 17 and 10 during human astrocytoma progression. Cancer Res 52:674–679
Giani C, Finocchiaro G (1994) Mutation rate of the CDKN2 gene in malignant gliomas. Cancer Res 54:6338–6339
He J, Allen JR, Collins VP, Allalunis-Turner J, Godbout R, Day RS III James CD (1994) CDK4 Amplification is an alternative mechanism to p16 gene homozygous deletion in glioma cell lines. Cancer Res 54:5804–5807
Hollstein MC, Sidransky D, Vogelstein B, Harris CC (1991) p53 Mutations in human cancers. Science 253:49–53
Hollstein MC, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith-Sorensen B, Montesano R, Harris CC (1994) Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acid Res 22:3551–3555
Hunter SB, Bandea C, Swan D, Abbott K, Varma VA (1993) Mutations in the p53 gene in human astrocytomas: detection by single-strand conformation polymorphism analysis and direct DNA sequencing. Mod Pathol 6:442–445
Hurtt MR, Moossy J, Donovan-Peluso M, Locker J (1992) Amplification of epidermal growth factor receptor gene in gliomas: histopathology and prognosis. J Neuropathol Exp Neurol 51:84–90
James CD, Collins VP (1992) Molecular genetic characterization of CNS tumor oncogenesis. Adv Cancer Res 58:121–142
James CD, Carlbom E, Dumanski JP, Hausen M, Nordenskjšld MD, Collins VP, Cavenee WK (1988) Clonal genomic alterations in glioma malignancy stages. Cancer Res 48:5546–5551
Jen J, Harper JW, Bigner SH, Bigner DD, Papadopoulos N, Markowitz S, Willson JKV, Kinzler KW, Vogelstein B (1994) Deletion of p16 and p15 in brain tumors. Cancer Res 54:6353–6358
Kleihues P, Burger PC, Scheithauer BW (1993) Histological typing of tumors of the central nervous system, 2nd edn. Springer, Berlin Heidelberg New York for World Health Organization, Geneva
Kleihues P, Burger PC, Scheithauer BW (1993) The new classification of brain tumors. Brain Pathol 3:255–268
Kleihues P, Hausen A zur, Schäuble B, Ohgaki H (1995) Tumours associated with p53 germline mutations. A synopsis of 91 families. (Submitted for publication)
Lang FF, Miller DC, Koslow M, Newcomb EW (1994) Pathways leading to glioblastoma multiforme: a molecular analysis of genetic alterations in 65 astrocytic tumors. J Neurosurg 81:427–436
Lang FF, Miller DC, Pisharody S, Newcomb EW (1994) High frequency of p53 protein accumulation without p53 gene mutation in human juvenile pilocytic, low grade and anaplastic astrocytomas. Oncogene 9:949–954
Levine AJ, Perry ME, Chang A, Silver A, Dittmer D, Wu M, Welsh D (1994) The 1993 Walter Hubert Lecture: the role of the p53 tumour-suppressor gene in tumorigenesis. Br J Cancer 69:409–416
Louis DN (1994) The p53 gene and protein in human brain tumors. J Neuropathol Exp Neurol 53:11–21
Louis DN, Von Deimling A, Chung RY, Rubio MP, Whaley JH, Eibl RH, Ohgaki H, Wiestler OD, Thor AD, Seizinger BR (1993) Comparative study of p53 gene and protein alterations in human astrocytomas. J Neuropathol Exp Neurol 52:31–38
Mashiyama S, Murakami Y, Yoshimoto T, Sekiya T, Hayashi K (1991) Detection of p53 gene mutations in human brain tumours by single strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene 6:1313–1318
Newcomb EW, Madonia WJ, Pisharody S, Lang FF, Koslow M, Miller DC (1993) A correlative study of p53 protein alteration and p53 gene mutation in glioblastoma multiforme. Brain Pathol 3:229–235
Ohgaki H, Eibl RH, Schwab M, Reichel M, Mariani L, Gehring M, Petersen I, Holl T, Wiestler OD, Kleihues P (1993) Mutations of the tumor suppressor gene p53 in neoplasms of human nervous system. Mol Carcinog 8:74–80
Rasheed BKA, McLendon RE, Herndon JE, Friedman HS, Friedman AH, Bigner DD, Bigner SH (1994) Alterations of the TP53 gene in human gliomas. Cancer Res 54:1324–1330
Reifenberger G, Liu L, Ichimura K, Schmidt EE, Collins VP (1993) Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res 53:2736–2739
Reifenberger G, Reifenberger J, Ichimura K, Meltzer PS, Collins VP (1994) Amplification of multiple genes from chromosomal region 12q13–14 in human malignant gliomas: preliminary mapping of the amplicons shows preferential involvement of CDK4, SAS, and MDM2. Cancer Res 54:4299–4303
Scheck AC, Coons SW (1993) Expression of the tumor suppressor gene DCC in human gliomas. Cancer Res 53:5605–5609
Schmidt EE, Ichimura K, Reifenberger G, Collins VP (1994) CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of gliomas. Cancer Res 54:6321–6324
Schoenberg BS (19XX) Nervous system. In: Schttenfeld D, Fraumeni JF (eds) Cancer epidemiology and prevention. Saunders, Philadelphia, pp 968–983
Sidransky D, Mikkelsen T, Schwechheimer K, Rosenblum ML, Cavanee W, Vogelstein B (1992) Clonal expansion of p53 mutant cells is associated with brain tumor progression. Nature 355:846–847
Thomas TL, Waxweiler RJ (1986) Brain tumours and occupational risk factors. A review. Scand J Work Environ Health 12:1–15
Venter DJ, Thomas DGT (1991) Multiple sequential molecular abnormalities in the evolution of human gliomas. Br J Cancer 36:753–757
Ueki K, Rubio M-P, Ramesh V, Correa KM, Rutter JL, Deimling A von, Buckler AJ, Gusella JF, Louis DN (1994) MTS1/CDKN2 gene mutations are rare in primary human astrocytomas with allelic loss of chromosome 9p. Hum Mol Genet 3:1841–1845
Von Deimling A, Eibl RH, Ohgaki H, Louis DN, Von Ammon K, Petersen I, Kleihues P, Chung RY, Wiestler OD, Seizinger BR (1992) p53 Mutations correlate with 17p allelic loss in grade II and grade III astrocytoma. Cancer Res 52:2897–2990
Von Deimling A, Louis DN, Von Ammon K, Petersen I, Hill T, Chung RY, Martuza RL, Schoenfeld DA, Yasargil MG, Wiestler OD, Seizinger BR (1992) Association of epidermal growth factor receptor gene amplification with loss of chromosome 10 in human glioblastoma multiforme. J Neurosurg 77:295–301
Von Deimling A, Louis DN, Von Ammon K, Petersen I, Wiestler OD, Seizinger BR (1992) Evidence for a tumor suppressor gene on chromosome 19q associated with human astrocytomas, oligodendrogliomas, and mixed gliomas. Cancer Res 52:4277–4299
Von Deimling A, Louis DN, Menon AG, Von Ammon K, Petersen P, Ellison D, Wiestler OD, Seizinger BR (1993) Deletions on the long arm of chromosome 17 in pilocytic astrocytoma. Acta Neuropathol 86:81–85
Von Deimling A, Von Ammon K, Schoenfeld D, Wiestler OD, Seizinger BR, Louis DN (1993) Subsets of glioblastoma multiforme defined by molecular genetic analysis. Brain Pathol 3:19–26
Walker DG, Duan W, Popovic EA, Kaye AH, Tomlinson FH, Lavin M (1995) Homozygous deletions of the multiple tumor suppressor gene 1 in the progression of human astrocytomas. Cancer Res 55:20–23
Watanabe K, Nagai M, Wakai S, Arai T, Kawashima K (1990) Loss of constitutional heterozygosity in chromosome 10 in human glioblastoma. Acta Neuropathol 80:251–254
Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B (1987) Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci 84:6899–6903
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohgaki, H., Kleihues, P., Schäuble, B. et al. Genetic alterations associated with the evolution and progression of astrocytic brain tumours. Vichows Archiv A Pathol Anat 427, 113–118 (1995). https://doi.org/10.1007/BF00196514
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00196514