Abstract
Visceral leishmaniasis is a zoonotic disease in many countries and dogs are considered the main domestic reservoir of Leishmania parasites, and the presence of infected animals represents a potential risk for human disease. In this chapter, we review the state-of-the-art of canine visceral leishmaniasis (CanL) vaccines, discussing the properties and problems associated with the few currently licensed and discontinued vaccines and looking forward to the development of new, more effective vaccines. Reducing the incidence of CanL through vaccination will improve canine health and welfare and contribute to preventing human VL.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Leishmaniases are neglected tropical diseases (NTDs) that affect the poorest populations in the world, and around 380 million people distributed in 98 countries throughout Asia, Africa, the Middle East, and Central and South America are exposed to the risks of infection annually [1]. The disease complex has an annual total incidence of 1.5–2.0 million cases, split between approximately 1.0–1.5 million cases of tegumentary leishmaniasis (TL) and 0.5 million cases of visceral leishmaniasis (VL) [1]. Risk factors for disease dissemination include poverty, population migration, malnutrition, poor hygiene, vector distribution and the status of host immunity [2]. Over 20 Leishmania parasite species are known, and they can be transmitted to mammals by approximately 70 different types of phlebotomine sandflies [3]. Based on the main clinical manifestations, leishmaniases are classified in two main groups: TL, which comprises the cutaneous, mucosal and cutaneous-diffuse forms of the disease, and VL, which is the most severe form of disease and may be fatal, if acute and not treated [4].
Human VL exists in zoonotic and anthroponotic forms, caused by Leishmania infantum and L. donovani, respectively [3]. The zoonotic form of VL can be found in the Mediterranean region and American continent, whereas the anthroponotic disease is found on the Asian and African continents, particularly India, Nepal, Bangladesh and East Africa (Somalia, the Sudan, South Sudan, Ethiopia). It has been suggested that, in endemic areas, there are five to ten times more cases of asymptomatic infection compared to clinically apparent VL in immunocompetent hosts [5]. The incubation period for symptomatic disease to develop can range from 10 days to 1 year, and clinical symptoms include fever, anorexia, weight loss, hepatosplenomegaly, haematological disorders, lymphadenopathy and pancytopenia [6]. Diagnosis of human VL is hampered by the variable sensitivity and/or specificity of the laboratory tests, whilst treatment is problematical due to toxicity of the drugs and their expense and the emergence of resistant parasite strains. More comprehensive information on human VL and TL epidemiology, global prevalence of diseases and their treatment is provided in Chap. 11 in this book.
In this chapter, we will focus on canine visceral leishmaniasis (CanL) and vaccine development. In countries where VL is zoonotic, dogs are considered the main domestic reservoirs of L. infantum parasites, and the presence of infected animals in these areas represents a potential risk for human disease. The canine disease shares similarity with human VL and provides a model to study the immunopathogenesis of L. infantum infection. This parasite species multiplies inside host macrophages in the liver, spleen, and bone marrow, and about 90% of infected dogs can remain asymptomatic or subclinical for years [7]. Since such animals usually do not progress to disease and remain asymptomatic, they contribute to the maintenance of the parasite’s transmission cycle between sandflies and humans. In symptomatic cases, dogs develop cutaneous changes, such as alopecia, onychogryphosis and exfoliative dermatitis, which are associated with organic alterations, such as splenomegaly, lymphadenomegaly, renal azotemia and neurological disorders, amongst others [8, 9].
Control measures to prevent CanL are necessary given the high prevalence of canine infection compared to human disease. However, available control measures are not considered adequate to disrupt the spread of the disease. In this sense, health control and surveillance measures include (1) the use of chemical insecticides, (2) environmental management for vector population control and vector-human contact reduction, (3) canine serological surveys, (4) euthanasia of positive cases and timely diagnosis and (5) adequate treatment of human cases to prevent severe forms of disease and death. In addition, antileishmanial treatment for dogs and vaccine use are strategies to reduce dog infectiousness and limit transmission of the parasite from canines to phlebotomine sand flies and to humans. However, distinct problems have limited the effectiveness of such control measures against CanL.
2 Diagnosis of CanL
The clinical diagnosis of CanL is facile in symptomatic dogs, due to the manifestation of cardinal signs and symptoms, but more difficult in asymptomatic animals and in those with few clinical signs known as oligosymptomatics [10]. For the latter in particular, laboratory tests are required. In order to provide sensitive and accurate diagnostic tests, parasitological, immunological and molecular tools have been developed to detect L. infantum in dogs. Classically, the diagnosis of CanL is performed with parasitological tests, where the direct demonstration of the parasites in samples collected from the animals is the strategy of choice. Leishmania amastigotes can be identified in stained smears of skin lesions, spleen, liver, bone marrow, and/or lymph node aspirates of the infected animals [11]. However, these examinations can yield false-negative results caused by the presence of low numbers of parasites in the smears and collected aspirates. Molecular techniques, such as polymerase chain reaction (PCR), have been used for diagnosis, and they are based on the in vitro amplification of specific-nucleotide sequences found in the parasites. CanL PCR tests show higher sensitivity and specificity to detect Leishmania contents compared to conventional parasitological diagnosis [12]. However, despite their high efficiency, PCR tests are expensive and require sophisticated equipment and trained professionals, which limits their use in field conditions, particularly in the least developed/low- to middle-income countries with endemic disease [13].
Serological diagnosis of CanL also has been done and is based on detecting IgG antibodies specific to Leishmania antigens. Laboratory tests such as enzyme-linked immunosorbent assay (ELISA), indirect fluorescent antibody test (IFAT) and direct agglutination test (DAT), amongst others, have been used for CanL diagnosis [14]. These tests are considered simpler and cheaper than parasitological methods, and sample collection is less invasive [9]. Soluble, crude and/or total antigenic extracts of Leishmania have been used for serodiagnosis of CanL: however, variable sensitivity and/or specificity has been observed, with false-positive results being found with sera from dogs infected by other pathogens, such as Trypanosoma cruzi, Ehrlichia canis, Babesia canis and Toxoplasma gondii, amongst others [13, 15]. In addition, asymptomatic dogs presenting low antileishmanial serology can be misdiagnosed as false negatives in serological trials [16]. New studies with modern biotechnological techniques have identified more refined and defined antigens to try and improve diagnosis of CanL, and a number of recombinant antigens have been evaluated [17]. However, further optimization is still needed to define the appropriate antigens to provide high-throughput performance for diagnosis of canine disease [18, 19].
The use of recombinant (r) parasite proteins has improved the sensitivity and specificity of the diagnostic tests. The rK39 kinesin-related protein presents immunodominant B-cell epitopes that are conserved amongst viscerotropic Leishmania species [20], and it has been evaluated for the serodiagnosis of CanL [21]. The rK39 antigen is suitable for detecting symptomatic disease cases, but lower sensitivity is found for detection of asymptomatic animals [22]. In Brazil, the rK39-based dipstick test has shown variable specificity when compared to conventional ELISA using parasite promastigote extracts [23]. Alternative kinesin-related proteins, such as rKLO8 and rK26, have been proposed also as capable of increasing diagnostic accuracy for CanL, and results combining such molecules have shown higher sensitivity and specificity [24]. A colloidal gold-based immunochromatographic test (ICT) based on detecting antibodies against a chimeric protein composed of B-cell epitopes from rK26 and rK39 proteins has been developed. This antigen showed potential application in screening surveys in exposed canine populations, with satisfactory diagnostic value when positive, negative and cross-reactive samples were evaluated [25].
A2 antigens were identified as a protein family of molecular weights between 45 and 100 kDa and are expressed in the amastigote stage of some Leishmania species, such as L. donovani, L. infantum and L. amazonensis [26]. The A2 gene product is composed of a variable number of repeated sequences of 10 amino acid residues [27]. Studies have shown that a rA2 antigen presents diagnostic efficacy for CanL [28, 29]. However, although ELISA assays have indicated that rA2 protein can distinguish between infected and non-infected dogs, A2 proteins have failed to distinguish between infected dogs and vaccinated and healthy animals, as well as animals infected with Ehrlichia canis and Babesia canis. This makes it difficult to use rA2 in serological trials [29, 30].
Ideally, immunological methods based on qualitative analysis using the naked eye could be developed that are user-friendly and have better field applications. Commercial kits for CanL identification have used biological fluids such as plasma, serum, whole blood or blood adsorbed onto filter paper. The specificity of the tests is high, as opposed to their sensitivity, which is variable and the main concern for clinical and epidemiological surveys [31, 32]. The use of combined antigens has been also evaluated as a method to increase the performance of CanL diagnostic tests. Magalhães et al. [33] used a mixture of three L. infantum recombinant protein antigens called Lci1, Lci12 and Lci13 for serodiagnosis of CanL, and results were promising. Another interesting approach is based on the use of multi-epitope chimeric proteins, in which specific B-cell epitopes are combined and assembled into a synthetic gene, leading to the production of a recombinant chimeric protein [34, 35], which can be used for lateral flow devices. Boarino et al. [36] produced a chimeric protein by fusing B-cell epitopes from K9, K39 and K26 antigens. ELISA assays showed sensitivity and specificity values of 96.0% and 99.0%, respectively, for CanL. Another chimeric protein composed of B-cell epitopes from the PQ10 and PQ20 proteins showed agreement from between 80.0% and 100% to detect asymptomatic CanL cases [19, 37].
Vale et al. [38] developed a chimeric protein based on the selection of specific B-cell epitopes of four L. infantum hypothetical proteins, which were previously shown to be antigenic in CanL or human VL. This chimeric protein had 100% sensitivity and 100% specificity for CanL, whilst a parasite antigenic preparation had sensitivity and specificity values of 26.0% and 96.4%, respectively. Thus, promising antigenic targets have been developed independently by several research groups, and these new candidates for serodiagnosis of CanL can help to identify infected dogs and to adopt effective control measures against the spread of disease.
3 Treatment of CanL
Therapeutic candidates have been developed for many years to try and treat CanL. However, their use requires administering veterinarians and problems associated with the parenteral administration of compounds include incomplete parasite clearance and undesirable toxic side effects [39, 40]. Treatment is also characterized by high rates of relapse, regardless of the antileishmanial drugs used and whether it is a single drug or combined drug therapy [41].
Miltefosine was originally developed as an anticancer agent in the 1990s and was first recorded for VL treatment in 2002 in India [42]. In 2016, the Brazilian Ministry of Health and the Ministry of Agriculture Livestock and Supply approved the registration of Milteforan® (Virbac, Brazil). Although there was a notable improvement in clinical symptoms when using this drug, it was not accompanied by parasitological clearance, suggesting that miltefosine treatment should not be recommended [43]. More recently, miltefosine treatment of L. infantum-infected dogs revealed clinical improvement of CanL with a reduction in parasite infectivity [44].
Allopurinol has parasitostatic activity, and its long-term administration maintains low parasite load, which contributes to preventing canine relapse [45]. Combination of allopurinol with miltefosine showed the most promise for CanL treatment [46]. However, induced resistance is also a problem associated with the use of allopurinol [47]. In most parts of the world, meglumine antimoniate is the most commonly used treatment for visceral leishmaniasis, and a combination of meglumine antimoniate and allopurinol is considered the most effective therapy for CanL [48]. However, treatment with the same human-used drugs is not recommended since it may induce parasite resistance and hamper human treatment [49]. Table 13.1 shows the drugs currently used to treat CanL.
The great challenges for CanL treatment are to identity a drug that (1) is not used to treat humans, (2) does not induce kidney damage or any other adverse effect, (3) controls parasite load, (4) interferes in the sandflies’ life cycle and (5) blocks parasite transmission. In this context, other treatment options should be studied, such as immunotherapy, to improve CanL treatment efficacy. Immunotherapy involves using compounds that can modulate host immune responses, aiming to achieve prophylactic and/or therapeutic efficacy [58,59,60]. These agents exert their effects by augmenting the host’s natural defences, restoring any impaired effector functions and stimulating a protective response against infection that results in parasitism control [61, 62]. The different protocols used for immunotherapy or immunochemotherapy generally improve clinical signs, with a possibility to further reduce the parasite burden by activating the immune system against Leishmania infection. Taken together, these results showed that immunotherapy is a promising strategy for treating CanL. However, parasite clearance was not completed reached, irrespective of the treatment, and this is the strongest negative aspect of such studies. The search for new immunotherapeutic targets to improve these types of treatment is of great interest, given that the aims are to improve parasite control and develop approaches to blocking CanL transmission.
4 Immune Responses in CanL
Immunoprophylaxis has been considered as a strategy for preventing canine disease, and efforts have been made to deduce the immune response profile generated in CanL. Immune control of L. infantum infection requires a balance between inflammatory and regulatory actions, and protective immunity is dependent on the development of an antigen- and parasite-specific Th1-type cellular immune response, primed by the production of cytokines, such as IFN-γ, IL-12, GM-CSF and others, by T cells [63, 64]. Conversely, Th2-type cells and T regulatory cells act to promote disease, since they produce anti-inflammatory cytokines that lead to inhibition of the Th1-type response, thus contributing to the deactivation of infected phagocytes and, consequently, loss of control of the inflammatory process and/or the development of active disease [65].
In symptomatic CanL, immunological changes involving T cells include the absence of delayed-type hypersensitivity reactions to parasite antigens, the reduction of T-cell numbers in the peripheral blood and a lower production of cytokines, such as IFN-γ and IL-2, by peripheral blood mononuclear cells (PBMC) of the infected animals [66]. For protection against infection, effector mechanisms associated with the activation of macrophages by IFN-γ, IL-12 and TNF-α cytokines to kill intracellular amastigotes via the l-arginine nitric oxide (NO) pathway have been described [67]. NO production and antileishmanial activity were detected in canine macrophage cell lines infected with L. infantum after incubation with these cytokines, as well as by macrophages from dogs immunized with killed parasite promastigotes [18]. The role of IL-12 cytokine in inducing the Th1-type response profile has also been described, and this molecule augments the production of IFN-γ by PBMC from dogs with experimental and natural symptomatic disease [68]. IL-12 was also detected in lymph node cells from dogs protected against L. infantum after immunization with DNA and vaccinia recombinant vectors expressing Leishmania homologue of activated C kinase protein [69]. In asymptomatic CanL, IL-12 and IFN-γ cytokines predominate and indicate that the Th1-type cellular profile is required for protection against active disease [70].
Regarding the humoral response, the production of antibodies specific to Leishmania antigens is found in dogs with symptomatic CanL [18]. Antibodies are required for parasite opsonization and phagocytosis by macrophages in infected animals [71]. In asymptomatic or subclinical infection, such processes occur at lower levels and can allow continued propagation of the parasites. As the parasite continues to propagate, there is an increase in the levels of circulating IgG antibodies, which can bind to Leishmania antigens leading to the formation of immune complexes, which trigger the production of IL-10 by infected macrophages [72]. There is concomitant hypergammaglobulinemia, followed by the deposition of the immune complexes that cause renal damage and, later on, renal failure, which is one of the most common causes of death in dogs caused by active disease [73]. Regarding antibody isotypes, IgG1 and IgG2 subclasses have been used as indicators for CanL, since a direct correlation between high levels of IgG1 anti-Leishmania antibodies and the appearance of clinical symptoms has been demonstrated in L. infantum-infected dogs. By contrast, IgG2 isotype antibodies are associated with asymptomatic infection and the predominance of a Th1-type response in dogs [74].
5 CanL Vaccines
The evidence of lifelong immunity against Leishmania infection has stimulated the development of prophylactic vaccination against leishmaniasis. With knowledge gained of the immune response mechanisms to protect against the parasite, an ideal CanL vaccine candidate should have the following attributes: it should (1) be safe and affordable to the hosts; (2) induce both CD4+ and CD8+ T-cell responses and long-term immunological memory, which could be boosted by natural infections, thereby reducing the number of repeat vaccine doses required for protection; and (3) be easy to produce and be stable at room temperature or at 4 °C, thus eliminating the need for storage at −20 °C to −80 °C [75]. Advances in recombinant technology have enabled many parasite proteins to be tested with different immune adjuvants, as vaccine candidates against VL. These proteins have showed variable success in mammalian models, which was dependent on the vaccine formulation, the choice of adjuvant and the animal model used for testing [76,77,78,79,80,81].
5.1 Leish-Tec® Vaccine
An amastigote stage-specific gene family from Leishmania called A2 was identified in some parasite species. The A2 gene family encodes for at least seven members, and the proteins have molecular weights (Mr) ranging from 45 to 100 kDa. These proteins are composed mainly of a repetitive amino acid sequence, with each repeat encoding for a stretch of ten amino acid residues that share partial identity with the S antigens of the Plasmodium falciparum V1 strain, which causes malaria in man [82, 83]. The A2 protein has emerged as an effective vaccine candidate, and when used as a recombinant protein with saponin as adjuvant, it protected mice against L. infantum infection [84,85,86]. A2-induced protective immunity was associated with the production of high levels of IFN-γ cytokine that were produced by T cells and low levels of IL-10 cytokine. Immunity was also characterized by elevated levels of A2 protein- and parasite-specific IgG2a antibodies, which contributed to significant reductions in the parasite load in organs of vaccinated mice, compared to unvaccinated controls. Based on this success in the murine model, the A2 antigen was formulated with saponin to produce a CanL vaccine called Leish-Tec® (Ceva Laboratórios Ltda., Brazil). Leish-Tec® is the CanL vaccine currently licensed for use in Brazil, and it was shown to induce protective immunity in beagle dogs challenged intravenously with high doses of live L. infantum parasites, as well reducing the infectiousness of the animals to sandflies, when xenodiagnoses were performed [87, 88]. In addition, the vaccine did not seroconvert vaccinated animals, an important requirement for CanL vaccines, since euthanasia of seropositive dogs is recommended by the public health authorities in Brazil [89].
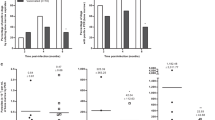
The study done between 2004 and 2008 in Porteirinha, Minas Gerais, an area of Brazil with endemic CanL, showed that the efficacy of Leish-Tec® in protecting exposed and vaccinated dogs was 71.4% (Confidence Interval (CI) 34.9–87.3%), when compared to unvaccinated and control animals [88]. Vaccination also led to reduced parasite transmission between dogs and humans. Moreover, if only vaccinated animals were considered, protection levels reached 96.4%, according to parasitological criteria adopted in the study. Vaccine efficacy amongst dogs that responded to vaccination with increased anti-A2 antibody levels was higher at 80.8% than the reported overall efficacy of 71.4%. In the study from Pereira et al. [90], seropositivity for anti-A2 IgG antibodies was detected in 98.0% of the vaccinated dogs, and this decreased to 81.13% at 6 months after vaccination but rising again to 98.0% after a vaccination booster. The anti-A2 IgG2/IgG1 ratio in the dogs was found to be higher than 1.0, suggesting the predominance of a Th1-type response in Leish-Tec®-vaccinated dogs.
Xenodiagnosis is an important tool for evaluating the potential for transmission from a vaccinated animal, although it is limited by low sensitivity and specificity in field conditions. In Leish-Tec®-vaccinated dogs, vaccine efficacy remained significantly high at 58.1% (95% CI: 26.0–76.3%), and vaccination induced a 46.6% reduction in parasite transmission to the sandflies that were feeding on anti-A2 seropositive vaccinated dogs [87]. Only 5.4% of animals vaccinated with Leish-Tec® were infectious to sandflies, as compared to a positive rate of 36.6% amongst control and unvaccinated dogs. Therefore, Leish-Tec® induced an appreciable reduction in Leishmania transmission to vectors and was protective against L. infantum infection. Thus, this vaccine shows promising protective effects but needs to be further optimized to be more effective in dogs under field conditions. Finally, it should be noted that imperfect vaccines pose a threat because they are not completely sterilizing, and they allow more parasite virulent strains to survive and transmit. If the evolved parasite strains then infect unvaccinated dogs, they will be more virulent than the strains that circulated before the vaccine was used [91].
5.2 Leishmune® Vaccine
Leishmania nucleoside hydrolases (NH) proteins are associated with the synthesis of parasite DNA and replication and relevant for early infection of mammalian hosts [92]. The L. donovani NH antigen has a Mr of 36 kDa (NH36), and there is high homology of NH proteins from distinct Leishmania species. NH36 was specifically recognized by sera of VL patients [93], and when used as an immunogen in murine models, it protected mice against infection with L. infantum [94], L. Mexicana [95], L. amazonensis [96] and L. major [97]. It was also found to protect dogs against infection with L. infantum [98].
Leishmune® (Fort Dodge Saúde Animal, Brazil) was then developed as a CanL vaccine based on the NH36 antigen. It is considered a second-generation vaccine, and it was licensed for prophylaxis against CanL in Brazil between 2004 and 2013 [75]. In a study with 550 Leishmune®-vaccinated dogs that were exposed to an endemic area of VL, only 1.0% (n = 5) of the animals died of disease, and 1.2% (n = 6) were symptomatic [99]. By contrast, there were 39.0% deaths within the untreated control group, and 20.6% of the dogs were symptomatic. The vaccine was prophylactic against CanL and protected 98.0% of vaccinated dogs and also reduced the parasite burden accessible for transmission to sandflies. The anti-fucose mannose ligand (FML) antibody response induced by the vaccine was mainly of the IgG2 subtype. Up to October 2011, a total of 150,000 healthy dogs were vaccinated in Brazil, and it was also observed that Leishmune® formulated with twice the concentration of saponin adjuvant had a therapeutic effect against naturally or experimentally acquired CanL [100].
Immunotherapy with saponin-enriched Leishmune® reduced not only CanL symptoms but also the rate of deaths and the parasite load in lymph nodes. Augmented immunochemotherapy using a combination of Leishmune®, allopurinol and amphotericin B promoted a sterile cure with negative PCR reactions for Leishmania DNA in dogs [101]. Leishmune® induced an immunological pattern characterized by enhanced levels of IFN-γ, NO and anti-L. infantum IgG2 antibody and an increased CD8+ T-cell response. The vaccine also induced early phenotypic changes in neutrophils and monocytes with increase in MHC II class and decrease in CD32+ and CD18+ activation markers, CD8+ T-cell activation and a selective pro-inflammatory response pattern. Sustained or increased proportions of CD4+ and CD21 B cells and increased proportions of CD8+ T cells and the diminished CD4+/CD25+ T cells have also been reported in dogs [102]. However, unlike the Leish-Tec® vaccine, Leishmune® induces a strong humoral response in vaccinated dogs, and this hampers the serological discrimination of infected animals. This fact made the public health authorities in Brazil discontinue Leishmune® as a prophylactic agent from 2014.
5.3 CaniLeish® Vaccine
The first vaccine registered in Europe against CanL was LiESP/QA-21 vaccine (CaniLeish®, Virbac, France). It is composed of purified excreted-secreted proteins of L. infantum (LiESP) produced by means of a patented cell-free, serum-free culture system invented by the Institut de Recherche pour le Développement (IRD), and adjuvanted with QA-21, a highly purified fraction of the Quillaja saponaria saponin [103]. The vaccine protocol is three doses, with each dose containing 100 μg LiESP and 60 μg QA-21, given subcutaneously with 21-day intervals and with a booster dose after 1 year to complete the immunization schedule. Leish-Tec® and Leishmune® share similar vaccinations schedules.
In a randomized clinical trial of healthy beagle dogs vaccinated with CaniLeish® and later challenged with live L. infantum, the vaccine induced the development of a strong Th1-type immune response in the vaccinated dogs that was associated with an increase in anti-parasite IgG2 levels, which were protective against infection [104]. Similar results were found in a double-blinded controlled study under field conditions, where a 2-year follow-up showed that CaniLeish® prevented infection in 68.4% of vaccinated dogs, and, in those animals developing active CanL, disease progression was lower and generally less severe than that observed in unvaccinated dogs [105, 106]. In addition, the number of parasites isolated from sandfly midguts feeding on vaccinated dogs was significantly reduced compared to unvaccinated dogs, thereby preventing vector transmission of infection.
5.4 LetiFend® Vaccine
Studies have shown that an immune response directed against Leishmania internal antigens may play a role in controlling disease [107]. L. infantum ribosomal protein extracts have been shown to induce protection against experimental infection in mice [77, 108, 109]. These proteins were recognized by antibodies in sera from mice [110], dogs [111] and humans [112] developing VL. The antigenicity of Leishmania nucleosomal histones proteins has been shown in CanL, and antibodies in sera from dogs developing CanL recognized specific B-cell epitopes from parasite ribosomal proteins LiP2A, LiP2B and LiP0, and from histone protein H2A. Based on these observations, a chimeric protein called protein Q, composed of five B-cell epitopes derived from these four Leishmania proteins, was developed and licensed as LetiFend® (Laboratorios LETI, Spain) in 2016 [113].
The LetiFend® vaccination protocol consists of one dose, followed by annual boosters, and the product should only be administered to dogs aged 6 months or older. The LetiFend® pre-licensing phase III trial included 275 vaccinated dogs and 274 controls dogs, which were exposed to natural infection in two CanL endemic areas located in France and Spain during a 2-year period [113]. These were privately owned dogs of different breeds and ages and kept outdoors in 19 dog kennels. Measurements of the humoral responses to vaccine and SLA, detection of parasite in lymph nodes and clinical evaluation of the animals were done at pre-determined time points. A case of confirmed CanL was defined as any dog presenting clinical signals compatible with CanL, combined with a positive serology to L. infantum antigen and the presence of parasite in the collected samples. Results showed that 4.7% of vaccinated dogs and 10.2% of control dogs developed CanL, and this difference was statistically significant. Only two study sites were selected to perform the analysis of vaccine efficacy due to an unexpectedly low incidence of infection in some dog kennels. According to the results of this field study, LetiFend® showed 72% efficacy in preventing CanL in vaccinated dogs [113]. No adverse effects were observed after vaccine administration during either laboratory or field studies [113, 114]. Furthermore, vaccination with LetiFend® in this field trial did not appear to elicit false-positive results in L. infantum serological diagnostic tests [115].
In another study using a large-scale dog population of different breeds and ages over a 24-month period, vaccination with LetiFend® reduced clinical signs related to the progression of CanL [116]. Data from this study also established a direct relationship between disease severity and circulating immune complexes (CIC) levels. Vaccinated dogs presented significantly lower CIC levels than the control unvaccinated group, which correlated to a lower parasitic load. Moreover, because of vaccination, changes in the protein composition of CIC were detected, including a significant increase in complement system proteins in the vaccinated animals. It was hypothesized that the higher amount of these proteins could be related to activation of this major player in innate immunity, followed by elimination of extracellular parasites. The vaccine induced specific IgG2a isotype antibodies in the vaccinated animals, which correlated with reduction in organ parasitism.
An important confounding factor for CanL control is the possible interference of vaccination schedules in diagnosing Leishmania infection. The impact of vaccination on the ability of common diagnostic methods to detect infection must be assessed prior to licensure of any new CanL vaccines. Difficulties in diagnosing CanL have been reported for dogs vaccinated with Leishmune® and CaniLeish® in Brazil [117] and Europe [105]. The negative impact of CanL vaccination on diagnosis and control of Leishmania infection is expected to be higher whenever vaccines with only low to moderate efficacy are widely implemented in endemic areas. In such cases, a significant proportion of vaccinated and potentially infected dogs would be expected, which, if left undetected, could represent an important reservoir of the parasite, indirectly inducing a rise in the incidence of infection both in vaccinated and unvaccinated animals. Table 13.2 shows the main CanL vaccines currently available to protect against parasite infection.
5.5 New Unlicensed Vaccine Candidates for CanL
Biotechnological tools have been used to try and identify new candidates for inclusion in vaccines for mammalian VL. For example, an immunoproteomics approach has led to the identification of several antigenic Leishmania proteins that have been evaluated for their biological function, as well as their performance as diagnostic markers, vaccine candidates and/or as potential drug targets [119,120,121]. In a previous immunoproteomic study, several parasite proteins, including known and hypothetical antigens, which were expressed in the amastigote and promastigote extracts, were identified by antibodies in sera from dogs developing CanL, and some of them were individually evaluated in ELISA for diagnosis of CanL [122]. The use of such candidates as vaccines was also postulated, due to the existence of specific CD4+ and CD8+ T-cell epitopes in their amino acid sequences, as well as by conservation amongst Leishmania species, but not in other Trypanosomatidae or mammalian hosts [122].
In this context, one of the hypothetical proteins identified in the cited study, namely, LiHyp1, which belongs to the super-oxygenase family in Leishmania, was evaluated as a vaccine candidate against VL. Immunization of BALB/c mice with recombinant LiHyp1 protein plus saponin adjuvant induced a Th1 immune response in the vaccinated animals, which was primed by protein- and parasite-specific IFN-γ, IL-12 and GM-CSF cytokine production, combined with the presence of low levels of IL-4 and IL-10 [123]. Animals subsequently infected with Leishmania parasites displayed significant reductions in the parasite load in their liver, spleen, bone marrow and draining lymph nodes, when compared to control unvaccinated groups. Protection was correlated with a parasite-specific and dependent IFN-γ production, mainly due to CD4+ T cells, which proved to be the major source of this cytokine in these animals. Similar immune and protection profiles were found with other hypothetical proteins, such as LiHyD [124], LiHyT [125], LiHyp6 [126] and LiHyV [127].
The use of chimeric protein-based vaccines containing polypeptides could provide benefits in terms of a more robust protective response against Leishmania infection [128,129,130]. In a recent study, MHC class I and II molecule-specific peptide epitopes were predicted within the amino acid sequences of three Leishmania proteins—one hypothetical, prohibitin and small glutamine-rich tetratricopeptide repeat-containing protein—and the information used to produce a chimeric protein. Immunization of mice with this chimeric protein plus adjuvant significantly lowered the parasite burden in internal organs, accompanied by increased levels of IFN-γ, IL-2, IL-12 and GM-CSF cytokines and IgG2a isotype antibody. In addition, the CD4+ and CD8+ T-cell subtypes contributed to IFN-γ production in the protected animals [30].
A chimeric protein called ChimeraT, containing specific T-cell epitopes from prohibitin, eukaryotic initiation factor 5a, LiHyp1 and LiHyp2 proteins, was constructed and evaluated in mice as a vaccine with saponin adjuvant or incorporation into a liposome [131, 132]. Both vaccine formulations significantly reduced the parasite load in mouse internal organs and stimulated significantly higher levels of IFN-γ, IL-12 and GM-CSF cytokines by both CD4+ and CD8+ T cells, with correspondingly low levels of IL-4 and IL-10 production. In addition, antibodies were predominantly IgG2a isotype, and homologous antigen-stimulated spleen cells produced significant nitrite (as a proxy for nitric oxide).
Other vaccine candidates against leishmaniasis have been also tested against VL. The parasite surface antigen-2 (PSA-2) comprises three polypeptides with Mr ranging from 50.0 to 96.0 kDa [133]. The immunogen was mixed with Corynebacterium parvum bacteria as the adjuvant, and this mixture induced protection in mice against Leishmania challenge via a Th1-type immune response [134]. The kinetoplastid membrane protein-11 (KMP-11), which is a highly conserved protein in distinct Leishmania spp., was evaluated as a vaccine candidate in hamsters against L. donovani infection [135]. The NH36 antigen was also shown to be protective in BALB/c mice against infection with L. infantum, L. mexicana or L. amazonensis, demonstrating the potential of this antigen as a heterologous vaccine against different parasite species [94].
In a previous study, KSAC was shown to be immunogenic and effective in inducing protection in mice against L. infantum. This chimeric protein, which is composed of the Leishmania homolog receptors for activated C kinase (LACK), glycoprotein 63 kDa (gp63), thiol-specific antioxidant (TSA), hydrophilic acylated surface protein B (HASPB), sterol 24-c-methyltransferase (SMT) and KMP-11, A2 and CPB proteins, induced protective responses in the vaccinated animals, which were associated with high production of IFN-γ combined with low levels of IL-4 and a decreased antileishmanial IgG1 response [130]. Another chimeric protein, Leish-111f, which is a combination of thiol-specific antioxidant (TSA), stress-inducible protein 1 (LmSTI-1) and the homolog of the eukaryotic translation initiation factor (eIF4A) proteins, when associated with immune adjuvants, was also able to protect BALB/c mice against Leishmania infection [136].
Another field that could be developed in relation to the discovery of new vaccine candidates against VL will be based on vector salivary proteins [137]. To date, evidence suggests that vector salivary molecules able to induce a Th1-type response in immunized animals could create a protective immunological environment at the bite site. This environment could have an impact when the parasites are injected, allowing the disease to be controlled and promoting a concomitant Leishmania-specific immunity [138]. The Th1-type immunological environment developed at the bite site to these antigens could promote a protective response against the parasite challenge. In this context, PdSP15, a 15 kDa salivary protein member of the family of small odorant binding proteins from Phlebotomus duboscqi, was evaluated as a vaccine candidate against leishmaniasis in a non-human primate [139]. Also, LJM19, an 11 kDa salivary protein with unknown function, and LJL143, a 38 kDa salivary protein with anticoagulant activity [140], both present in the saliva of Lutzomyia longipalpis, were shown to be protective against VL [141]. A summary of some of the single recombinant protein or polyprotein-based vaccine candidates for VL is shown in Table 13.3. Thus, distinct vaccine candidates have been identified and tested in animal models to develop new and effective vaccines to protect against Leishmania infection in both dogs and humans. Further studies are certainly necessary to develop these experimental vaccines for phase I studies.
6 Conclusions
CanL is a serious disease that afflicts canids, causing death if untreated. Infected dogs can be a focus of transmission via vector sandflies to other dogs and humans, in countries where VL is a zoonosis. Preventing new infections in dogs can help to stop the current increase of VL in humans. In this context, effective prophylactic measures to control CanL are imperative, and vaccines are probably the most economical way to control neglected tropical diseases. As described in this chapter, there are some licensed vaccines available for use in countries where CanL is prevalent. However, these vaccines are still considered to be suboptimal, and their efficacy data are unreliable, which is due mainly to the lack of study design standardization, methodological shortcomings and substantial differences found in the characteristics of the study dog populations. All these shortcomings preclude authoritative comparisons between the licensed vaccines. Additional studies are required to prove the efficacy of these vaccines and the new CanL vaccines that are in pre-clinical development.
References
World-Health-Organisation. 2021. http://www.who.int/topics/leishmaniasis/en/. Accessed 30 Mar 2020.
Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. https://doi.org/10.1371/journal.pone.0035671.
Grimaldi G Jr, Tesh RB. Leishmaniases of the New World: current concepts and implications for future research. Clin Microbiol Rev. 1993;6(3):230–50. https://doi.org/10.1128/cmr.6.3.230.
Akilov OE, Khachemoune A, Hasan T. Clinical manifestations and classification of Old World cutaneous leishmaniasis. Int J Dermatol. 2007;46(2):132–42. https://doi.org/10.1111/j.1365-4632.2007.03154.x.
Das VNR, Bimal S, Siddiqui NA, Kumar A, Pandey K, Sinha SK, et al. Conversion of asymptomatic infection to symptomatic visceral leishmaniasis: a study of possible immunological markers. PLoS Negl Trop Dis. 2020;14(6):e0008272. https://doi.org/10.1371/journal.pntd.0008272.
Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392(10151):951–70. https://doi.org/10.1016/S0140-6736(18)31204-2.
Duthie MS, Lison A, Courtenay O. Advances toward diagnostic tools for managing zoonotic visceral Leishmaniasis. Trends Parasitol. 2018;34(10):881–90. https://doi.org/10.1016/j.pt.2018.07.012.
Vinuelas J, Garcia-Alonso M, Ferrando L, Navarrete I, Molano I, Miron C, et al. Meningeal leishmaniosis induced by Leishmania infantum in naturally infected dogs. Vet Parasitol. 2001;101(1):23–7. https://doi.org/10.1016/s0304-4017(01)00413-7.
Travi BL, Cordeiro-da-Silva A, Dantas-Torres F, Miro G. Canine visceral leishmaniasis: diagnosis and management of the reservoir living among us. PLoS Negl Trop Dis. 2018;12(1):e0006082. https://doi.org/10.1371/journal.pntd.0006082.
Marcondes M, Day MJ. Current status and management of canine leishmaniasis in Latin America. Res Vet Sci. 2019;123:261–72. https://doi.org/10.1016/j.rvsc.2019.01.022.
Teixeira AIP, Silva DM, Vital T, Nitz N, de Carvalho BC, Hecht M, et al. Improving the reference standard for the diagnosis of canine visceral leishmaniasis: a challenge for current and future tests. Mem Inst Oswaldo Cruz. 2019;114:e180452. https://doi.org/10.1590/0074-02760180452.
Caldas S, Marcelino AP, Faria G, de Oliveira SF, Ataide ACZ, Cunha LM, et al. Visceral leishmaniasis: a practical strategy for quantitative molecular diagnosis in naturally infected dogs. Parasitol Res. 2020;119(5):1683–90. https://doi.org/10.1007/s00436-020-06654-y.
Pessoa ESR, Vaitkevicius-Antao V, de Andrade TAS, de Oliveira Silva AC, de Oliveira GA, Trajano-Silva LAM, et al. The diagnosis of canine visceral leishmaniasis in Brazil: confronting old problems. Exp Parasitol. 2019;199:9–16. https://doi.org/10.1016/j.exppara.2019.02.012.
Lopes EG, Seva AP, Ferreira F, Nunes CM, Keid LB, Hiramoto RM, et al. Serological and molecular diagnostic tests for canine visceral leishmaniasis in Brazilian endemic area: one out of five seronegative dogs are infected. Epidemiol Infect. 2017;145(12):2436–44. https://doi.org/10.1017/S0950268817001443.
Troncarelli MZ, Camargo JB, Machado JG, Lucheis SB, Langoni H. Leishmania spp. and/or Trypanosoma cruzi diagnosis in dogs from endemic and nonendemic areas for canine visceral leishmaniasis. Vet Parasitol. 2009;164(2–4):118–23. https://doi.org/10.1016/j.vetpar.2009.06.027.
Rocha MF, Michalsky EM, de Oliveira L-SF, Valadao JL, Franca-Silva JC, Pinheiro LC, et al. Dogs with divergent serology for visceral leishmaniasis as sources of Leishmania infection for Lutzomyia longipalpis phlebotomine sand flies—an observational study in an endemic area in Brazil. PLoS Negl Trop Dis. 2020;14(2):e0008079. https://doi.org/10.1371/journal.pntd.0008079.
Farahmand M, Nahrevanian H. Application of recombinant proteins for Serodiagnosis of visceral leishmaniasis in humans and dogs. Iran Biomed J. 2016;20(3):128–34. https://doi.org/10.7508/ibj.2016.03.001.
de Almeida Leal GG, Roatt BM, de Oliveira Aguiar-Soares RD, Carneiro CM, Giunchetti RC, Teixeira-Carvalho A, et al. Immunological profile of resistance and susceptibility in naturally infected dogs by Leishmania infantum. Vet Parasitol. 2014;205(3–4):472–82. https://doi.org/10.1016/j.vetpar.2014.08.022.
Faria AR, Pires SDF, Reis AB, Coura-Vital W, Silveira J, Sousa GM, et al. Canine visceral leishmaniasis follow-up: a new anti-IgG serological test more sensitive than ITS-1 conventional PCR. Vet Parasitol. 2017;248:62–7. https://doi.org/10.1016/j.vetpar.2017.10.020.
Matlashewski G, Das VN, Pandey K, Singh D, Das S, Ghosh AK, et al. Diagnosis of visceral leishmaniasis in Bihar India: comparison of the rK39 rapid diagnostic test on whole blood versus serum. PLoS Negl Trop Dis. 2013;7(5):e2233. https://doi.org/10.1371/journal.pntd.0002233.
Ritmeijer K, Melaku Y, Mueller M, Kipngetich S, O’Keeffe C, Davidson RN. Evaluation of a new recombinant K39 rapid diagnostic test for Sudanese visceral leishmaniasis. Am J Trop Med Hyg. 2006;74(1):76–80.
Lemos EM, Laurenti MD, Moreira MA, Reis AB, Giunchetti RC, Raychaudhuri S, et al. Canine visceral leishmaniasis: performance of a rapid diagnostic test (Kalazar detect) in dogs with and without signs of the disease. Acta Trop. 2008;107(2):205–7. https://doi.org/10.1016/j.actatropica.2008.04.023.
Quinnell RJ, Carson C, Reithinger R, Garcez LM, Courtenay O. Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis: longitudinal study and meta-analysis. PLoS Negl Trop Dis. 2013;7(1):e1992. https://doi.org/10.1371/journal.pntd.0001992.
Martinez Abad LP, Almeida CS, Mattos AMM, Mendonca ACP, Alves MJM, Pinheiro AC, et al. Diagnostic accuracy of rKLO8 versus rK26 ELISAs for screening of canine visceral leishmaniasis. Acta Trop. 2017;166:133–8. https://doi.org/10.1016/j.actatropica.2016.11.021.
Freire ML, Assis TSM, Avelar DM, Rabello A, Cota G. Evaluation of a new brand of immunochromatographic test for visceral leishmaniasis in Brazil made available from 2018. Rev Inst Med Trop Sao Paulo. 2018;60:e49. https://doi.org/10.1590/s1678-9946201860049.
Carvalho FA, Charest H, Tavares CA, Matlashewski G, Valente EP, Rabello A, et al. Diagnosis of American visceral leishmaniasis in humans and dogs using the recombinant Leishmania donovani A2 antigen. Diagn Microbiol Infect Dis. 2002;43(4):289–95. https://doi.org/10.1016/s0732-8893(02)00410-8.
Ghedin E, Zhang WW, Charest H, Sundar S, Kenney RT, Matlashewski G. Antibody response against a Leishmania donovani amastigote-stage-specific protein in patients with visceral leishmaniasis. Clin Diagn Lab Immunol. 1997;4(5):530–5. https://doi.org/10.1128/cdli.4.5.530-535.1997.
Farahmand M, Khalaj V, Mohebali M, Khalili G, Naderi S, Ghaffarinejad P, et al. Comparison of recombinant A2-ELISA with rKE16 dipstick and direct agglutination tests for diagnosis of visceral leishmaniasis in dogs in northwestern Iran. Rev Soc Bras Med Trop. 2015;48(2):188–93. https://doi.org/10.1590/0037-8682-0285-2014.
Mendes TM, Roma EH, Costal-Oliveira F, Dhom-Lemos LC, Toledo-Machado CM, Bruna-Romero O, et al. Epitope mapping of recombinant Leishmania donovani virulence factor A2 (recLdVFA2) and canine leishmaniasis diagnosis using a derived synthetic bi-epitope. PLoS Negl Trop Dis. 2017;11(5):e0005562. https://doi.org/10.1371/journal.pntd.0005562.
Dias DS, Ribeiro PAF, Martins VT, Lage DP, Costa LE, Chavez-Fumagalli MA, et al. Vaccination with a CD4(+) and CD8(+) T-cell epitopes-based recombinant chimeric protein derived from Leishmania infantum proteins confers protective immunity against visceral leishmaniasis. Transl Res. 2018;200:18–34. https://doi.org/10.1016/j.trsl.2018.05.001.
Banu SS, Ahmed BN, Shamsuzzaman AKM, Lee R. Evaluation of recombinant K39 antigen and various promastigote antigens in sero-diagnosis of visceral leishmaniasis in Bangladesh. Parasite Epidemiol Control. 2016;1(3):219–28. https://doi.org/10.1016/j.parepi.2016.07.003.
Duarte MC, Lage DP, Martins VT, Costa LE, Salles BCS, Carvalho A, et al. Performance of Leishmania braziliensis enolase protein for the serodiagnosis of canine and human visceral leishmaniosis. Vet Parasitol. 2017;238:77–81. https://doi.org/10.1016/j.vetpar.2017.03.024.
Magalhaes FB, Castro Neto AL, Nascimento MB, Santos WJT, Medeiros ZM, Lima Neto AS, et al. Evaluation of a new set of recombinant antigens for the serological diagnosis of human and canine visceral leishmaniasis. PLoS One. 2017;12(9):e0184867. https://doi.org/10.1371/journal.pone.0184867.
Fraga DB, Pacheco LV, Borja LS, Tuy PG, Bastos LA, Solca Mda S, et al. The rapid test based on Leishmania infantum chimeric rK28 protein improves the diagnosis of canine visceral leishmaniasis by reducing the detection of false-positive dogs. PLoS Negl Trop Dis. 2016;10(1):e0004333. https://doi.org/10.1371/journal.pntd.0004333.
Anfossi L, Di Nardo F, Profiti M, Nogarol C, Cavalera S, Baggiani C, et al. A versatile and sensitive lateral flow immunoassay for the rapid diagnosis of visceral leishmaniasis. Anal Bioanal Chem. 2018;410(17):4123–34. https://doi.org/10.1007/s00216-018-1067-x.
Boarino A, Scalone A, Gradoni L, Ferroglio E, Vitale F, Zanatta R, et al. Development of recombinant chimeric antigen expressing immunodominant B epitopes of Leishmania infantum for serodiagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2005;12(5):647–53. https://doi.org/10.1128/CDLI.12.5.647-653.2005.
Faria AR, de Castro VL, Coura-Vital W, Reis AB, Damasceno LM, Gazzinelli RT, et al. Novel recombinant multiepitope proteins for the diagnosis of asymptomatic Leishmania infantum-infected dogs. PLoS Negl Trop Dis. 2015;9(1):e3429. https://doi.org/10.1371/journal.pntd.0003429.
Vale DL, Lage DP, Machado AS, Freitas CS, de Oliveira D, Galvani NC, et al. Serodiagnosis of canine leishmaniasis using a novel recombinant chimeric protein constructed with distinct B-cell epitopes from antigenic Leishmania infantum proteins. Vet Parasitol. 2021;296:109513. https://doi.org/10.1016/j.vetpar.2021.109513.
Valladares JE, Riera C, Alberola J, Gallego M, Portus M, Cristofol C, et al. Pharmacokinetics of meglumine antimoniate after administration of a multiple dose in dogs experimentally infected with Leishmania infantum. Vet Parasitol. 1998;75(1):33–40. https://doi.org/10.1016/s0304-4017(97)00193-3.
Miro G, Cardoso L, Pennisi MG, Oliva G, Baneth G. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: part two. Trends Parasitol. 2008;24(8):371–7. https://doi.org/10.1016/j.pt.2008.05.003.
Ribeiro RR, Michalick MSM, da Silva ME, Dos Santos CCP, Frezard FJG, da Silva SM. Canine leishmaniasis: an overview of the current status and strategies for control. Biomed Res Int. 2018;2018:3296893. https://doi.org/10.1155/2018/3296893.
Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012;67(11):2576–97. https://doi.org/10.1093/jac/dks275.
Andrade HM, Toledo VP, Pinheiro MB, Guimaraes TM, Oliveira NC, Castro JA, et al. Evaluation of miltefosine for the treatment of dogs naturally infected with L. infantum (=L. chagasi) in Brazil. Vet Parasitol. 2011;181(2–4):83–90. https://doi.org/10.1016/j.vetpar.2011.05.009.
Proverbio D, Spada E, de Giorgi GB, Perego AR. Proteinuria reduction after treatment with miltefosine and allopurinol in dogs naturally infected with leishmaniasis. Vet World. 2016;9(8):904–8. https://doi.org/10.14202/vetworld.2016.904-908.
Koutinas AF, Saridomichelakis MN, Mylonakis ME, Leontides L, Polizopoulou Z, Billinis C, et al. A randomised, blinded, placebo-controlled clinical trial with allopurinol in canine leishmaniosis. Vet Parasitol. 2001;98(4):247–61. https://doi.org/10.1016/s0304-4017(01)00399-5.
Foglia Manzillo V, Paparcone R, Cappiello S, De Santo R, Bianciardi P, Oliva G. Resolution of tongue lesions caused by Leishmania infantum in a dog treated with the association miltefosine-allopurinol. Parasit Vectors. 2009;2(Suppl 1):S6. https://doi.org/10.1186/1756-3305-2-S1-S6.
Yasur-Landau D, Jaffe CL, Doron-Faigenboim A, David L, Baneth G. Induction of allopurinol resistance in Leishmania infantum isolated from dogs. PLoS Negl Trop Dis. 2017;11(9):e0005910. https://doi.org/10.1371/journal.pntd.0005910.
Solano-Gallego L, Koutinas A, Miro G, Cardoso L, Pennisi MG, Ferrer L, et al. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol. 2009;165(1–2):1–18. https://doi.org/10.1016/j.vetpar.2009.05.022.
Travi BL. Ethical and epidemiological dilemmas in the treatment of dogs for visceral leishmaniasis in Latin America. Biomedica. 2014;34(1):7–12. https://doi.org/10.1590/S0120-41572014000100002.
Berman JD. Chemotherapy for leishmaniasis: biochemical mechanisms, clinical efficacy, and future strategies. Rev Infect Dis. 1988;10(3):560–86. https://doi.org/10.1093/clinids/10.3.560.
Balana-Fouce R, Reguera RM, Cubria JC, Ordonez D. The pharmacology of leishmaniasis. Gen Pharmacol. 1998;30(4):435–43. https://doi.org/10.1016/s0306-3623(97)00268-1.
Mateo M, Maynard L, Vischer C, Bianciardi P, Miro G. Comparative study on the short term efficacy and adverse effects of miltefosine and meglumine antimoniate in dogs with natural leishmaniosis. Parasitol Res. 2009;105(1):155–62. https://doi.org/10.1007/s00436-009-1375-3.
Oliva G, Gradoni L, Ciaramella P, De Luna R, Cortese L, Orsini S, et al. Activity of liposomal amphotericin B (AmBisome) in dogs naturally infected with Leishmania infantum. J Antimicrob Chemother. 1995;36(6):1013–9. https://doi.org/10.1093/jac/36.6.1013.
Miro G, Oliva G, Cruz I, Canavate C, Mortarino M, Vischer C, et al. Multicentric, controlled clinical study to evaluate effectiveness and safety of miltefosine and allopurinol for canine leishmaniosis. Vet Dermatol. 2009;20(5–6):397–404. https://doi.org/10.1111/j.1365-3164.2009.00824.x.
Saridomichelakis MN, Mylonakis ME, Leontides LS, Billinis C, Koutinas AF, Galatos AD, et al. Periodic administration of allopurinol is not effective for the prevention of canine leishmaniosis (Leishmania infantum) in the endemic areas. Vet Parasitol. 2005;130(3–4):199–205. https://doi.org/10.1016/j.vetpar.2005.04.013.
Poli A, Sozzi S, Guidi G, Bandinelli P, Mancianti F. Comparison of aminosidine (paromomycin) and sodium stibogluconate for treatment of canine leishmaniasis. Vet Parasitol. 1997;71(4):263–71. https://doi.org/10.1016/s0304-4017(97)00014-9.
Reguera RM, Moran M, Perez-Pertejo Y, Garcia-Estrada C, Balana-Fouce R. Current status on prevention and treatment of canine leishmaniasis. Vet Parasitol. 2016;227:98–114. https://doi.org/10.1016/j.vetpar.2016.07.011.
Khadem F, Uzonna JE. Immunity to visceral leishmaniasis: implications for immunotherapy. Future Microbiol. 2014;9(7):901–15. https://doi.org/10.2217/fmb.14.43.
Roatt BM, Aguiar-Soares RD, Coura-Vital W, Ker HG, Moreira N, Vitoriano-Souza J, et al. Immunotherapy and immunochemotherapy in visceral leishmaniasis: promising treatments for this neglected disease. Front Immunol. 2014;5:272. https://doi.org/10.3389/fimmu.2014.00272.
Singh OP, Sundar S. Immunotherapy and targeted therapies in treatment of visceral leishmaniasis: current status and future prospects. Front Immunol. 2014;5:296. https://doi.org/10.3389/fimmu.2014.00296.
Okwor I, Uzonna JE. Immunotherapy as a strategy for treatment of leishmaniasis: a review of the literature. Immunotherapy. 2009;1(5):765–76. https://doi.org/10.2217/imt.09.40.
Taslimi Y, Zahedifard F, Rafati S. Leishmaniasis and various immunotherapeutic approaches. Parasitology. 2018;145(4):497–507. https://doi.org/10.1017/S003118201600216X.
Bogdan C. Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell Microbiol. 2008;10(6):1221–34. https://doi.org/10.1111/j.1462-5822.2008.01146.x.
Kedzierski L, Evans KJ. Immune responses during cutaneous and visceral leishmaniasis. Parasitology. 2014;141:1–19. https://doi.org/10.1017/S003118201400095X.
Rodrigues V, Cordeiro-da-Silva A, Laforge M, Silvestre R, Estaquier J. Regulation of immunity during visceral Leishmania infection. Parasit Vectors. 2016;9:118. https://doi.org/10.1186/s13071-016-1412-x.
Dias DS, Ribeiro PAF, Martins VT, Lage DP, Ramos FF, Dias ALT, et al. Recombinant prohibitin protein of Leishmania infantum acts as a vaccine candidate and diagnostic marker against visceral leishmaniasis. Cell Immunol. 2018;323:59–69. https://doi.org/10.1016/j.cellimm.2017.11.001.
Oliveira-da-Silva JA, Lage DP, Ramos FF, Machado AS, Tavares GSV, Mendonca DVC, et al. Leishmania infantum pyridoxal kinase evaluated in a recombinant protein and DNA vaccine to protects against visceral leishmaniasis. Mol Immunol. 2020;124:161–71. https://doi.org/10.1016/j.molimm.2020.06.010.
Strauss-Ayali D, Baneth G, Shor S, Okano F, Jaffe CL. Interleukin-12 augments a Th1-type immune response manifested as lymphocyte proliferation and interferon gamma production in Leishmania infantum-infected dogs. Int J Parasitol. 2005;35(1):63–73. https://doi.org/10.1016/j.ijpara.2004.10.015.
Ramiro MJ, Zarate JJ, Hanke T, Rodriguez D, Rodriguez JR, Esteban M, et al. Protection in dogs against visceral leishmaniasis caused by Leishmania infantum is achieved by immunization with a heterologous prime-boost regime using DNA and vaccinia recombinant vectors expressing LACK. Vaccine. 2003;21(19–20):2474–84. https://doi.org/10.1016/s0264-410x(03)00032-x.
Manna L, Reale S, Viola E, Vitale F, Foglia Manzillo V, Pavone LM, et al. Leishmania DNA load and cytokine expression levels in asymptomatic naturally infected dogs. Vet Parasitol. 2006;142(3–4):271–80. https://doi.org/10.1016/j.vetpar.2006.06.028.
Goto Y, Howard RF, Bhatia A, Trigo J, Nakatani M, Netto EM, et al. Distinct antigen recognition pattern during zoonotic visceral leishmaniasis in humans and dogs. Vet Parasitol. 2009;160(3–4):215–20. https://doi.org/10.1016/j.vetpar.2008.10.097.
Schaut RG, Lamb IM, Toepp AJ, Scott B, Mendes-Aguiar CO, Coutinho JF, et al. Regulatory IgDhi B cells suppress T cell function via IL-10 and PD-L1 during progressive visceral leishmaniasis. J Immunol. 2016;196(10):4100–9. https://doi.org/10.4049/jimmunol.1502678.
Parody N, Cacheiro-Llaguno C, Osuna C, Renshaw-Calderon A, Alonso C, Carnes J. Circulating immune complexes levels correlate with the progression of canine leishmaniosis in naturally infected dogs. Vet Parasitol. 2019;274:108921. https://doi.org/10.1016/j.vetpar.2019.108921.
de Oliveira MC, Paraguai de Souza E, Borja-Cabrera GP, Maria Melo Batista L, Aparecida dos Santos M, Ellner Parra L, et al. IgG1/IgG2 antibody dichotomy in sera of vaccinated or naturally infected dogs with visceral leishmaniosis. Vaccine. 2003;21(19–20):2589–97. https://doi.org/10.1016/s0264-410x(03)00046-x.
Jain K, Jain NK. Vaccines for visceral leishmaniasis: a review. J Immunol Methods. 2015;422:1–12. https://doi.org/10.1016/j.jim.2015.03.017.
Fujiwara RT, Vale AM, Franca da Silva JC, da Costa RT, Quetz Jda S, Martins Filho OA, et al. Immunogenicity in dogs of three recombinant antigens (TSA, LeIF and LmSTI1) potential vaccine candidates for canine visceral leishmaniasis. Vet Res. 2005;36(5–6):827–38. https://doi.org/10.1051/vetres:2005033.
Iborra S, Parody N, Abanades DR, Bonay P, Prates D, Novais FO, et al. Vaccination with the Leishmania major ribosomal proteins plus CpG oligodeoxynucleotides induces protection against experimental cutaneous leishmaniasis in mice. Microbes Infect. 2008;10(10–11):1133–41. https://doi.org/10.1016/j.micinf.2008.06.002.
Kumari S, Kumar A, Samant M, Singh N, Dube A. Discovery of novel vaccine candidates and drug targets against visceral leishmaniasis using proteomics and transcriptomics. Curr Drug Targets. 2008;9(11):938–47. https://doi.org/10.2174/138945008786786091.
Poot J, Janssen LH, van Kasteren-Westerneng TJ, van der Heijden-Liefkens KH, Schijns VE, Heckeroth A. Vaccination of dogs with six different candidate leishmaniasis vaccines composed of a chimerical recombinant protein containing ribosomal and histone protein epitopes in combination with different adjuvants. Vaccine. 2009;27(33):4439–46. https://doi.org/10.1016/j.vaccine.2009.05.043.
Bhowmick S, Ravindran R, Ali N. IL-4 contributes to failure, and colludes with IL-10 to exacerbate Leishmania donovani infection following administration of a subcutaneous leishmanial antigen vaccine. BMC Microbiol. 2014;14:8. https://doi.org/10.1186/1471-2180-14-8.
de Oliveira ES, Vieira de Carvalho T, Meirelles Miranda B, Carneiro da Silva A, Viana Fialho Martins T, Licursi de Oliveira L, et al. Lipophosphoglycan-3 protein from Leishmania infantum chagasi plus saponin adjuvant: a new promising vaccine against visceral leishmaniasis. Vaccine. 2021;39(2):282–91. https://doi.org/10.1016/j.vaccine.2020.11.064.
Charest H, Matlashewski G. Developmental gene expression in Leishmania donovani: differential cloning and analysis of an amastigote-stage-specific gene. Mol Cell Biol. 1994;14(5):2975–84. https://doi.org/10.1128/mcb.14.5.2975-2984.1994.
Farahmand M, Atashi Shirazi H, Nahrevanian H, Hajjaran H. Molecular analysis of A2-genes encoding stage-specific S antigen-like proteins among isolates from Iranian cutaneous and visceral leishmaniasis. Iran J Basic Med Sci. 2011;14(5):407–13.
Coelho EA, Tavares CA, Carvalho FA, Chaves KF, Teixeira KN, Rodrigues RC, et al. Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect Immun. 2003;71(7):3988–94. https://doi.org/10.1128/iai.71.7.3988-3994.2003.
Mizbani A, Taheri T, Zahedifard F, Taslimi Y, Azizi H, Azadmanesh K, et al. Recombinant Leishmania tarentolae expressing the A2 virulence gene as a novel candidate vaccine against visceral leishmaniasis. Vaccine. 2009;28(1):53–62. https://doi.org/10.1016/j.vaccine.2009.09.114.
He J, Huang F, Zhang J, Chen H, Chen Q, Zhang J, et al. DNA prime-protein boost vaccine encoding HLA-A2, HLA-A24 and HLA-DR1 restricted epitopes of CaNA2 against visceral leishmaniasis. Immunology. 2019;156(1):94–108. https://doi.org/10.1111/imm.13007.
Fernandes CB, Junior JT, de Jesus C, Souza BM, Larangeira DF, Fraga DB, et al. Comparison of two commercial vaccines against visceral leishmaniasis in dogs from endemic areas: IgG, and subclasses, parasitism, and parasite transmission by xenodiagnosis. Vaccine. 2014;32(11):1287–95. https://doi.org/10.1016/j.vaccine.2013.12.046.
Regina-Silva S, Feres AM, Franca-Silva JC, Dias ES, Michalsky EM, de Andrade HM, et al. Field randomized trial to evaluate the efficacy of the Leish-Tec(R) vaccine against canine visceral leishmaniasis in an endemic area of Brazil. Vaccine. 2016;34(19):2233–9. https://doi.org/10.1016/j.vaccine.2016.03.019.
Grimaldi G Jr, Teva A, Dos-Santos CB, Santos FN, Pinto ID, Fux B, et al. Field trial of efficacy of the Leish-tec(R) vaccine against canine leishmaniasis caused by Leishmania infantum in an endemic area with high transmission rates. PLoS One. 2017;12(9):e0185438. https://doi.org/10.1371/journal.pone.0185438.
Pereira IE, Silva KP, Menegati LM, Pinheiro AC, Assuncao EAO, Araujo MLP, et al. Performance of recombinant proteins in diagnosis and differentiation of canine visceral leishmaniasis infected and vaccinated dogs. Eur J Microbiol Immunol (Bp). 2020;10(3):165–71. https://doi.org/10.1556/1886.2020.00018.
Palatnik-de-Sousa CB, Nico D. The delay in the licensing of protozoal vaccines: a comparative history. Front Immunol. 2020;11:204. https://doi.org/10.3389/fimmu.2020.00204.
Figueroa-Villar JD, Sales EM. The importance of nucleoside hydrolase enzyme (NH) in studies to treatment of Leishmania: a review. Chem Biol Interact. 2017;263:18–27. https://doi.org/10.1016/j.cbi.2016.12.004.
Santana DM, Borja-Cabrera GP, Paraguai de Souza E, Sturm NR, Palatnik de Sousa CB, Campbell DA. Nucleoside hydrolase from Leishmania (L.) donovani is an antigen diagnostic for visceral leishmaniasis. Mol Biochem Parasitol. 2002;120(2):315–9. https://doi.org/10.1016/s0166-6851(02)00010-5.
Aguilar-Be I, da Silva ZR, Paraguai de Souza E, Borja-Cabrera GP, Rosado-Vallado M, Mut-Martin M, et al. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infect Immun. 2005;73(2):812–9. https://doi.org/10.1128/IAI.73.2.812-819.2005.
Chale-Balboa WG, Mut-Martin M, Ramirez-Sierra MJ, Garcia-Miss MR, Dumonteil E. A combination DNA vaccine encoding nucleoside hydrolase 36 and glycoprotein 63 protects female but not male hamsters against Leishmania mexicana. Parasite. 2009;16(3):227–30. https://doi.org/10.1051/parasite/2009163227.
Souza LOP, Palatnik de Sousa CB. The nucleoside hydrolase DNA vaccine VR1012NH36 in prophylactic vaccination against mice tegumentary leishmaniasis. Procedia Vaccinol. 2009;1(1):120–3. https://doi.org/10.1016/j.provac.2009.07.022.
Al-Wabel MA, Tonui WK, Cui L, Martin SK, Titus RG. Protection of susceptible BALB/c mice from challenge with Leishmania major by nucleoside hydrolase, a soluble exo-antigen of Leishmania. Am J Trop Med Hyg. 2007;77(6):1060–5.
Borja-Cabrera GP, Santos FB, Picillo E, Gravino AE, Manna L, Palatnik-de-Sousa CB. Nucleoside hydrolase DNA vaccine against canine visceral leishmaniasis. Proc Vaccinol. 2009;1(1):104–9. https://doi.org/10.1016/j.provac.2009.07.019.
Borja-Cabrera GP, Santos FN, Bauer FS, Parra LE, Menz I, Morgado AA, et al. Immunogenicity assay of the Leishmune vaccine against canine visceral leishmaniasis in Brazil. Vaccine. 2008;26(39):4991–7. https://doi.org/10.1016/j.vaccine.2008.07.029.
Borja-Cabrera GP, Santos FN, Santos FB, Trivellato FA, Kawasaki JK, Costa AC, et al. Immunotherapy with the saponin enriched-Leishmune vaccine versus immunochemotherapy in dogs with natural canine visceral leishmaniasis. Vaccine. 2010;28(3):597–603. https://doi.org/10.1016/j.vaccine.2009.09.071.
Santos FN, Borja-Cabrera GP, Miyashiro LM, Grechi J, Reis AB, Moreira MA, et al. Immunotherapy against experimental canine visceral leishmaniasis with the saponin enriched-Leishmune vaccine. Vaccine. 2007;25(33):6176–90. https://doi.org/10.1016/j.vaccine.2007.06.005.
de Lima VM, Ikeda FA, Rossi CN, Feitosa MM, Vasconcelos RO, Nunes CM, et al. Diminished CD4+/CD25+ T cell and increased IFN-gamma levels occur in dogs vaccinated with Leishmune in an endemic area for visceral leishmaniasis. Vet Immunol Immunopathol. 2010;135(3–4):296–302. https://doi.org/10.1016/j.vetimm.2009.12.008.
Moreno J, Vouldoukis I, Martin V, McGahie D, Cuisinier AM, Gueguen S. Use of a LiESP/QA-21 vaccine (CaniLeish) stimulates an appropriate Th1-dominated cell-mediated immune response in dogs. PLoS Negl Trop Dis. 2012;6(6):e1683. https://doi.org/10.1371/journal.pntd.0001683.
Oliva G, Nieto J, Foglia Manzillo V, Cappiello S, Fiorentino E, Di Muccio T, et al. A randomised, double-blind, controlled efficacy trial of the LiESP/QA-21 vaccine in naive dogs exposed to two Leishmania infantum transmission seasons. PLoS Negl Trop Dis. 2014;8(10):e3213. https://doi.org/10.1371/journal.pntd.0003213.
Velez R, Domenech E, Cairo J, Gallego M. The impact of canine leishmaniosis vaccination with Canileish(R) in Leishmania infantum infection seroprevalence studies. Acta Trop. 2020;202:105259. https://doi.org/10.1016/j.actatropica.2019.105259.
Velez R, Domenech E, Rodriguez-Cortes A, Barrios D, Tebar S, Fernandez-Arevalo A, et al. Evaluation of canine leishmaniosis vaccine CaniLeish(R) under field conditions in native dog populations from an endemic Mediterranean area-a randomized controlled trial. Acta Trop. 2020;205:105387. https://doi.org/10.1016/j.actatropica.2020.105387.
Requena JM, Alonso C, Soto M. Evolutionarily conserved proteins as prominent immunogens during Leishmania infections. Parasitol Today. 2000;16(6):246–50. https://doi.org/10.1016/s0169-4758(00)01651-3.
Chavez-Fumagalli MA, Costa MAF, Oliveira DM, Ramirez L, Costa LE, Duarte MC, et al. Vaccination with the Leishmania infantum ribosomal proteins induces protection in BALB/c mice against Leishmania chagasi and Leishmania amazonensis challenge. Microb Infect. 2010;12(12–13):967–77. https://doi.org/10.1016/j.micinf.2010.06.008.
Ramirez L, Iborra S, Cortes J, Bonay P, Alonso C, Barral-Netto M, et al. BALB/c mice vaccinated with Leishmania major ribosomal proteins extracts combined with CpG oligodeoxynucleotides become resistant to disease caused by a secondary parasite challenge. J Biomed Biotechnol. 2010;2010:181690. https://doi.org/10.1155/2010/181690.
Iniesta V, Corraliza I, Carcelen J, Gomez Gordo L, Fernandez-Cotrina J, Parejo JC, et al. Leishmania major infection in susceptible and resistant mice elicit a differential humoral response against a total soluble fraction and defined recombinant antigens of the parasite. Parasitol Res. 2008;102(5):887–93. https://doi.org/10.1007/s00436-007-0844-9.
Coelho EA, Ramirez L, Costa MA, Coelho VT, Martins VT, Chavez-Fumagalli MA, et al. Specific serodiagnosis of canine visceral leishmaniasis using Leishmania species ribosomal protein extracts. Clin Vaccine Immunol. 2009;16(12):1774–80. https://doi.org/10.1128/CVI.00295-09.
Soto M, Requena JM, Quijada L, Alonso C. Specific serodiagnosis of human leishmaniasis with recombinant Leishmania P2 acidic ribosomal proteins. Clin Diagn Lab Immunol. 1996;3(4):387–91. https://doi.org/10.1128/cdli.3.4.387-391.1996.
Fernandez Cotrina J, Iniesta V, Monroy I, Baz V, Hugnet C, Maranon F, et al. A large-scale field randomized trial demonstrates safety and efficacy of the vaccine LetiFend(R) against canine leishmaniosis. Vaccine. 2018;36(15):1972–82. https://doi.org/10.1016/j.vaccine.2018.02.111.
Carcelen J, Iniesta V, Fernandez-Cotrina J, Serrano F, Parejo JC, Corraliza I, et al. The chimerical multi-component Q protein from Leishmania in the absence of adjuvant protects dogs against an experimental Leishmania infantum infection. Vaccine. 2009;27(43):5964–73. https://doi.org/10.1016/j.vaccine.2009.07.069.
Iniesta V, Fernandez-Cotrina J, Solano-Gallego L, I Monroy, Gomez-Luque A, Munoz-Madrid R. Vaccination with LetiFend, a novel canine leishmaniasis vaccine, does not interfere with serological diagnostic tests. In: Proceedings of the X Southern European Veterinary Conference/51 Congreso Nacional Avepa. Granada, Spain; 2016. p. Poster SEVC00678.
Cacheiro-Llaguno C, Parody N, Renshaw-Calderon A, Osuna C, Alonso C, Carnes J. Vaccination with LetiFend(R) reduces circulating immune complexes in dogs experimentally infected with L. infantum. Vaccine. 2020;38(4):890–6. https://doi.org/10.1016/j.vaccine.2019.10.078.
Marcondes M, Ikeda FA, Vieira RF, Day MJ, Lima VM, Rossi CN, et al. Temporal IgG subclasses response in dogs following vaccination against Leishmania with Leishmune(R). Vet Parasitol. 2011;181(2–4):153–9. https://doi.org/10.1016/j.vetpar.2011.04.004.
Moreno J. Assessment of vaccine-induced immunity against canine visceral leishmaniasis. Front Vet Sci. 2019;6:168. https://doi.org/10.3389/fvets.2019.00168.
Chenik M, Lakhal S, Ben Khalef N, Zribi L, Louzir H, Dellagi K. Approaches for the identification of potential excreted/secreted proteins of Leishmania major parasites. Parasitology. 2006;132(Pt 4):493–509. https://doi.org/10.1017/S0031182005009546.
Paape D, Barrios-Llerena ME, Le Bihan T, Mackay L, Aebischer T. Gel free analysis of the proteome of intracellular Leishmania mexicana. Mol Biochem Parasitol. 2010;169(2):108–14. https://doi.org/10.1016/j.molbiopara.2009.10.009.
Costa MM, Andrade HM, Bartholomeu DC, Freitas LM, Pires SF, Chapeaurouge AD, et al. Analysis of Leishmania chagasi by 2-D difference gel electrophoresis (2-D DIGE) and immunoproteomic: identification of novel candidate antigens for diagnostic tests and vaccine. J Proteome Res. 2011;10(5):2172–84. https://doi.org/10.1021/pr101286y.
Coelho VT, Oliveira JS, Valadares DG, Chavez-Fumagalli MA, Duarte MC, Lage PS, et al. Identification of proteins in promastigote and amastigote-like Leishmania using an immunoproteomic approach. PLoS Negl Trop Dis. 2012;6(1):e1430. https://doi.org/10.1371/journal.pntd.0001430.
Martins VT, Chavez-Fumagalli MA, Costa LE, Canavaci AM, Martins AM, Lage PS, et al. Antigenicity and protective efficacy of a Leishmania amastigote-specific protein, member of the super-oxygenase family, against visceral leishmaniasis. PLoS Negl Trop Dis. 2013;7(3):e2148. https://doi.org/10.1371/journal.pntd.0002148.
Lage DP, Martins VT, Duarte MC, Garde E, Chavez-Fumagalli MA, Menezes-Souza D, et al. Prophylactic properties of a Leishmania-specific hypothetical protein in a murine model of visceral leishmaniasis. Parasite Immunol. 2015;37(12):646–56. https://doi.org/10.1111/pim.12287.
Martins VT, Lage DP, Duarte MC, Costa LE, Garde E, Rodrigues MR, et al. A new Leishmania-specific hypothetical protein, LiHyT, used as a vaccine antigen against visceral leishmaniasis. Acta Trop. 2016;154:73–81. https://doi.org/10.1016/j.actatropica.2015.11.006.
Martins VT, Chavez-Fumagalli MA, Lage DP, Duarte MC, Garde E, Costa LE, et al. Antigenicity, immunogenicity and protective efficacy of three proteins expressed in the promastigote and amastigote stages of Leishmania infantum against visceral leishmaniasis. PLoS One. 2015;10(9):e0137683. https://doi.org/10.1371/journal.pone.0137683.
Martins VT, Duarte MC, Chavez-Fumagalli MA, Menezes-Souza D, Coelho CSP, de Magalhaes-Soares DF, et al. A Leishmania-specific hypothetical protein expressed in both promastigote and amastigote stages of Leishmania infantum employed for the serodiagnosis of, and as a vaccine candidate against, visceral leishmaniasis. Parasit Vectors. 2015;8:363. https://doi.org/10.1186/s13071-015-0964-5.
Molano I, Alonso MG, Miron C, Redondo E, Requena JM, Soto M, et al. A Leishmania infantum multi-component antigenic protein mixed with live BCG confers protection to dogs experimentally infected with L. infantum. Vet Immunol Immunopathol. 2003;92(1–2):1–13. https://doi.org/10.1016/s0165-2427(02)00315-x.
Gradoni L, Foglia Manzillo V, Pagano A, Piantedosi D, De Luna R, Gramiccia M, et al. Failure of a multi-subunit recombinant leishmanial vaccine (MML) to protect dogs from Leishmania infantum infection and to prevent disease progression in infected animals. Vaccine. 2005;23(45):5245–51. https://doi.org/10.1016/j.vaccine.2005.07.001.
Goto Y, Bhatia A, Raman VS, Liang H, Mohamath R, Picone AF, et al. KSAC, the first defined polyprotein vaccine candidate for visceral leishmaniasis. Clin Vaccine Immunol. 2011;18(7):1118–24. https://doi.org/10.1128/CVI.05024-11.
Lage DP, Ribeiro PAF, Dias DS, Mendonca DVC, Ramos FF, Carvalho LM, et al. A candidate vaccine for human visceral leishmaniasis based on a specific T cell epitope-containing chimeric protein protects mice against Leishmania infantum infection. NPJ Vaccines. 2020;5:75. https://doi.org/10.1038/s41541-020-00224-0.
Lage DP, Ribeiro PAF, Dias DS, Mendonça DVC, Ramos FF, Carvalho LM, et al. Liposomal formulation of ChimeraT, a multiple T-cell epitope-containing recombinant protein, is a candidate vaccine for human visceral leishmaniasis. Vaccines (Basel). 2020;8(2):289. https://doi.org/10.3390/vaccines8020289.
Handman E, Osborn AH, Symons F, van Driel R, Cappai R. The Leishmania promastigote surface antigen 2 complex is differentially expressed during the parasite life cycle. Mol Biochem Parasitol. 1995;74(2):189–200. https://doi.org/10.1016/0166-6851(95)02500-6.
Sjolander A, Baldwin TM, Curtis JM, Bengtsson KL, Handman E. Vaccination with recombinant parasite surface antigen 2 from Leishmania major induces a Th1 type of immune response but does not protect against infection. Vaccine. 1998;16(20):2077–84. https://doi.org/10.1016/s0264-410x(98)00075-9.
Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F. Leishmaniasis vaccine candidates for development: a global overview. Indian J Med Res. 2006;123(3):423–38.
Coler RN, Skeiky YA, Bernards K, Greeson K, Carter D, Cornellison CD, et al. Immunization with a polyprotein vaccine consisting of the T-cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infect Immun. 2002;70(8):4215–25. https://doi.org/10.1128/IAI.70.8.4215-4225.2002.
Duarte MC, Lage DP, Martins VT, Chavez-Fumagalli MA, Roatt BM, Menezes-Souza D, et al. Recent updates and perspectives on approaches for the development of vaccines against visceral leishmaniasis. Rev Soc Bras Med Trop. 2016;49(4):398–407. https://doi.org/10.1590/0037-8682-0120-2016.
Kamhawi S, Aslan H, Valenzuela JG. Vector saliva in vaccines for visceral leishmaniasis: a brief encounter of high consequence? Front Public Health. 2014;2:99. https://doi.org/10.3389/fpubh.2014.00099.
Oliveira F, Rowton E, Aslan H, Gomes R, Castrovinci PA, Alvarenga PH, et al. A sand fly salivary protein vaccine shows efficacy against vector-transmitted cutaneous leishmaniasis in nonhuman primates. Sci Transl Med. 2015;7(290):290ra90. https://doi.org/10.1126/scitranslmed.aaa3043.
Collin N, Assumpcao TC, Mizurini DM, Gilmore DC, Dutra-Oliveira A, Kotsyfakis M, et al. Lufaxin, a novel factor Xa inhibitor from the salivary gland of the sand fly Lutzomyia longipalpis blocks protease-activated receptor 2 activation and inhibits inflammation and thrombosis in vivo. Arterioscler Thromb Vasc Biol. 2012;32(9):2185–98. https://doi.org/10.1161/ATVBAHA.112.253906.
Gomes R, Teixeira C, Teixeira MJ, Oliveira F, Menezes MJ, Silva C, et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci U S A. 2008;105(22):7845–50. https://doi.org/10.1073/pnas.0712153105.
Gupta R, Kumar V, Kushawaha PK, Tripathi CP, Joshi S, Sahasrabuddhe AA, et al. Characterization of glycolytic enzymes—rAldolase and rEnolase of Leishmania donovani, identified as Th1 stimulatory proteins, for their immunogenicity and immunoprophylactic efficacies against experimental visceral leishmaniasis. PLoS One. 2014;9(1):e86073. https://doi.org/10.1371/journal.pone.0086073.
Poot J, Spreeuwenberg K, Sanderson SJ, Schijns VE, Mottram JC, Coombs GH, et al. Vaccination with a preparation based on recombinant cysteine peptidases and canine IL-12 does not protect dogs from infection with Leishmania infantum. Vaccine. 2006;24(14):2460–8. https://doi.org/10.1016/j.vaccine.2005.12.039.
Khoshgoo N, Zahedifard F, Azizi H, Taslimi Y, Alonso MJ, Rafati S. Cysteine proteinase type III is protective against Leishmania infantum infection in BALB/c mice and highly antigenic in visceral leishmaniasis individuals. Vaccine. 2008;26(46):5822–9. https://doi.org/10.1016/j.vaccine.2008.08.065.
Santos-Gomes GM, Rodrigues A, Teixeira F, Carreira J, Alexandre-Pires G, Carvalho S, et al. Immunization with the Leishmania infantum recombinant cyclophilin protein 1 confers partial protection to subsequent parasite infection and generates specific memory T cells. Vaccine. 2014;32(11):1247–53. https://doi.org/10.1016/j.vaccine.2014.01.024.
Jaffe CL, Rachamim N, Sarfstein R. Characterization of two proteins from Leishmania donovani and their use for vaccination against visceral leishmaniasis. J Immunol. 1990;144(2):699–706.
Kushawaha PK, Gupta R, Sundar S, Sahasrabuddhe AA, Dube A. Elongation factor-2, a Th1 stimulatory protein of Leishmania donovani, generates strong IFN-gamma and IL-12 response in cured Leishmania-infected patients/hamsters and protects hamsters against Leishmania challenge. J Immunol. 2011;187(12):6417–27. https://doi.org/10.4049/jimmunol.1102081.
Stager S, Smith DF, Kaye PM. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J Immunol. 2000;165(12):7064–71. https://doi.org/10.4049/jimmunol.165.12.7064.
Wilson ME, Young BM, Andersen KP, Weinstock JV, Metwali A, Ali KM, et al. A recombinant Leishmania chagasi antigen that stimulates cellular immune responses in infected mice. Infect Immun. 1995;63(5):2062–9. https://doi.org/10.1128/iai.63.5.2062-2069.1995.
Baharia RK, Tandon R, Sharma T, Suthar MK, Das S, Siddiqi MI, et al. Recombinant NAD-dependent SIR-2 protein of Leishmania donovani: immunobiochemical characterization as a potential vaccine against visceral leishmaniasis. PLoS Negl Trop Dis. 2015;9(3):e0003557. https://doi.org/10.1371/journal.pntd.0003557.
Agallou M, Smirlis D, Soteriadou KP, Karagouni E. Vaccination with Leishmania histone H1-pulsed dendritic cells confers protection in murine visceral leishmaniasis. Vaccine. 2012;30(34):5086–93. https://doi.org/10.1016/j.vaccine.2012.05.075.
Ramirez L, Corvo L, Duarte MC, Chavez-Fumagalli MA, Valadares DG, Santos DM, et al. Cross-protective effect of a combined L5 plus L3 Leishmania major ribosomal protein based vaccine combined with a Th1 adjuvant in murine cutaneous and visceral leishmaniasis. Parasit Vectors. 2014;7:3. https://doi.org/10.1186/1756-3305-7-3.
Tewary P, Pandya J, Mehta J, Sukumaran B, Madhubala R. Vaccination with Leishmania soluble antigen and immunostimulatory oligodeoxynucleotides induces specific immunity and protection against Leishmania donovani infection. FEMS Immunol Med Microbiol. 2004;42(2):241–8. https://doi.org/10.1016/j.femsim.2004.05.008.
Saljoughian N, Taheri T, Zahedifard F, Taslimi Y, Doustdari F, Bolhassani A, et al. Development of novel prime-boost strategies based on a tri-gene fusion recombinant L. tarentolae vaccine against experimental murine visceral leishmaniasis. PLoS Negl Trop Dis. 2013;7(4):e2174. https://doi.org/10.1371/journal.pntd.0002174.
Coler RN, Duthie MS, Hofmeyer KA, Guderian J, Jayashankar L, Vergara J, et al. From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin Transl Immunol. 2015;4(4):e35. https://doi.org/10.1038/cti.2015.6.
Duthie MS, Favila M, Hofmeyer KA, Tutterrow YL, Reed SJ, Laurance JD, et al. Strategic evaluation of vaccine candidate antigens for the prevention of visceral leishmaniasis. Vaccine. 2016;34(25):2779–86. https://doi.org/10.1016/j.vaccine.2016.04.067.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Coelho, E.A.F., Christodoulides, M. (2023). Vaccines for Canine Leishmaniasis. In: Christodoulides, M. (eds) Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges . Springer, Cham. https://doi.org/10.1007/978-3-031-24355-4_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-24355-4_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24354-7
Online ISBN: 978-3-031-24355-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)