Abstract
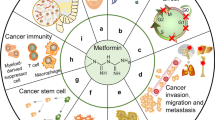
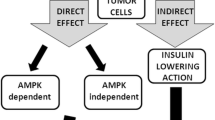
Metformin is considered, in conjunction with lifestyle modification, as a first-line treatment modality for type 2 diabetes mellitus (DM). Recently, several clinical studies have reported reduced incidence of neoplastic diseases in DM type 2 patients treated with metformin, as compared to diet or other antidiabetic agents. Moreover, in vitro studies have disclosed significant antiproliferative and proapoptotic effects of metformin on different types of cancer. Metformin acts by activating AMP-activated protein kinase (AMPK), a key player in the regulation of energy homeostasis. Moreover, by activating AMPK, metformin inhibits the mammalian target of rapamycin complex 1 (mTORC1) resulting in decreased cancer cell proliferation. Concomitantly, metformin induces activation of LKB1 (serine/threonine kinase 11), a tumor suppressor gene, which is required for the phosphorylation and activation of AMPK. These new encouraging experimental data supporting the anti-cancer effects of metformin urgently require further clinical studies in order to establish its use as a synergistic therapy targeting the AMPK/mTOR signaling pathway.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Nathan DM, Buse JB, Davidson MB, et al, 2006 Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32: 193–203.
Saydah SH, Loria CM, Eberhardt MS, Brancati FL, 2003 Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidem 157: 1092–1100.
Michels KB, Solomon CG, Hu FB, et al, 2003 Type 2 diabetes and subsequent incidence of breast cancer in the Nurses Health Study. Diabetes Care 26: 1752–1758.
Will JC, Galuska DA, Vinicor F, Calle EE, 1998 Colorectal cancer: another complication of diabetes mellitus? Am J Epidem 147: 816–825.
Everhart J, Wright D, 1995 Diabetes mellitus as risk factor for pancreatic cancer: a meta-analysis. JAMA 273: 1605–1609.
Gapstur SM, Gann PH, Colangelo LA, et al, 2001 Postload plasma glucose concentration and 27-year prostate cancer mortality (United States). Cancer Causes Control 12: 763–772.
Evans JM, Donnelly LA, Emslie-Smith AM, et al, 2005 Metformin and reduced risk of cancer in diabetic patients. BMJ 330: 1304–1305.
Bowker SL, Majumdar SR, Veugelers P, Johnson JA, 2006 Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29: 254–258.
Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JMM, 2009 New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 32: 1620–1625.
Moore MA, Park CB, Tsuda H, 1998 Implications of the hyperinsulinemia-diabetes-cancer link for preventive efforts. Eur J Cancer Prev 7: 89–107.
Hemkens LG, Grouven U, Bender R, 2009 Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 52: 1732–1744.
Futterweit W, Mechanick JI, 1988 Polycystic ovarian disease: etiology, diagnosis, and treatment. Compr Ther 14: 12–20.
Galluzzo A, Amato MC, Giordano C, 2008 Insulin resistance and polycystic ovary syndrome. Nutr Metab Cardiovas Dis 18: 511–518.
Coulam CB, Annegers JF, Kranz JS, 1983 Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol 61: 403–407.
Anderson KE, Sellers TA, Chen PL, et al, 1997 Association of Stein-Leventhal syndrome with the incidence of postmenopausal breast carcinoma in a large prospective study of women in Iowa. Cancer 79: 494–499.
Pierpoint T, McKeigue PM, Isaacs AJ, et al, 1998 Mortality of women with polycystic ovary syndrome at long term follow up. J Clin Epidemiol 51: 581–586.
Balen A, 2001 Polycystic ovary syndrome and cancer. Hum Reprod Update 6: 522–525.
Diabetes Prevention Program Research Group, 2002 Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403.
Ehrmann DA, 2005 Polycystic ovary syndrome. N Engl J Med 24: 1223–1236.
Campbell IW, Ritz P 2007 Understanding the glucose-lowering actions of metformin. In: Bailey CJ, Campbell IW, Chan JCN, Davidson JA, Howlett HCS, Ritz P (Eds), Metformin The Gold Standard, John Wiley & Sons, Ltd, Chichester, UK; pp, 77–88.
Detaille D, Guigas B, Leverve X, Wiernspreger N, Devos P, 2002 Obligatory role of membrane events in the regulatory effect of metformin on the respiratory chain function. Biochem Pharmacol 63: 1259–1272.
Giannarelli R, Aragona M, Coppelli A, Del Prato S, 2003 Reducing insulin resistance with metformin: the evidence today. Diabetes Metab 29: 628–635.
Matthaei S, Reibold JP, Hamman A, et al, 1993 In vivo metformin treatment ameliorates insulin resistance: evidence for potentiation of insulin-induced translocation and increased functional activity of glucose transporters in obese (fa/fa) Zucker rats adipocytes. Endocrinology 133: 304–311.
Musi N, Hirshman MF, Nygren J, et al, 2002 Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51: 2074–2081.
Bowker SL, Majumdar SR, Veugelers P, Johnson JA, 2006 Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29: 1990–1991.
Isakovic A, Harhaji L, Stevanovic D, et al, 2007 Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci 64: 1290–1302.
Buzzai M, Jones RG, Amaravadi RK, et al, 2007 Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 67: 6745–6752.
Zakikhani M, Dowling R, Fantus G, et al, 2006 Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 66: 10269–10273.
Ben Sahra I, Laurent K, Loubat A, et al, 2008 The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 27: 3576–3586.
Hadad S, Fleming S, Thompson A, 2008 Targeting AMPK: a new therapeutic opportunity in breast cancer. Crit Rev Oncol Hematol 67: 1–7.
Carling D, 2004 The AMP-activated protein kinase cascade — a unifying system for energy control. Trends Biochem Sci 29: 18–24.
Hardie DG, Scott JW, Pan DA, Hudson ER, 2003 Management of cellular energy by the AMP-activated protein kinase system. FEBS Letters 546: 113–120.
Alessi DR, Sakamoto K, Bayascas JR, 2006 LKB-1-dependent signaling pathways. Annu Rev Biochem 75: 137–163.
Hutber CA, Hardie DG, Winder WW, 1997 Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol 272: E 262–266.
Winder WW, Hardie DG, 1999 AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277: E1–10.
Willer A, 2003 Reduction of the individual cancer risk by physical exercise. Onkologie 26: 283–289.
Woods A, Johnstone SR, Dickerson K, et al, 2003 LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13: 2004–2008.
Shaw RJ, Lamia KA, Vasquez D, et al, 2005 The kinase LKB-1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646.
Thomson DM, Brown JD, Fillmore N, et al, 2007 LKB-1 and the regulation of malonyl-Co A and fatty acid oxidation in muscle. Am J Physiol Endocrinol Metab 293: E1572–1579.
Hawley SA, Boudeau J, Reid JL, et al, 2003 Complexes between LKB-1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2: 28.
Hoyer-Hansen M, Jaattela M, 2007 AMP-activated protein kinase: a universal regulator of autophagy? Autophagy 3: 381–383.
Chaiyapan W, Sangkhathat S, Kanngurn S, et al, 2010 Immunohistological evidence for Wnt-signaling activation in Peutz-Jeghers polyposis. Pediatr Surg Int 26: 173–177.
Kikuchi A, Yamamoto H, 2008 Tumor formation due to abnormalities in the β-catenin-independent pathway of Wnt signaling. Cancer Sci 99: 202–208.
Liu C, Tu Y, Sun X, et al, 2010 Wnt/ beta-Catenin pathway in human glioma: expression pattern and clinical/prognostic correlations. Clin Exp Med Aug 31 (e-pub ahead of print).
Inoki K, Corradetti MN, Guan KL, 2005 Dysregulation of TSC-mTOR pathway in human disease. Nat Gen 37: 19–24.
Van Slegtenhorst M, Nellist M, Nagelkerken B, et al, 1998 Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum Mol Genet 7: 1053–1057.
Mita MM, Mita A, Rowinsky EK, 2003 Mammalian target of rapamycin: a new molecular target for breast cancer. Clin Breast Cancer 4: 126–137.
Bose S, Chandran S, Mirocha JM, Bose N, 2006 The akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol 19: 238–245.
Shaw RJ, Bardeesy N, Manning BD, et al, 2004 The LKB-1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6: 91–99.
Zakikhani M, Blouin MJ, Piura E, Pollak MN, 2010 Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Res Treat published online: 05 February 2010.
Kuhajda FP, Pizer ES, Li JN, et al, 2000 Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci USA 97: 3450–3454.
Zhou G, Myers R, Li Y, et al, 2001 Role of AMP-activated protein kinase in mechanism of metfomin action. J Clin Invest 108: 1167–1174.
Xiang X, Saha AK, Wen R, et al, 2004 AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun 321: 161–167.
Jones RG, Plas DR, Kubek S, et al, 2005 AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18: 283–293.
Rajawat YS, Bossis I, 2008 Autophagy in aging and in neurodegenerative disorders. Hormones (Athens) 7: 46–61.
Giese A, Bjerkvig R, Berens M, Westphal M, 2003 Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21: 1624–1636.
Rattan R, Giri S, Singh A, Singh I, 2006 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem 280: 39582–39593.
Laderoute KR, Amin K, Calaogan JM, et al, 2006 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol 26: 5336–5347.
Shaw MM, Gurr WK, McCrimmon RJ, et al, 2007 5′-AMP-activated protein kinase alpha deficiency enhances stress-induced apoptosis in BHK and PC12 cells. J Cell Mol Med 11: 286–298.
Baumann P, Mandl-Weber S, Emmerich B, et al, 2007 Inhibition of adenosine monophosphate-activated protein kinase induces apoptosis in multiple myeloma cells. Anticancer Drugs 18: 405–410.
Vucicevic L, Misirkic M, Janetovic K, et al, 2009 AMP-activated protein kinase-dependent and -independent mechanisms underlying in vitro antiglioma action of compound C. Biochem Pharmacol 77: 1684–1693.
Wolf I, Sedetzki S, Catane R, et al, 2005 Diabetes mellitus and breast cancer. Lancet Oncol 6: 103–111.
Wolf I, Sedetzki S, Gluck I, et al, 2006 Association between diabetes mellitus and adverse characteristics of breast cancer at presentation. Eur J Cancer 42: 1077–1082.
Sachdev D, Singh R, Fujita-Yamaguchi Y, et al, 2006 Down-regulation of insulin receptor by antibodies against the type I insulin-like growth factor receptor: implication for anti-insulin-like growth factor therapy in breast cancer. Cancer Res 66: 2391–2402.
Goodwin PJ, Ennis M, Pritchard KI, et al, 2002 Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 20: 42–51.
Millikan RC, Newman B, Tse CK, et al, 2008 Epidemiology of basal-like breast cancer. Breast Cancer Res Treat 109: 123–139.
Yang XR, Sherman ME, Rimm DL, et al, 2007 Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev 16: 439–443.
Sorlie T, Wang Y, Xiao C, et al, 2006 Distinct molecular mechanisms underlying clinically relevant subtypes of breast cancer: gene expression analyses across three different platforms. BMC genomics 7: 127.
Liu B, Fan Z, Edgerton SM, et al, 2009 Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle 8: 2031–2040.
Alimova IN, Liu B, Fan Z, et al, 2009 Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 6: 909–915.
Zhuang Y, Miskimins WK, 2008 Cell cycle arrest in metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal 3: 18–28.
Neve RM, Chin K, Fridlyand J, et al, 2006 A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10: 515–527.
Phoenix KN, Vumbaca F, Claffey KP, 2009 Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERa negative MDA-MB-435 breast cancer model. Breast Cancer Res 113: 101–111.
Jiralerspong S, Giordano SH, Palla SL, et al, 2009 Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 27: 3297–3302.
Garcia A, Tisman G, 2010 Metformin, B12, and enhanced breast cancer response to chemotherapy. J Clin Oncol 28: p.e19.
Hwang JT, Ha J, Park IJ, et al, 2007 Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett 247: 115–121.
Hwang JT, Kim YM, Surh YJ, et al, 2006 Selenium regulates cyclooxygenase-2 and extracellular signal-regulated kinase signaling pathways by activating AMP-activated protein kinase in colon cancer cells. Cancer Res 66: 10057–10063.
Degenhardt K, Mathew R, Beaudoin B, et al, 2006 Autophagy promotes tumor cells survival and restricts necrosis, inflammation and tumorigenesis. Cancer Cell 10: 51–64.
Koepsell H, Lips K, Volk C, 2007 Polyspecific organic transporters: structure, function, physiologic roles, and biopharmaceutical implications. Pharm Res 24: 1227–1251.
Shu Y, Sheardown S, Brown C, et al, 2007 Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 117: 1422–1431.
Nies At, Koepsell H, Winter S, et al, 2009 Expression of organic cation transporterts OCT1(SLA22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 50: 1227–1240.
Tzvetkov MV, Vormfelde SV, Balen D, et al, 2009 The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3on the renal clearance of metformin. Clin Pharmacol Therap 86: 299–306.
Algire C, Zakikhani M, Blouin MJ, et al, 2008 Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLCI carcinoma growth. Endocr Relat Cancer 15: 833–839.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Micic, D., Cvijovic, G., Trajkovic, V. et al. Metformin: Its emerging role in oncology. Hormones 10, 5–15 (2011). https://doi.org/10.14310/horm.2002.1288
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14310/horm.2002.1288