Abstract
In the spectrum of breast malignancies, triple-negative breast cancer is the most widely spreading subtype of breast cancer due to a low availability of therapeutic remedies. Recently, antibody–drug conjugates dramatically resolved the landscape for the treatment of triple-negative breast cancer. This review mainly focuses on the chemistry, structure, mechanism of action, and role of antibody–drug conjugates in triple-negative breast cancer. Datopotecan Deruxtecan (Dato-DXd) is a new-generation ADC showing encouraging results for TNBC. In this review, we have also emphasized TROP-2-directed Datopotamab deruxtecan ADCs to treat triple-negative breast cancer, its synthesis, mechanism of action, pharmacokinetics, pharmacodynamics, adverse events, and their ongoing clinical trials.
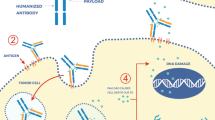
Graphical abstract











Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
Abbreviations
- ADCs:
-
Antibody–drug conjugates
- AML:
-
Acute myeloid leukemia
- mAbs:
-
Monoclonal antibodies
- GO:
-
Gemtuzumab ozogamicin
- AEs:
-
Adverse events
- OS:
-
Overall survival
- BSC:
-
Best supportive care
- MMAE:
-
Monomethyl auristatin E
- HL:
-
Hodgkin lymphoma
- ALCL:
-
Anaplastic large cell lymphoma
- MF:
-
Mycosis fungoides
- C-ALCL:
-
Cutaneous anaplastic large cell lymphoma
- PTCL:
-
Peripheral T-cell lymphomas
- ORR:
-
Objective response rate
- PFS:
-
Progression-free survival
- CR:
-
Complete response
- T-DM1:
-
Trastuzumab emtansine DM1 emtansine
- UC:
-
Urothelial carcinoma
- InO:
-
Inotuzumab ozogamicin
- ALL:
-
Acute lymphoblastic leukemia
- SoC:
-
Standard-of-care
- MP:
-
Moxetumomab pasudotox
- HCL:
-
Hairy cell leukemia
- PV:
-
Polatuzumab vedotin
- DLBCL:
-
Diffuse large B-cell lymphoma
- NHL:
-
Non-Hodgkin lymphoma
- LBCL:
-
Large B cell lymphoma
- EV:
-
Enfortumab vedotin
- la/mUC:
-
Locally advanced or metastatic urothelial
- QOL:
-
Quality of life
- BPI-SF:
-
Brief Pain Inventory Short Form
- T-DXd:
-
Trastuzumab deruxtecan
- SG:
-
Sacituzumab govitecan
- Trop-2:
-
Trophoblast cell surface antigen 2
- TNBC:
-
Triple-negative breast cancer
- CRC:
-
Colorectal cancer
- SCLC:
-
Small cell lung cancer
- GC:
-
Gastric cancer
- ICIs:
-
Immune checkpoint inhibitors
- TRAEs:
-
Treatment-related adverse events
- RC48:
-
Disitamab vedotin
- NMPA:
-
National Medical Products Administration
- DCR:
-
Disease control rate
- TV:
-
Tisotumab vedotin
- TF:
-
Tissue factor
- NSCLC:
-
Non-small cell lung cancer
- r/mCC:
-
Recurrent or metastatic cervical cancer
- LT:
-
Loncastuximab tesirine
- PBD:
-
Pyrrolobenzodiazepine
- MIRV:
-
Mirvetuximab soravtansin
- FRα:
-
Folate receptor α
- PE38:
-
Pseudomonas exotoxin A
- DM4:
-
Maytansinoid
- DXd:
-
Deruxtecan
- DAR:
-
Drug-to-antibody ratio
- PARP:
-
Poly-ADP ribose polymerase
- AR:
-
Androgen receptor
- Wnt:
-
Wingless-related integration site
- PI3K:
-
Phosphoinositide 3-kinase
- mTOR:
-
Mechanistic target of rapamycin
- TGF-β:
-
Transforming growth factor beta
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- IM:
-
Immunomodulatory
- LAR:
-
Luminal androgen receptor
- BL-1:
-
Basal-like 1
- BL-2:
-
Basal-like 2
- M:
-
Mesenchymal
- MSL:
-
Mesenchymal stem-like
References
Zraik IM, Heß-Busch Y. Management of chemotherapy side effects and their long-term sequelae. Urol A. 2021;60:862–71. https://doi.org/10.1007/s00120-021-01569-7.
Wang Z, Li H, Gou L, Li W, Wang Y. Antibody–drug conjugates: recent advances in payloads. Acta Pharm Sin B. 2023;13:4025–59. https://doi.org/10.1016/j.apsb.2023.06.015.
Ponziani S, Di Vittorio G, Pitari G, Cimini AM, Ardini M, Gentile R, Iacobelli S, Sala G, Capone E, Flavell DJ, Ippoliti R, Giansanti F. Antibody-drug conjugates: the new frontier of chemotherapy. Int J Mol Sci. 2020;21:5510. https://doi.org/10.3390/ijms21155510.
Biomedicines | Free Full-Text | Advances and limitations of antibody drug conjugates for cancer. n.d. https://www.mdpi.com/2227-9059/9/8/872. Accessed 1 May 2024.
Xu X, Zhang J, Wang T, Li J, Rong Y, Wang Y, Bai C, Yan Q, Ran X, Wang Y, Zhang T, Sun J, Jiang Q. Emerging non-antibody-drug conjugates (non-ADCs) therapeutics of toxins for cancer treatment. Acta Pharm Sin B. 2023. https://doi.org/10.1016/j.apsb.2023.11.029.
Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–80. https://doi.org/10.1038/nrc2394.
Cure of xenografted human carcinomas by BR96-doxorubicin immunoconjugates | Science. n.d. https://doi.org/10.1126/science.8327892. Accessed 1 May 2024.
Rosenberg B, Van Camp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–9. https://doi.org/10.1038/205698a0.
Paclitaxel (Taxol) | N Engl J Med. n.d. https://doi.org/10.1056/NEJM199504133321507. Accessed 1 May 2024.
Johnson DA, Laguzza BC. Antitumor xenograft activity with a conjugate of a vinca derivative and the squamous carcinoma-reactive monoclonal antibody PF1/D. Can Res. 1987;47:3118–22.
Dillman RO, Johnson DE, Shawler DL, Koziol JA. Superiority of an acid-labile daunorubicin-monoclonal antibody immunoconjugate compared to free drug1. Can Res. 1988;48:6097–102.
Tolcher AW, Sugarman S, Gelmon KA, Cohen R, Saleh M, Isaacs C, Young L, Healey D, Onetto N, Slichenmyer W. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J Clin Oncol. 1999;17:478–478. https://doi.org/10.1200/JCO.1999.17.2.478.
Niell BL, Jochelson MS, Amir T, Brown A, Adamson M, Baron P, Bennett DL, Chetlen A, Dayaratna S, Freer PE, Ivansco LK, Klein KA, Malak SF, Mehta TS, Moy L, Neal CH, Newell MS, Richman IB, Schonberg M, Small W, Ulaner GA, Slanetz PJ. ACR Appropriateness Criteria® female breast cancer screening: 2023 update. J Am Coll Radiol. 2023;21(2024):S126–43. https://doi.org/10.1016/j.jacr.2024.02.019.
Sharma P. Update on the treatment of early-stage triple-negative breast cancer. Curr Treat Opt Oncol. 2018;19:22. https://doi.org/10.1007/s11864-018-0539-8.
Nounou MI, ElAmrawy F, Ahmed N, Abdelraouf K, Goda S, Syed-Sha-Qhattal H. Breast cancer: conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer (Auckl). 2015;92s:BCBCR.S29420. https://doi.org/10.4137/BCBCR.S29420.
Molecular portraits of human breast tumours | Nature. n.d. https://www.nature.com/articles/35021093. Accessed 9 June 2024.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale A-L. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci. 2001;98:10869–74. https://doi.org/10.1073/pnas.191367098.
JCI—identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. n.d. https://www.jci.org/articles/view/45014?elq=2f1e11aad7e740cf9a3d8bfd51c3b4f4. Accessed 9 June 2024.
Wang D-Y, Jiang Z, Ben-David Y, Woodgett JR, Zacksenhaus E. Molecular stratification within triple-negative breast cancer subtypes. Sci Rep. 2019;9:19107. https://doi.org/10.1038/s41598-019-55710-w.
De Soto JA, Wang X, Tominaga Y, Wang R-H, Cao L, Qiao W, Li C, Xu X, Skoumbourdis AP, Prindiville SA, Thomas CJ, Deng C-X. The inhibition and treatment of breast cancer with poly(ADP-ribose) polymerase (PARP-1) inhibitors. Int J Biol Sci. 2006;2:179–85.
Hu X-C, Zhang J, Xu B-H, Cai L, Ragaz J, Wang Z-H, Wang B-Y, Teng Y-E, Tong Z-S, Pan Y-Y, Yin Y-M, Wu C-P, Jiang Z-F, Wang X-J, Lou G-Y, Liu D-G, Feng J-F, Luo J-F, Sun K, Gu Y-J, Wu J, Shao Z-M. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16:436–46. https://doi.org/10.1016/S1470-2045(15)70064-1.
TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal-like) breast cancer | J Clin Oncol. n.d. https://doi.org/10.1200/jco.2008.26.15_suppl.1009. Accessed 9 June 2024.
Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, Blackwell K, Rugo H, Nabell L, Forero A, Stearns V, Doane AS, Danso M, Moynahan ME, Momen LF, Gonzalez JM, Akhtar A, Giri DD, Patil S, Feigin KN, Hudis CA, Traina TA, on behalf of the Translational Breast Cancer Research Consortium (TBCRC 011). Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19:5505–12. https://doi.org/10.1158/1078-0432.CCR-12-3327.
β-Catenin is required for the tumorigenic behavior of triple-negative breast cancer cells | PLoS ONE. n.d. https://doi.org/10.1371/journal.pone.0117097. Accessed 9 June 2024.
Bhola NE, Balko JM, Dugger TC, Kuba MG, Sánchez V, Sanders M, Stanford J, Cook RS, Arteaga CL. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123:1348–58. https://doi.org/10.1172/JCI65416.
Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer: evidence from a phase 1 trial of mTOR inhibition in combination with liposomal doxorubicin and bevacizumab | Breast Cancer | JAMA Oncology | JAMA Network. n.d. https://jamanetwork.com/journals/jamaoncology/article-abstract/2587051. Accessed 9 June 2024.
Nanda R, Chow LQM, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34:2460–7. https://doi.org/10.1200/JCO.2015.64.8931.
Yin L, Duan J-J, Bian X-W, Yu S. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22:61. https://doi.org/10.1186/s13058-020-01296-5.
Jeyachandran S, Chandrashekar K, Ganesan G, Alagarsamy L, Subbaraj GK, Kulanthaivel L. Triple-negative breast cancer (TNBC): clinical features and therapeutic targets. In: Pathak S, Banerjee A, Bisgin A, editors. Handbook of animal models and its uses in cancer research. Singapore: Springer Nature; 2022. p. 1–14. https://doi.org/10.1007/978-981-19-1282-5_41-1.
Al Jarroudi O, El Bairi K, Curigliano G, Afqir S. Antibody–drug conjugates: a new therapeutic approach for triple-negative breast cancer. In: Al Jarroudi O, El Bairi K, Curigliano G, editors. Breast cancer research and treatment: innovative concepts. Cham: Springer International Publishing; 2023. p. 1–27. https://doi.org/10.1007/978-3-031-33602-7_1.
Liu K, Li M, Li Y, Li Y, Chen Z, Tang Y, Yang M, Deng G, Liu H. A review of the clinical efficacy of FDA-approved antibody–drug conjugates in human cancers. Mol Cancer. 2024;23:62. https://doi.org/10.1186/s12943-024-01963-7.
Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody–drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18:3–19. https://doi.org/10.1158/1541-7786.MCR-19-0582.
Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? | Br J Cancer. n.d. https://www.nature.com/articles/bjc2017367. Accessed 7 June 2024.
Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018;9:33–46. https://doi.org/10.1007/s13238-016-0323-0.
Optimizing conjugation chemistry, antibody conjugation site, and surface density in antibody–nanogel conjugates (ANCs) for cell-specific drug delivery. Bioconjug Chem. 2023; 34707–718. https://doi.org/10.1021/acs.bioconjchem.3c00034.
King TA, Walsh SJ, Kapun M, Wharton T, Krajcovicova S, Glossop MS, Spring DR. Disulfide re-bridging reagents for single-payload antibody-drug conjugates. Chem Commun. 2023;59:9868–71. https://doi.org/10.1039/D3CC02980H.
Ahangarpour M, Brimble MA, Kavianinia I. Late-stage desulfurization enables rapid and efficient solid-phase synthesis of cathepsin-cleavable linkers for antibody–drug conjugates. Bioconjug Chem. 2024. https://doi.org/10.1021/acs.bioconjchem.4c00199.
Nguyen TD, Bordeau BM, Balthasar JP. Mechanisms of ADC toxicity and strategies to increase ADC tolerability. Cancers. 2023;15:713. https://doi.org/10.3390/cancers15030713.
Maiti R, Patel B, Patel N, Patel M, Patel A, Dhanesha N. Antibody drug conjugates as targeted cancer therapy: past development, present challenges and future opportunities. Arch Pharm Res. 2023;46:361–88. https://doi.org/10.1007/s12272-023-01447-0.
Sheyi R, de la Torre BG, Albericio F. Linkers: an assurance for controlled delivery of antibody-drug conjugate. Pharmaceutics. 2022;14:396. https://doi.org/10.3390/pharmaceutics14020396.
Othman J, Dillon R. Time to add gemtuzumab ozogamicin to intensive chemotherapy for NPM1-mutated acute myeloid leukaemia? Lancet Haematol. 2023;10:e478–9. https://doi.org/10.1016/S2352-3026(23)00092-3.
Kegyes D, Ghiaur G, Bancos A, Tomuleasa C, Gale RP. Immune therapies of B-cell acute lymphoblastic leukaemia in children and adults. Crit Rev Oncol Hematol. 2024;196: 104317. https://doi.org/10.1016/j.critrevonc.2024.104317.
Wang L, Liu L, Zhang Z, Li F, Ruan Y, He Y, Huang J, Zheng X. Cost-effectiveness of sacituzumab govitecan versus single-agent chemotherapy for patients with metastatic triple-negative breast cancer in China. Clin Breast Cancer. 2024. https://doi.org/10.1016/j.clbc.2024.04.010.
Bowers JT, Anna J, Bair SM, Annunzio K, Epperla N, Pullukkara JJ, Gaballa S, Spinner MA, Li S, Messmer MR, Nguyen J, Ayers EC, Wagner CB, Hu B, Di M, Huntington SF, Furqan F, Shah NN, Chen C, Ballard HJ, Hughes ME, Chong EA, Nasta SD, Barta SK, Landsburg DJ, Svoboda J. Brentuximab vedotin plus AVD for Hodgkin lymphoma: incidence and management of peripheral neuropathy in a multisite cohort. Blood Adv. 2023;7:6630–8. https://doi.org/10.1182/bloodadvances.2023010622.
Liao MZ, Lu D, Lu T, Gibiansky L, Deng R, Samineni D, Dere R, Lin A, Hirata J, Shen B-Q, Zhang D, Li D, Li C, Miles D. Clinical pharmacology strategies to accelerate the development of polatuzumab vedotin and summary of key findings. Adv Drug Deliv Rev. 2024;207: 115193. https://doi.org/10.1016/j.addr.2024.115193.
Spira A, Zhou X, Chen L, Gnanasakthy A, Wang L, Ungar D, Curiel R, Liao L, Radford J, Kahl B. Health-related quality of life, symptoms, and tolerability of loncastuximab tesirine in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2022;22:158–68. https://doi.org/10.1016/j.clml.2021.09.001.
Rodriguez-Otero P, Tamariz LE, San-Miguel JF. Single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma. Lancet Haematol. 2023;10:e786–7. https://doi.org/10.1016/S2352-3026(23)00278-8.
Antonarelli G, Corti C, Tarantino P, Salimbeni BT, Zagami P, Marra A, Trapani D, Tolaney S, Cortes J, Curigliano G. Management of patients with HER2-positive metastatic breast cancer after trastuzumab deruxtecan failure. ESMO Open. 2023;8: 101608. https://doi.org/10.1016/j.esmoop.2023.101608.
Gogia P, Ashraf H, Bhasin S, Xu Y. Antibody–drug conjugates: a review of approved drugs and their clinical level of evidence. Cancers. 2023;15:3886. https://doi.org/10.3390/cancers15153886.
Baah S, Laws M, Rahman KM. Antibody–drug conjugates—a tutorial review. Molecules. 2021;26:2943. https://doi.org/10.3390/molecules26102943.
Aretin M-B. Antibody–drug conjugates—the magic bullet? Memo. 2022;15:125–8. https://doi.org/10.1007/s12254-021-00780-8.
Domínguez-Llamas S, Caro-Magdaleno M, Mataix-Albert B, Avilés-Prieto J, Romero-Barranca I, Rodríguez-de-la-Rúa E. Adverse events of antibody–drug conjugates on the ocular surface in cancer therapy. Clin Transl Oncol. 2023;25:3086–100. https://doi.org/10.1007/s12094-023-03261-y.
Bashraheel SS, Domling A, Goda SK. Update on targeted cancer therapies, single or in combination, and their fine tuning for precision medicine. Biomed Pharmacother. 2020;125: 110009. https://doi.org/10.1016/j.biopha.2020.110009.
Ocular adverse events associated with antibody–drug conjugates in human clinical trials | J Ocul Pharmacol Ther. n.d. https://doi.org/10.1089/jop.2015.0064. Accessed 6 June 2024.
Lambert JM, Morris CQ. Antibody-drug conjugates (ADCs) for personalized treatment of solid tumors: a review. Adv Ther. 2017;34:1015–35. https://doi.org/10.1007/s12325-017-0519-6.
Molecules | Free Full-Text | Antibody–drug conjugates for cancer therapy. n.d. https://www.mdpi.com/1420-3049/25/20/4764. Accessed 6 June 2024.
Lapusan S, Vidriales MB, Thomas X, de Botton S, Vekhoff A, Tang R, Dumontet C, Morariu-Zamfir R, Lambert JM, Ozoux M-L, Poncelet P, San Miguel JF, Legrand O, DeAngelo DJ, Giles FJ, Marie J-P. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Invest New Drugs. 2012;30:1121–31. https://doi.org/10.1007/s10637-011-9670-0.
Teicher BA, Chari RVJ. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17:6389–97. https://doi.org/10.1158/1078-0432.CCR-11-1417.
Human antibodies from transgenic animals | Nat Biotechnol. n.d. https://www.nature.com/articles/nbt1135. Accessed 6 June 2024.
Marmé F. Antibody-drug conjugates for breast cancer. Oncology Research and Treatment. 2021;45:26–36. https://doi.org/10.1159/000521499.
Trastuzumab emtansine for residual invasive HER2-positive breast cancer | N Engl J Med. n.d. https://doi.org/10.1056/nejmoa1814017. Accessed 6 July 2024.
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, Lee KS, Niikura N, Park YH, Xu B, Wang X, Gil-Gil M, Li W, Pierga J-Y, Im S-A, Moore Halle CF, Rugo Hope S, Yerushalmi R, Zagouri F, Gombos A, Kim S-B, Liu Q, Luo T, Saura C, Schmid P, Sun T, Gambhire D, Yung L, Wang Y, Singh J, Vitazka P, Meinhardt G, Harbeck N, Cameron DA. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. https://doi.org/10.1056/NEJMoa2203690.
Keskinkilic M, Sacks R. Antibody-drug conjugates in triple negative breast cancer. Clin Breast Cancer. 2024;24:163–74. https://doi.org/10.1016/j.clbc.2024.01.008.
Dri A, Arpino G, Bianchini G, Curigliano G, Danesi R, De Laurentiis M, Del Mastro L, Fabi A, Generali D, Gennari A, Guarneri V, Santini D, Simoncini E, Zamagni C, Puglisi F. Breaking barriers in triple negative breast cancer (TNBC)—unleashing the power of antibody-drug conjugates (ADCs). Cancer Treat Rev. 2024;123: 102672. https://doi.org/10.1016/j.ctrv.2023.102672.
Qiu S, Zhang J, Wang Z, Lan H, Hou J, Zhang N, Wang X, Lu H. Targeting Trop-2 in cancer: recent research progress and clinical application. Biochim Biophys Acta. 2023;1878:188902. https://doi.org/10.1016/j.bbcan.2023.188902.
Oncogene-mediated metabolic gene signature predicts breast cancer outcome | npj Breast Cancer. n.d. https://www.nature.com/articles/s41523-021-00341-6. Accessed 14 June 2024.
Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9:28989–9006. https://doi.org/10.18632/oncotarget.25615.
Schipilliti FM, Drittone D, Mazzuca F, La Forgia D, Guven DC, Rizzo A. Datopotamab deruxtecan: a novel antibody drug conjugate for triple-negative breast cancer. Heliyon. 2024;10: e28385. https://doi.org/10.1016/j.heliyon.2024.e28385.
Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, Hirai T, Atsumi R, Nakada T, Hayakawa I, Abe Y, Agatsuma T. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–108. https://doi.org/10.1158/1078-0432.CCR-15-2822.
Ocean AJ, Starodub AN, Bardia A, Vahdat LT, Isakoff SJ, Guarino M, Messersmith WA, Picozzi VJ, Mayer IA, Wegener WA, Maliakal P, Govindan SV, Sharkey RM, Goldenberg DM. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: safety and pharmacokinetics. Cancer. 2017;123:3843–54. https://doi.org/10.1002/cncr.30789.
Nakada T, Masuda T, Naito H, Yoshida M, Ashida S, Morita K, Miyazaki H, Kasuya Y, Ogitani Y, Yamaguchi J, Abe Y, Honda T. Novel antibody drug conjugates containing exatecan derivative-based cytotoxic payloads. Bioorg Med Chem Lett. 2016;26:1542–5. https://doi.org/10.1016/j.bmcl.2016.02.020.
Okajima D, Yasuda S, Maejima T, Karibe T, Sakurai K, Aida T, Toki T, Yamaguchi J, Kitamura M, Kamei R, Fujitani T, Honda T, Shibutani T, Muramatsu S, Nakada T, Goto R, Takahashi S, Yamaguchi M, Hamada H, Noguchi Y, Murakami M, Abe Y, Agatsuma T. Datopotamab deruxtecan, a novel TROP2-directed antibody–drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther. 2021;20:2329–40. https://doi.org/10.1158/1535-7163.MCT-21-0206.
Conilh L, Sadilkova L, Viricel W, Dumontet C. Payload diversification: a key step in the development of antibody–drug conjugates. J Hematol Oncol. 2023;16:3. https://doi.org/10.1186/s13045-022-01397-y.
Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, Brufsky A, Sardesai SD, Kalinsky K, Zelnak AB, Weaver R, Traina T, Dalenc F, Aftimos P, Lynce F, Diab S, Cortés J, O’Shaughnessy J, Diéras V, Ferrario C, Schmid P, Carey LA, Gianni L, Piccart MJ, Loibl S, Goldenberg DM, Hong Q, Olivo MS, Itri LM, Rugo HS. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–41. https://doi.org/10.1056/NEJMoa2028485.
Rugo HS, Tolaney SM, Loirat D, Punie K, Bardia A, Hurvitz SA, O’Shaughnessy J, Cortés J, Diéras V, Carey LA, Gianni L, Piccart MJ, Loibl S, Goldenberg DM, Hong Q, Olivo M, Itri LM, Kalinsky K. Safety analyses from the phase 3 ASCENT trial of sacituzumab govitecan in metastatic triple-negative breast cancer. npj Breast Cancer. 2022;8:1–10. https://doi.org/10.1038/s41523-022-00467-1.
Heist RS, Sands J, Bardia A, Shimizu T, Lisberg A, Krop I, Yamamoto N, Kogawa T, Al-Hashimi S, Fung SSM, Galor A, Pisetzky F, Basak P, Lau C, Meric-Bernstam F. Clinical management, monitoring, and prophylaxis of adverse events of special interest associated with datopotamab deruxtecan. Cancer Treat Rev. 2024;125: 102720. https://doi.org/10.1016/j.ctrv.2024.102720.
Rugo HS, Bardia A, Marmé F, Cortés J, Schmid P, Loirat D, Tredan O, Ciruelos EM, Dalenc F, Pardo PG, Jhaveri K, Delaney RJ, Valdez T, Wang H, Verret W, Tolaney SM. LBA76 overall survival (OS) results from the phase III TROPiCS-02 study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with HR+/HER2- metastatic breast cancer (mBC). Ann Oncol. 2022;33:S1386. https://doi.org/10.1016/j.annonc.2022.08.012.
Krop I, Juric D, Shimizu T, Tolcher A, Spira A, Mukohara T, Lisberg AE, Kogawa T, Papadopoulos KP, Hamilton E, Damodaran S, Greenberg J, Gu W, Kobayashi F, Guevara F, Jikoh T, Kawasaki Y, Meric-Bernstam F, Bardia A. Abstract GS1–05: datopotamab deruxtecan in advanced/metastatic HER2- breast cancer: results from the phase 1 TROPION-PanTumor01 study. Cancer Res. 2022;82:GS1-05. https://doi.org/10.1158/1538-7445.SABCS21-GS1-05.
Schmid P, Jung KH, Wysocki PJ, Jassem J, Ma CX, Fernandes R, Huisden R, Stewart R, Vukovic P, Nunes AT, Nowecki Z. 166MO Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): initial results from BEGONIA, a phase Ib/II study. Ann Oncol. 2022;33:S199. https://doi.org/10.1016/j.annonc.2022.03.185.
Bardia A, Krop IE, Kogawa T, Juric D, Tolcher AW, Hamilton EP, Mukohara T, Lisberg A, Shimizu T, Spira AI, Tsurutani J, Damodaran S, Papadopoulos KP, Greenberg J, Kobayashi F, Zebger-Gong H, Wong R, Kawasaki Y, Nakamura T, Meric-Bernstam F. Datopotamab deruxtecan in advanced or metastatic HR+/HER2- and triple-negative breast cancer: results from the Phase I TROPION-PanTumor01 Study. J Clin Oncol. 2024. https://doi.org/10.1200/JCO.23.01909.
Bardia A, Jhaveri K, Kalinsky K, Pernas S, Tsurutani J, Xu B, Hamilton E, Im S-A, Nowecki Z, Sohn J, Laurentiis MD, Jañez NM, Adamo B, Lee KS, Jung KH, Rubovszky G, Tseng L-M, Lu Y-S, Yuan Y, Maxwell MJ, Haddad V, Khan SS, Rugo HS, Pistilli B. TROPION-Breast01: datopotamab deruxtecan vs chemotherapy in pre-treated inoperable or metastatic HR+/HER2- breast cancer. Future Oncol. 2024;20:423–36. https://doi.org/10.2217/fon-2023-0188.
Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer | N Engl J Med. n.d. https://doi.org/10.1056/nejmoa2115022. Accessed 14 June 2024.
Hurvitz SA, Hegg R, Chung W-P, Im S-A, Jacot W, Ganju V, Chiu JWY, Xu B, Hamilton E, Madhusudan S, Iwata H, Altintas S, Henning J-W, Curigliano G, Perez-Garcia JM, Kim S-B, Petry V, Huang C-S, Li W, Frenel J-S, Antolin S, Yeo W, Bianchini G, Loi S, Tsurutani J, Egorov A, Liu Y, Cathcart J, Ashfaque S, Cortés J. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. The Lancet. 2023;401:105–17. https://doi.org/10.1016/S0140-6736(22)02420-5.
Shastry M, Jacob S, Rugo HS, Hamilton E. Antibody-drug conjugates targeting TROP-2: clinical development in metastatic breast cancer. The Breast. 2022;66:169–77. https://doi.org/10.1016/j.breast.2022.10.007.
M-Rabet M, Cabaud O, Josselin E, Finetti P, Castellano R, Farina A, Agavnian-Couquiaud E, Saviane G, Collette Y, Viens P, Gonçalves A, Ginestier C, Charafe-Jauffret E, Birnbaum D, Olive D, Bertucci F, Lopez M. Nectin-4: a new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann Oncol. 2017;28:769–76. https://doi.org/10.1093/annonc/mdw678.
Guo P, Huang J, Zhu B, Huang AC, Jiang L, Fang J, Moses MA. A rationally designed ICAM1 antibody drug conjugate eradicates late-stage and refractory triple-negative breast tumors in vivo. Sci Adv. 2023;9:eabq7866. https://doi.org/10.1126/sciadv.abq7866.
Zhu S, Wu Y, Song B, Yi M, Yan Y, Mei Q, Wu K. Recent advances in targeted strategies for triple-negative breast cancer. J Hematol Oncol. 2023;16:100. https://doi.org/10.1186/s13045-023-01497-3.
Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blättler WA, Lambert JM, Chari RVJ, Lutz RJ, Wong WLT, Jacobson FS, Koeppen H, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90. https://doi.org/10.1158/0008-5472.CAN-08-1776.
Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128:347–56. https://doi.org/10.1007/s10549-010-1090-x.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh D-Y, Diéras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. https://doi.org/10.1056/NEJMoa1209124.
Krop IE, Kim S-B, González-Martín A, LoRusso PM, Ferrero J-M, Smitt M, Yu R, Leung ACF, Wildiers H. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–99. https://doi.org/10.1016/S1470-2045(14)70178-0.
Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, Krop IE, Blackwell K, Hoersch S, Xu J, Green M, Gianni L. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–42. https://doi.org/10.1016/S1470-2045(17)30312-1.
Sussman D, Smith LM, Anderson ME, Duniho S, Hunter JH, Kostner H, Miyamoto JB, Nesterova A, Westendorf L, Van Epps HA, Whiting N, Benjamin DR. SGN–LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther. 2014;13:2991–3000. https://doi.org/10.1158/1535-7163.MCT-13-0896.
Tsai M, Han HS, Montero AJ, Tkaczuk KH, Assad H, Pusztai L, Hurvitz SA, Wilks ST, Specht JM, Nanda R, Mita M, O’Shaughnessy J, Krop IE, Medgyesy D, Abraham J, Modi S, Li H, Wu S, Garfin PM, Burris HA. 259P Weekly ladiratuzumab vedotin monotherapy for metastatic triple-negative breast cancer. Ann Oncol. 2021;32:S474–5. https://doi.org/10.1016/j.annonc.2021.08.542.
Koster K-L, Huober J, Joerger M. New antibody-drug conjugates (ADCs) in breast cancer—an overview of ADCs recently approved and in later stages of development. Explor Target Anti-Tumor Ther. 2022;3:27. https://doi.org/10.37349/etat.2022.00069.
Meisel JL, Pluard TJ, Vinayak S, Stringer-Reasor EM, Brown-Glaberman U, Dillon PM, Basho RK, Varadarajan R, O’Shaughnessy J, Han HS, Sinha R, Fox JR, Villanueva R, Chen LC, Wu S, Li H, Tran S, Manso L. Phase 1b/2 study of ladiratuzumab vedotin (LV) in combination with pembrolizumab for first-line treatment of triple-negative breast cancer (SGNLVA-002, trial in progress). J Clin Oncol. 2022;40:TPS1127. https://doi.org/10.1200/JCO.2022.40.16_suppl.TPS1127.
Grinda T, Rassy E, Pistilli B. Antibody–drug conjugate revolution in breast cancer: the road ahead. Curr Treat Options in Oncol. 2023;24:442–65. https://doi.org/10.1007/s11864-023-01072-5.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SNS: writing review and editing, conceptualization. GGN: writing original draft and review. ANS & VAJ: conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this work.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sawant, S., Naik, G.G., Sahu, A.N. et al. Understanding the chemistry & pharmacology of antibody–drug conjugates in triple-negative breast cancer with special reference to exatecan derivatives. Med Oncol 41, 301 (2024). https://doi.org/10.1007/s12032-024-02542-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02542-y