Abstract
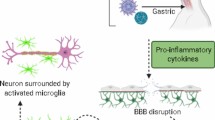
Parkinson’s disease (PD) is a globally prevalent neurodegenerative disorder, characterized by progressive neuronal loss in the substantia nigra and formation of Lewy bodies. These pathological characteristics are clinically translated into motor symptoms, such as bradykinesia, rigidity, resting tremors, and postural instability. Emerging data from epidemiological studies suggest a possible association between PD and hepatitis C virus (HCV) infection, which affects up to 71 million individuals worldwide. Preclinical studies have shown that HCV can penetrate and replicate within the brain macrophages and microglial cells, increasing their production of pro-inflammatory cytokines that can directly cause neuronal toxicity. Other studies reported that interferon, previously used to treat HCV infection, can increase the risk of PD through inhibition of the nigrostriatal dopaminergic transmission or induction of neuroinflammation. In this article, we provide a comprehensive review on the possible association between HCV infection and PD and highlight recommendations for further research and practice in this regard.

Similar content being viewed by others
References
Savitt JM, Dawson VL, Dawson TM (2006) Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest 116:1744–1754
Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci 28:57–87
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79:368–376. doi:10.1136/jnnp.2007.131045
De Lau LML, Breteler MMB (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535
Ahmed H, Abushouk AI, Gabr M, Negida A, Abdel-Daim MM (2017) Parkinson’s disease and pesticides: a meta-analysis of disease connection and genetic alterations. Biomed Pharmacother 90:638–649
Takahashi M, Yamada T (1999) Viral etiology for Parkinson’s disease—a possible role of influenza A virus infection. Jpn J Infect Dis 52:89–98
Marttila RJ, Arstila P, Nikoskelainen J, Halonen PE, Rinne UK (1977) Viral antibodies in the sera from patients with Parkinson disease. Eur Neurol 15:25–33
The Polaris Observatory HCV collaborators (2017) Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol & Hepatol 2:161–176
Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW (2010) Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 138:513–521, 521.e1–6. doi:10.1053/j.gastro.2009.09.067
Blonski W, Reddy KR (2008) Hepatitis C virus infection and hepatocellular carcinoma. Clin Liver Dis 12:661–674. doi:10.1016/j.cld.2008.03.007
Heckmann JG, Kayser C, Heuss D, Manger B, Blum HE, Neundörfer B (1999) Neurological manifestations of chronic hepatitis C. J Neurol 246:486–491
Sène D, Limal N, Cacoub P (2004) Hepatitis C virus-associated extrahepatic manifestations: a review. Metab Brain Dis 19:357–381
Laskus T, Radkowski M, Adair DM, Wilkinson J, Scheck AC, Rakela J (2005) Emerging evidence of hepatitis C virus neuroinvasion. AIDS 19(Suppl 3):S140–S144
Wu WY-Y, Kang K-H, Chen SL-S, Chiu SY-H, Yen AM-F, Fann JC-Y et al (2015) Hepatitis C virus infection: a risk factor for Parkinson’s disease. J Viral Hepat 22:784–791. doi:10.1111/jvh.12392
Tsai H-H, Liou H-H, Muo C-H, Lee C-Z, Yen R-F, Kao C-H (2016) Hepatitis C virus infection as a risk factor for Parkinson disease: a nationwide cohort study. Neurology 86:840–846. doi:10.1212/WNL.0000000000002307
Wangensteen KJ, Krawitt EL, Hamill RW, Boyd JT (2016) Parkinsonism in patients with chronic hepatitis C treated with interferons. Clin Neuropharmacol 39:1–5. doi:10.1097/WNF.0000000000000120
Gowans EJ (2000) Distribution of markers of hepatitis C virus infection throughout the body. Semin Liver Dis 20:85–102
Radkowski M, Wilkinson J, Nowicki M, Adair D, Vargas H, Ingui C et al (2002) Search for hepatitis C virus negative-strand RNA sequences and analysis of viral sequences in the central nervous system: evidence of replication. J Virol 76:600–608
Adair DM, Wilkinson J, Scheck AC, Radkowski M, Rakela J, Laskus T (2004) Differential display and microarray analysis show differentially expressed genes in central nervous system in HCV infected patients and laser capture microscopy points to brain microglia as cells harboring HCV. Hepatology, vol. 40, John Wiley & Sons Inc 111 River St, Hoboken, NJ 07030 USA, p. 433A
Fletcher NF, Wilson GK, Murray J, Hu K, Lewis A, Reynolds GM et al (2012) Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology 142:634–643.e6
Lucchese G, Kanduc D (2014) Single amino acid repeats connect viruses to neurodegeneration. Curr Drug Discov Technol 11:214–219
Qian L, Flood PM, Hong J-S (2010) Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J Neural Transm 117:971–979
Forton DM, Allsop JM, Main J, Foster GR, Thomas HC, Taylor-Robinson SD (2001) Evidence for a cerebral effect of the hepatitis C virus. Lancet 358:38–39
Lyons PD, Benveniste EN (1998) Cleavage of membrane-associated ICAM-1 from astrocytes: involvement of a metalloprotease. Glia 22:103–112
Gill SS, Hou Y, Ghane T, Pulido OM (2008) Regional susceptibility to domoic acid in primary astrocyte cells cultured from the brain stem and hippocampus. Mar Drugs 6:25–38
Sheridan GK, Dev KK (2012) S1P1 receptor subtype inhibits demyelination and regulates chemokine release in cerebellar slice cultures. Glia 60:382–392. doi:10.1002/glia.22272
Fiala M, Avagyan H, Merino JJ, Bernas M, Valdivia J, Espinosa-Jeffrey A et al (2013) Chemotactic and mitogenic stimuli of neuronal apoptosis in patients with medically intractable temporal lobe epilepsy. Pathophysiol: Off J Int Soc Pathophysiol 20:59–69. doi:10.1016/j.pathophys.2012.02.003
Lane TE, Liu MT, Chen BP, Asensio VC, Samawi RM, Paoletti AD et al (2000) A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol 74:1415–1424
Chao C, Ghorpade A (2009) Production and roles of glial tissue inhibitor of metalloproteinases-1 in human immunodeficiency virus-1-associated dementia neuroinflammation: a review. Am J Infect Dis 5:314–320
Stetler-Stevenson WG (2008) Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Science Signaling 1:re6. doi:10.1126/scisignal.127re6
Weissenborn K, Ennen JC, Bokemeyer M, Ahl B, Wurster U, Tillmann H et al (2006) Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut 55:1624–1630. doi:10.1136/gut.2005.080267
Hoofnagle JH, Mullen KD, Jones DB, Rustgi V, Di Bisceglie A, Peters M et al (1986) Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. N Engl J Med 315:1575–1578
Chung RT, Baumert TF (2014) Curing chronic hepatitis C—the arc of a medical triumph. N Engl J Med 370:1576–1578. doi:10.1056/NEJMp1400986
Ahmed H, Abushouk AI, Gadelkarim M, Mohamed A, Gabr M, Negida A (2017) Efficacy of daclatasvir plus peginterferon alfa and ribavirin for patients with chronic hepatitis C genotype 4 infection. Bangladesh J Pharmacol 12:12–22
Raison CL, Demetrashvili M, Capuron L, Miller AH (2005) Neuropsychiatric adverse effects of interferon-α. CNS Drugs 19:105–123
LaRochelle JS, Karp BI (2004) Restless legs syndrome due to interferon-α. Mov Disord 19:730–731
Quarantini LC, Miranda-Scippa A, Parana R, Sampaio AS, Bressan RA (2007) Acute dystonia after injection of pegylated interferon alpha-2b. Mov Disord 22:747–748
Mizoi Y, Kaneko H, Oharazawa A, Kuroiwa H (1997) Parkinsonism in a patient receiving interferon alpha therapy for chronic hepatitis C. Rinsho Shinkeigaku 37:54–56
Shuto H, Kataoka Y, Horikawa T, Fujihara N, Oishi R (1997) Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain Res 747:348–351
Mount MP, Lira A, Grimes D, Smith PD, Faucher S, Slack R et al (2007) Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci 27:3328–3337. doi:10.1523/JNEUROSCI.5321-06.2007
Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V et al (2009) Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119:182–192. doi:10.1172/JCI36470
De Almeida CMO, Galvão MDLDS, Ferreira PLDC, Braga WS (2009) Interferon-induced parkinsonism in a patient with chronic hepatitis C. Arq Neuropsiquiatr 67:715–716. doi:10.1590/S0004-282X2009000400031
Çubukçu HC, Yurtdaş M, Durak ZE, Aytaç B, Güneş HN, Çokal BG et al (2016) Oxidative and nitrosative stress in serum of patients with Parkinson’s disease. Neurol Sci 37:1793–1798. doi:10.1007/s10072-016-2663-1
Akıl E, Bulut A, Kaplan İ, Özdemir HH, Arslan D, Aluçlu MU (2015) The increase of carcinoembryonic antigen (CEA), high-sensitivity C-reactive protein, and neutrophil/lymphocyte ratio in Parkinson’s disease. Neurol Sci 36:423–428. doi:10.1007/s10072-014-1976-1
Ataç Uçar C, Gökçe Çokal B, Ünal Artık HA, İnan LE, Yoldaş TK (2017) Comparison of neutrophil–lymphocyte ratio (NLR) in Parkinson’s disease subtypes. Neurol Sci 38:287–293. doi:10.1007/s10072-016-2758-8
Taylor JM, Main BS, Crack PJ (2013) Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson’s disease. Neurochem Int 62:803–819. doi:10.1016/j.neuint.2012.12.016
Block ML, Hong J-S (2007) Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans 35:1127–1132. doi:10.1042/BST0351127
Garcia-Esparcia P, Llorens F, Carmona M, Ferrer I (2014) Complex deregulation and expression of cytokines and mediators of the immune response in Parkinson’s disease brain is region dependent. Brain Pathol 24:584–598. doi:10.1111/bpa.12137
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397. doi:10.1016/S1474-4422(09)70062-6
Dexter DT, Jenner P (2013) Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 62:132–144. doi:10.1016/j.freeradbiomed.2013.01.018
Chen H-H, Liu PF-C, Tsai H-H, Yen R-F, Liou H-H (2016) Re: Wangensteen et al. of a letter on ‘Hepatitis C virus infection: a risk factor for Parkinson’s disease. J Viral Hepat 23:560–560. doi:10.1111/jvh.12521
Kim J, Jang ES, Ok K, Oh ES, Kim KJ, Jeon B et al (2016) Association between hepatitis C virus infection and Parkinson’s disease. Mov Disord 31:1584–1585
Pakpoor J, Noyce A, Selkihova M, Lees A (2017) Viral hepatitis and Parkinson disease: a national record-linkage study. Neurology 88:1630–1633
Munhoz RP, Bertucci Filho D, Teive HAG (2017) Not all drug-induced parkinsonism are the same: the effect of drug class on motor phenotype. Neurol Sci 38:319–324. doi:10.1007/s10072-016-2771-y
Ahmed H, Elgebaly A, Abushouk AI, Hammad AM, Attia A, Negida A (2016) Safety and efficacy of sofosbuvir plus ledipasvir with and without ribavirin for chronic HCV genotype-1 infection: a systematic review and meta-analysis. Antivir Ther. doi:10.3851/imp3083
Ahmed H, Abushouk AI, Menshawy A, Attia A, Negida A, Loutfy SA (2017) FRI-224-ribavirin free versus ribavirin containing therapy with all second generation direct acting antivirals for the treatment of hepatitis C virus genotype 1 infection; a pooled analysis of 4501 patients. J Hepatol 66:S508–S509
Bettiol SS, Rose TC, Hughes CJ, Smith LA (2015) Alcohol consumption and Parkinson’s disease risk: a review of recent findings. J Parkinson Dis 5:425–442
Hernán MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ (2002) A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol 52:276–284
Benito-León J (2017) Viral hepatitis and the risk of Parkinson disease. Neurology 88:1596–1597
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Funding sources
None.
Rights and permissions
About this article
Cite this article
Abushouk, A.I., El-Husseny, M.W.A., Magdy, M. et al. Evidence for association between hepatitis C virus and Parkinson’s disease. Neurol Sci 38, 1913–1920 (2017). https://doi.org/10.1007/s10072-017-3077-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-3077-4