Abstract
Purpose
While MEK inhibitors demonstrated activity in metastatic triple negative breast cancer (mTNBC) preclinical studies, preclinical, and clinical studies implicate rapid development of resistance limiting clinical benefit. The purpose of this study was to determine response rate for Trametinib alone and in combination with Uprosertib in patients with mTNBC previously treated with chemotherapy.
Methods
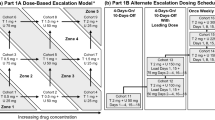
This was an open-label, two-part, phase II, single-arm, multicenter study. Patients first received Trametinib monotherapy (2 mg daily; Part I) then at progression transitioned to Trametinib (1.5 mg) plus Uprosertib (50 mg; Part II).
Results
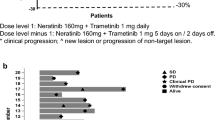
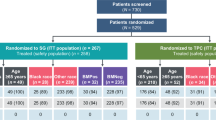
Between October 2013 and January 2017, 37 patients were enrolled to Part I. Subsequently, 19 patients entered Part II. Of the 37 patients receiving Trametinib monotherapy, 2 patients achieved partial response (PR) for an ORR of 5.4% (2/37) and an additional 6/37 (16.2%) achieved stable disease (SD). The clinical benefit rate (PR+SD) for patients receiving monotherapy was 21.6% (8/37). Of the 19 patients in Part II, 3 patients achieved PR for an ORR to Part II of 15.8% (3/19) and an additional 3 achieved SD. Median progression-free survival (PFS) was 7.7 weeks for Part I and 7.8 weeks for Part II. Circulating tumor DNA (ctDNA) clearance at C2D1 of Trametinib monotherapy was associated with improved PFS and overall survival.
Conclusion
In patients with mTNBC, Trametinib monotherapy demonstrated limited efficacy and addition of Uprosertib was associated with numerically greater objective responses but no difference in PFS. Translational analyses suggest ctDNA clearance as a potential early biomarker of response.



Similar content being viewed by others
Data availability
Bhuvaneswari Ramaswamy and Daniel G. Stover had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73:17–48. https://doi.org/10.3322/CAAC.21763
Obidiro O, Battogtokh G, Akala EO (2023) Triple negative breast cancer treatment options and limitations: future outlook. Pharmaceutics. https://doi.org/10.3390/PHARMACEUTICS15071796
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434. https://doi.org/10.1158/1078-0432.CCR-06-3045
Santana-Davila R, Perez EA (2010) Treatment options for patients with triple-negative breast cancer. J Hematol Oncol 3:1–11. https://doi.org/10.1186/1756-8722-3-42/TABLES/1
Bertucci F, Finetti P, Cervera N et al (2008) How basal are triple-negative breast cancers? Int J cancer 123:236–240. https://doi.org/10.1002/IJC.23518
Liedtke C, Mazouni C, Hess KR et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275–1281. https://doi.org/10.1200/JCO.2007.14.4147
Zhu S, Wu Y, Song B et al (2023) Recent advances in targeted strategies for triple-negative breast cancer. J Hematol Oncol 161(16):1–36. https://doi.org/10.1186/S13045-023-01497-3
Huppert LA, Gumusay O, Rugo HS (2022) Emerging treatment strategies for metastatic triple-negative breast cancer. Ther Adv Med Oncol. https://doi.org/10.1177/17588359221086916
Li Y, Zhang H, Merkher Y et al (2022) Recent advances in therapeutic strategies for triple-negative breast cancer. J Hematol Oncol. https://doi.org/10.1186/S13045-022-01341-0
Hoeflich KP, O’Brien C, Boyd Z et al (2009) In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res 15:4649–4664. https://doi.org/10.1158/1078-0432
Flaherty KT, Robert C, Hersey P et al (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367:107–114. https://doi.org/10.1056/NEJMOA1203421
Zhao Y, Adjei AA (2014) The clinical development of MEK inhibitors. Nat Rev Clin Oncol 11:385–400. https://doi.org/10.1038/NRCLINONC.2014.83
Kun E, Tsang YTM, Ng CW et al (2021) MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat Rev. https://doi.org/10.1016/J.CTRV.2020.102137
Gershenson DM, Miller A, Brady WE et al (2022) Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet 399:541–553. https://doi.org/10.1016/S0140-6736(21)02175-9
Borthakur G, Popplewell L, Boyiadzis M et al (2016) Activity of the oral MEK inhibitor trametinib in RAS-mutant relapsed or refractory myeloid malignancies. Cancer 122:1871. https://doi.org/10.1002/CNCR.29986
Desikan SP, Ravandi F, Pemmaraju N et al (2022) A phase ii study of azacitidine, venetoclax, and trametinib in relapsed or refractory acute myeloid leukemia harboring RAS pathway-activating mutations. Acta Haematol 145:529–536. https://doi.org/10.1159/000525566
Ragon BK, Odenike O, Baer MR et al (2019) Oral MEK 1/2 inhibitor trametinib in combination with AKT inhibitor GSK2141795 in patients with acute myeloid leukemia with RAS mutations: a phase ii study. Clin Lymphoma Myeloma Leuk 19:431. https://doi.org/10.1016/J.CLML.2019.03.015
Khan ZM, Real AM, Marsiglia WM et al (2020) Structural basis for the action of the drug trametinib at KSR-bound MEK. Nature 588:509–514. https://doi.org/10.1038/S41586-020-2760-4
Shariati M, Meric-Bernstam F (2019) Targeting AKT for cancer therapy. Expert Opin Investig Drugs 28:977. https://doi.org/10.1080/13543784.2019.1676726
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/J.EJCA.2008.10.026
(2006) Common terminology criteria for adverse events v3.0 (CTCAE) components and organization CATEGORY
Stover DG, Coloff JL, Barry WT et al (2016) The role of proliferation in determining response to neoadjuvant chemotherapy in breast cancer: a gene expression-based meta-analysis. Clin Cancer Res 22:6039–6050. https://doi.org/10.1158/1078-0432.CCR-16-0471
Subramanian A, Tamayo P, Mootha VK et al (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550. https://doi.org/10.1073/PNAS.0506580102
Adalsteinsson VA, Ha G, Freeman SS et al (2017) Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat Commun 81(8):1–13. https://doi.org/10.1038/s41467-017-00965-y
Gori S, Sidoni A, Colozza M et al (2009) EGFR, pMAPK, pAkt and PTEN status by immunohistochemistry: correlation with clinical outcome in HER2-positive metastatic breast cancer patients treated with trastuzumab. Ann Oncol Off J Eur Soc Med Oncol 20:648–654. https://doi.org/10.1093/ANNONC/MDN681
Balko JM, Cook RS, Vaught DB et al (2012) Profiling of residual breast cancers after neoadjuvant chemotherapy identifies DUSP4 deficiency as a mechanism of drug resistance. Nat Med 18:1052–1059. https://doi.org/10.1038/NM.2795
Stover DG, Parsons HA, Ha G et al (2018) Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J Clin Oncol 36:543–553. https://doi.org/10.1200/JCO.2017.76.0033
Nabet BY, Esfahani MS, Moding EJ et al (2020) Noninvasive early identification of therapeutic benefit from immune checkpoint inhibition. Cell 183:363-376.e13. https://doi.org/10.1016/J.CELL.2020.09.001
Parikh AR, Mojtahed A, Schneider JL et al (2020) Serial ctDNA monitoring to predict response to systemic therapy in metastatic gastrointestinal cancers. Clin Cancer Res 26:1877–1885. https://doi.org/10.1158/1078-0432.CCR-19-3467
Hrebien S, Citi V, Garcia-Murillas I et al (2019) Early ctDNA dynamics as a surrogate for progression-free survival in advanced breast cancer in the BEECH trial. Ann Oncol Off J Eur Soc Med Oncol 30:945–952. https://doi.org/10.1093/ANNONC/MDZ085
O’Leary B, Hrebien S, Morden JP et al (2018) Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun 91(9):1–10. https://doi.org/10.1038/s41467-018-03215-x
Reichert ZR, Morgan TM, Li G et al (2023) Prognostic value of plasma circulating tumor DNA fraction across four common cancer types: a real-world outcomes study. Ann Oncol 34:111–120. https://doi.org/10.1016/j.annonc.2022.09.163
Gouda MA, Subbiah V (2023) Expanding the benefit: dabrafenib/trametinib as tissue-agnostic therapy for braf v600e-positive adult and pediatric solid tumors. Am Soc Clin Oncol Educ book Am Soc Clin Oncol Annu Meet 43:e404770. https://doi.org/10.1200/EDBK_404770
Ribas A, Butler M, Lutzky J et al (2015) Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol 33:3003–3003. https://doi.org/10.1200/JCO.2015.33.15_SUPPL.3003
Liu L, Mayes PA, Eastman S et al (2015) The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res 21:1639–1651. https://doi.org/10.1158/1078-0432.CCR-14-2339
Liu Y, Zhang X, Wang G, Cui X (2021) Triple combination therapy with PD-1/PD-L1, BRAF, and MEK inhibitor for stage III–IV melanoma: a systematic review and meta-analysis. Front Oncol 11:693655. https://doi.org/10.3389/FONC.2021.693655/BIBTEX
Tolcher AW, Kurzrock R, Valero V et al (2020) Phase I dose-escalation trial of the oral AKT inhibitor uprosertib in combination with the oral MEK1/MEK2 inhibitor trametinib in patients with solid tumors. Cancer Chemother Pharmacol 85:673–683. https://doi.org/10.1007/S00280-020-04038-8
Algazi AP, Esteve-Puig R, Nosrati A et al (2018) Dual MEK/AKT inhibition with trametinib and GSK2141795 does not yield clinical benefit in metastatic NRAS-mutant and wild-type melanoma. Pigment Cell Melanoma Res 31:110–114. https://doi.org/10.1111/PCMR.12644
Westin SN, Sill MW, Coleman RL et al (2019) Safety lead-in of the MEK inhibitor trametinib in combination with GSK2141795, an AKT inhibitor, in patients with recurrent endometrial cancer: An NRG Oncology/GOG study. Gynecol Oncol 155:420–428. https://doi.org/10.1016/J.YGYNO.2019.09.024
Liu JF, Gray KP, Wright AA et al (2019) Results from a single arm, single stage phase II trial of trametinib and GSK2141795 in persistent or recurrent cervical cancer. Gynecol Oncol 154:95–101. https://doi.org/10.1016/J.YGYNO.2019.05.003
Seo T, Noguchi E, Yoshida M et al (2020) Response to dabrafenib and trametinib of a patient with metaplastic breast carcinoma harboring a BRAF V600E mutation. Case Rep Oncol Med 2020:1–6. https://doi.org/10.1155/2020/2518383
Lim B, Peterson CB, Davis A et al (2021) ONC201 and an MEK Inhibitor trametinib synergistically inhibit the growth of triple-negative breast cancer cells. Biomedicines. https://doi.org/10.3390/BIOMEDICINES9101410
Zawistowski JS, Bevill SM, Goulet DR et al (2017) Enhancer remodeling during adaptive bypass to MEK inhibition is attenuated by pharmacologic targeting of the P-TEFb complex. Cancer Discov 7:302–321. https://doi.org/10.1158/2159-8290.CD-16-0653
Goulet DR, Foster JP, Zawistowski JS et al (2020) Discrete adaptive responses to MEK inhibitor in subpopulations of triple-negative breast cancer. Mol Cancer Res 18:1685–1698. https://doi.org/10.1158/1541-7786.MCR-19-1011
Sanz-Garcia E, Zhao E, Bratman SV, Siu LL (2022) Monitoring and adapting cancer treatment using circulating tumor DNA kinetics: current research, opportunities, and challenges. Sci Adv. https://doi.org/10.1126/SCIADV.ABI8618
Magbanua MJM, Swigart LB, Wu HT et al (2021) Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol Off J Eur Soc Med Oncol 32:229–239. https://doi.org/10.1016/J.ANNONC.2020.11.007
Lehmann BD, Bauer JA, Chen X et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121:2750–2767. https://doi.org/10.1172/JCI45014
Creighton CJ, Hilger AM, Murthy S et al (2006) Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer Res 66:3903–3911. https://doi.org/10.1158/0008-5472.CAN-05-4363
Acknowledgements
This study is in memorial of senior author, Dr. Bhuvaneswari Ramaswamy, who envisioned, designed, and oversaw the conduct of this study reflecting her unwavering passion and dedication to difficult to treat types of breast cancer. The authors would like to acknowledge Catherine Carson, Celia Garr, Katherine Weber, and Danielle Nickell for clinical support making this research possible.
Funding
N01 Grant NIH Contract No. HHSN261201100070C (BR), NCI UH3CA239105 (DGS) and Pelotonia Grant. NCI was involved in the design and conduct of the study and in review of the manuscript. Funders were not involved in collection, management, analysis, and interpretation of the data; preparation or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design, acquisition of data, analysis and interpretation of data, editing, and final approval. VP, HB, DGS, BR contributed to the drafting of the article. The critical revision of the article was performed by DGS and BR.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflicts of interest related to the current analyses.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daniel G. Stover, Bhuvaneswari Ramaswamy equally directed the work.
This work was presented at the 2024 Annual Meeting of the American Association of Cancer Research; San Diego, CA, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prasath, V., Boutrid, H., Wesolowski, R. et al. Phase II study of MEK inhibitor trametinib alone and in combination with AKT inhibitor GSK2141795/uprosertib in patients with metastatic triple negative breast cancer. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07551-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07551-z