Abstract
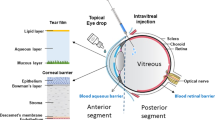
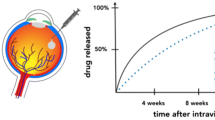
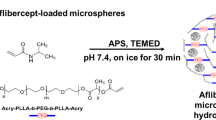
In general, it is difficult to achieve effective levels of drugs in the vitreous and the retina via topical and/or systemic administration. Intraocular drug delivery systems that achieve longer duration of pharmacological effect with lower administration frequency are urgently needed. Intraocular sustained drug release via implantable devices or injectable particles has been investigated for the treatment of various vitreoretinal disorders. Several non-biodegradable implants are available in clinical practice or in the late developmental phase: Vitrasert® (ganciclovir intravitreal implant) for cytomegalovirus retinitis, Retisert™ (fluocinolone acetonide intravitreal implant) for non-infectious uveitis, Iluvien™ (fluocinolone acetonide intravitreal implant) for diabetic macular oedema, and NT-501 (a polymer implant containing human retinal epithelial cells genetically modified to secrete ciliary neurotrophic factor) for non-neovascular (dry) age-related macular degeneration and/or retinitis pigmentosa. Many biodegradable formulations, including different shapes of rods, nail-like plugs, discs, or micro- or nano-particles, have also been investigated, but are not available as yet for the treatment of vitreoretinal disorders. The most developed biodegradable device, Ozurdex™ (dexamethasone intravitreal implant), is approved as first-line therapy for the treatment of macular oedema following branch retinal vein occlusion or central retinal vein occlusion.
In this article, we review the progress of major biodegradable drug delivery systems currently in clinical trials or in experimental stages for the treatment of vitreoretinal disorders.







Similar content being viewed by others
References
Maurice DM, Mishima S. Ocular pharmacokinetics. In: Sears ML, editor. Pharmacology of the eye. New York: Springer-Verlag, 1984: 19–116
Kunou N, Ogura Y, Honda Y, et al. Biodegradable scleral implant for controlled intraocular delivery of betamethasone phosphate. J Biomed Mater Res 2000 Sep 15; 51(4): 635–41
Sakurai E, Matsuda Y, Ozeki H, et al. Scleral plug of biodegradable polymers containing ganciclovir for experimental cytomegalovirus retinitis. Invest Ophthalmol Vis Sci 2001; 42(9): 2043–8
Okabe J, Kimura H, Kunou N, et al. Biodegradable intrascleral implant for sustained intraocular delivery of betamethasone phosphate. Invest Ophthalmol Vis Sci 2003; 44(2): 740–4
Gopferich A. Mechanisms of polymer degradation and erosion. Biomaterials 1996; 17(2): 103–14
Gopferich A. Erosion of composite polymer matrices. Biomaterials 1997; 18(5): 397–403
von Burkersroda F, Schedl L, Gopferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002; 23(21): 4221–31
Brunner A, Mader K, Gopferich A. pH and osmotic pressure inside biodegradable microspheres during erosion. Pharm Res 1999; 16(6): 847–53
Lucke A, Kiermaier J, Gopferich A. Peptide acylation by poly(alpha-hydroxy esters). Pharm Res 2002; 19(2): 175–81
Ibrahim MA, Ismail A, Fetouh MI, et al. Stability of insulin during the erosion of poly(lactic acid) and poly(lactic-co-glycolic acid) microspheres. J Control Release 2005; 106(3): 241–52
Miller RA, Brady JM, Cutright DE. Degradation rates of oral resorbable implants (polylactates and poly-glycolates): rate modification with changes in PLA/PGA copolymer ratios. J Biomed Mater Res 1977; 11(5): 711–9
Kuno N, Ogura Y, Hashizoe M, et al. Controlled intraocular delivery of ganciclovir with use of biodegradable scleral implants in rabbits. J Control Release 1995; 37: 143–50
Kuno N, Ogura Y, Yasukawa T, et al. Long-term sustained release of ganciclovir from biodegradable scleral implant for the treatment of cytomegalovirus retinitis. J Control Release 2000; 68(2): 263–71
Hora MS, Rana RK, Nunberg JH, et al. Release of human serum albumin from poly(lactide-co-glycolide) microspheres. Pharm Res 1990; 7(11): 1190–4
Sanders LM, Kent JS, McRae GI, et al. Controlled release of a luteinizing hormone-releasing hormone analogue from poly(d,l-lactide-co-glycolide) microspheres. J Pharm Sci 1984; 73(9): 1294–7
Pitt CG, Gratzl MM, Kimmel GL, et al. Aliphatic polyesters: II. The degradation of poly(DL-lactide), poly(µ-ca-prolactone) and their copolymers in vivo. Biomaterials 1981; 2: 215–20
Li SM, Garreau H, Vert M. Structure-property relationships in the case of degradation of massive aliphatic poly (α-hydroxy acids) in aqueous media. Part 1: poly (DL-lactic acid). J Mater Sci Mater Med 1990; 1: 123–30
Li SM, Garreau H, Vert M. Structure-property relationships in the case of degradation of massive aliphatic poly (α-hydroxy acids) in aqueous media. Part 2: degradation of lactide-glycolide copolymers, PLA37.5GA25 and PLA75GA25. J Mater Sci Mater Med 1990; 1: 131–9
Li SM, Garreau H, Vert M. Structure-property relationships in the case of degradation of massive aliphatic poly (α-hydroxy acids) in aqueous media. Part 3: influence of the morphology of poly (L-lactic acid). J Mater Sci Mater Med 1990; 1: 198–206
Giordano GG, Chevez-Barrios P, Refojo MF, et al. Biodegradation and tissue reaction to intravitreous biodegradable poly(D,L-lactic-co-glycolic)acid microspheres. Curr Eye Res 1995; 14(9): 761–8
Herrero-Vanrell R, Refojo MF. Biodegradable microspheres for vitreoretinal drug delivery. Adv Drug Deliv 2001; 52: 5–16
Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev 1997; 28: 5–24
Leong KW, Brott BC, Langer R. Bioerodible poly-anhydrides as drug-carrier matrices. I: characterization, degradation, and release characteristics. J Biomed Mater Res 1985; 19(8): 941–55
Brem H, Kader A, Epstein JI, et al. Biocompatibility of a biodegradable, controlled-release polymer in the rabbit brain. Sel Cancer Ther 1989; 5(2): 55–65
Brem H, Tamargo RJ, Olivi A, et al. Biodegradable polymers for controlled delivery of chemotherapy with and without radiation therapy in the monkey brain. J Neurosurg 1994; 80(2): 283–90
Jampel HD, Leong KW, Dunkelburger GR, et al. Glaucoma filtration surgery in monkeys using 5-fluorouridine in polyanhydride disks. Arch Ophthalmol 1990; 108(3): 430–5
Jampel HD, Thibault D, Leong KW, et al. Glaucoma filtration surgery in nonhuman primates using Taxol and etoposide in polyanhydride carriers. Invest Ophthalmol Vis Sci 1993; 34(11): 3076–83
Lee DA, Flores RA, Anderson PJ, et al. Glaucoma filtration surgery in rabbits using bioerodible polymers and 5-fluorouracil. Ophthalmology 1987; 94(12): 1523–30
Lee DA, Leong KW, Panek WC, et al. The use of bioerodible polymers and 5-fluorouracil in glaucoma filtration surgery. Invest Ophthalmol Vis Sci 1988; 29(11): 1692–7
Bausch & Lomb. Vitrasert® (ganciclovir intravitreal implant) 4.5 mg [online]. Available from URL: http://www.bausch.com/en_US/ecp/pharma/product/vitrasert.aspx [Accessed 2009 Jun 23]
Bausch & Lomb. Vitrasert®: sterile intravitreal implant with Cytovene® (ganiciclovir, 4.5 mg) [online]. Available from URL: http://www.bausch.com [Accessed 2009 Jun 23]
Jaffe GJ, Ben-Nun J, Guo H, et al. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology 2000; 107(11): 2024–33
Jaffe GJ, Yang CH, Guo H, et al. Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Invest Ophthalmol Vis Sci 2000; 41(11): 3569–75
Jaffe GJ, McCallum RM, Branchaud B, et al. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology 2005; 112(7): 1192–8
Retisert® (fluocinolone acetonide intravitreal implant) 0.59mg: prescribing information. Rochester (NY): Bausch & Lomb Inc., 2009 Mar [online]. Available from URL: http://www.bausch.com/en_US/downloads/ecp/pharma/general/retisert_prescinfopdf.pdf [Accessed 2009 Jun 23]
Retisert® (fluocinolone acetonide intravitreal implant) 0.59mg — sterile: prescribing information. Rochester (NY): Bausch & Lomb Inc., 2007 Apr [online]. Available from URL: http://www.bauschsurgical.com/vitreoretinal/pdf/prescribing_information_new.pdf [Accessed 2009 Jun 23]
Alimera Sciences. Iluvien: addressing the ophthalmic crisis of diabetes [online]. Available from URL: http://www.alimerasciences.com/Products/IluvienOverview/tabid/82/Default.aspx [Accessed 2009 Jun 23]
Kane FE, Burdan J, Cuino A, et al. Iluvien: a new sustained delivery technology for posterior eye disease. Expert Opin Drug Deliv 2008; 5(9): 1039–46
Tao W, Wen R, Goddard MB, et al. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci 2002; 43(10): 3292–8
Bush RA, Lei B, Tao W, et al. Encapsulated cell-based intraocular delivery of ciliary neurotrophic factor in normal rabbit: dose-dependent effects on ERG and retinal histology. Invest Ophthalmol Vis Sci 2004; 45(7): 2420–30
Neurotech. About encapsulated cell technology [online]. Available from URL: http://www.neurotechusa.com/ect/about_encapsulated_cell_technology.asp [Accessed 2009 Jun 23]
Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A 2006; 103(10): 3896–901
Neurotech. Positive results from Neurotech’s NT-501 phase 2 dry AMD (geographic atrophy) study demonstrate proof of concept [online]. Available from URL: http://www.neurotechusa.com/news_events/pr_2009-03-26.asp [Accessed 2009 Jun 23]
SurModics. Intravitreal drug delivery capabilities [online]. Available from URL: http://www.surmodics.com/clinical-ophthalmology-intravitreal.html [Accessed 2009 Jun 23]
Dugel PU, Fliott D, Cantrill HL, et al. I-Vation™ TA: 24-month clinical results of the phase I safety and preliminary efficacy study [abstract no. 4332]. Association for Research in Vision and Ophthalmology Annual Meeting; 2009 May 3–7; Fort Lauderdale (FL)
Holland GN, Pepose JS, Pettit TH, et al. Acquired immune deficiency syndrome: ocular manifestations. Ophthalmology 1983; 90(8): 859–73
Palestine AG, Rodrigues MM, Macher AM, et al. Ophthalmic involvement in acquired immunodeficiency syndrome. Ophthalmology 1984; 91(9): 1092–9
Hennis HL, Scott AA, Apple DJ. Cytomegalovirus retinitis. Surv Ophthalmol 1989; 34(3): 193–203
Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 1997; 277(5322): 112–6
Mitchell SM, Membrey WL, Youle MS, et al. Cytomegalovirus retinitis after the initiation of highly active antiretroviral therapy: a 2 year prospective study. Br J Ophthalmol 1999; 83(6): 652–5
Stephenson J. The art of ‘HAART’: researchers probe the potential and limits of aggressive HIV treatments. JAMA 1997; 277(8): 614–6
Vrabec TR, Baldassano VF, Whitcup SM. Discontinuation of maintenance therapy in patients with quiescent cytomegalovirus retinitis and elevated CD4+ counts. Ophthalmology 1998; 105(7): 1259–64
Ogura Y, Ikada Y, inventors. Biodegradable scleral plug. US patent 5707643. 1998
Ryan SJ, Stout JT, Dugel PU. Subretinal neovascularization. In: Ryan SJ, editor. Retina. St Louis (MO): Mosby, 1994: 1027–47
Klein R, Klein BEK, Linton KLP. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1992; 99: 933–43
Macugen® (pegaptanib sodium injection): prescribing information. Eyetech Inc. [online]. Available from URL: http://www.macugen.com/macugenUSPI.pdf [Accessed 2009 Jun 23]
Lucentis® (ranibizumab injection): prescribing information. Genentech Inc. [online]. Available from URL: http://www.lucentis.com/lucentis/pdf/prescribing_information.pdf [Accessed 2009 Jun 23]
Bashshur ZF, Haddad ZA, Schakal A, et al. Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: a one-year prospective study. Am J Ophthalmol 2008; 145: 249–56
Arevalo JF, Fromow-Guerra J, Sanchez JG, et al. Primary intravitreal bevacizumab for subfoveal choroidal neovascularization in age-related macular degeneration: results of the Pan-American Collaborative Retina Study Group at 12 months follow-up. Retina 2008; 28: 1387–94
Avila MP, Farah ME, Santos A, et al. Twelve-month short-term safety and visual acuity results from a multicentre, prospective study of epiretinal strontium-90 brachytherapy with bevacizumab for the treatment of subfoveal choroidal neovascularization secondary to age-related macular degeneration. Br J Ophthalmol 2008; 93: 305–9
Visudyne® (verteporfin for injection): prescribing information. Novartis Pharmaceuticals [online]. Available from URL: http://www.pharma.us.novartis.com/product/pi/pdf/visudyne.pdf [Accessed 2009 Jun 23]
Allison BA, Pritchard PH, Levy JG. Evidence for low-density lipoprotein receptor-mediated uptake of benzo-porphyrin derivative. Br J Cancer 1994; 69(5): 833–9
Amin K, Wasan KM, Albrecht RM, et al. Cell association of liposomes with high fluid anionic phospholipid content is mediated specifically by LDL and its receptor, LDLr. J Pharm Sci 2002; 91(5): 1233–44
Lucentis Utilizing Visudyne (LUV Trial) combination therapy in the treatment of age-related macular degeneration [ClinicalTrials.gov identifier NCT00423189]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2009 Jun 23]
Photodynamic therapy combined with bevacizumab versus bevacizumab alone for neovascular age-related macular degeneration (ARMAST) [ClinicalTrials.gov identifier NCT00696592]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2009 Jun 23]
Safety and efficacy of oral PTK787 in patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration (AMD) (ADVANCE) [ClinicalTrials.gov identifier NCT00138632]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2009 Jun 23]
TAC-PF, Avastin® in combination with photodynamic therapy to treat age related macular degeneration (VER-TACL) [ClinicalTrials.gov identifier NCT00464347]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2009 Jun 23]
Reduced fluence Visudyne-Anti-VEGF-Dexamethasone In Combination for AMD Lesions (RADICAL) [Clinical Trials.gov identifier NCT00492284]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2009 Jun 23]
Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 2000; 65(1–2): 271–84
Maeda H, Matsumura Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit Rev Ther Drug Carrier Syst 1989; 6(3): 193–210
Ideta R, Yanagi Y, Tamaki Y, et al. Effective accumulation of polyion complex micelle to experimental choroidal neovascularization in rats. FEBS Lett 2004; 557(1–3): 21–5
Ideta R, Tasaka F, Yanagi Y, et al. Photodynamic therapy for choroidal neovascularization using polyion complex micelle [abstract no. 33]. 5th International Symposium on Ocular Pharmacology and Therapeutics; 2004 Mar 11–14; Monte Carlo
Yasukawa T, Kimura H, Tabata Y, et al. Targeted delivery of anti-angiogenic agent TNP-470 using water-soluble polymer in the treatment of choroidal neovascularization. Invest Ophthalmol Vis Sci 1999; 40(11): 2690–6
Kamizuru H, Kimura H, Yasukawa T, et al. Monoclonal antibody-mediated drug targeting to choroidal neovascularization in the rat. Invest Ophthalmol Vis Sci 2001 Oct; 42(11): 2664–72
Yasukawa T, Kimura H, Tabata Y, et al. Targeting of interferon to choroidal neovascularization by use of dextran and metal coordination. Invest Ophthalmol Vis Sci 2002; 43(3): 842–8
Beeley NR, Rossi JV, Mello-Filho PA, et al. Fabrication, implantation, elution, and retrieval of a steroid-loaded polycaprolactone subretinal implant. J Biomed Mater Res A 2005; 73(4): 437–44
Ali F. A review of diabetic macular edema. Digit J Ophthalmol 2002; 3(6) [online]. Available from URL: http://www.djo.harvard.edu/site.php?url=/physicians/oa/387 [Accessed 2009 Sep 15]
Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy: IV. Diabetic macular edema. Ophthalmology 1984; 91: 1464–74
Bandello F, Pognuz R, Polito A, et al. Diabetic macular edema: classification, medical and laser therapy. Semin Ophthalmol 2003; 18: 251–8
Early Treatment Diabetic Retinopathy Study Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985; 103: 1796–806
Jermak CM, Dellacroce JT, Heffez J, et al. Triamcinolone acetonide in ocular therapeutics. Surv Ophthalmol 2007; 52: 503–22
De Carvalho RPP. Pharmacokinetics of a novel intra-vitreous dexamethasone biodegradable drug delivery system (DEX-BDD) [abstract no. 3874]. Association for Research in Vision and Ophthalmology Annual Meeting; 2002 May 5–10; Fort Lauderdale (FL)
Kuppermann BD, Blumenkranz MS, Haller JA, et al. An intravitreous dexamethasone bioerodible drug delivery system for the treatment of persistent diabetic macular edema [abstract no. 4289]. Association for Research in Vision and Ophthalmology Annual Meeting; 2003 May 4–9; Fort Lauderdale (FL)
Haller JA, Blumenkranz MS, Williams GA, et al. Treatment of persistent macular edema associated with central and branch retinal vein occlusion with extended delivery of intravitreal dexamethasone [abstract no. 4311]. Association for Research in Vision and Ophthalmology Annual Meeting; 2003 May 4–9; Fort Lauderdale (FL)
Williams GA, Blumenkranz MS, Haller JA, et al. Treatment of persistent macular edema (PME) associated with uveitis or Irvine-Gass syndrome (IGS) with an intravitreal bioerodible sustained dexamethasone release implant; a prospective controlled multi-center clinical trial [abstract no. 4309]. Association for Research in Vision and Ophthalmology Annual Meeting; 2003 May 4–9; Fort Lauderdale (FL)
Allergan. Allergan receives FDA approval for OZURDEX™ biodegradable, injectable steroid implant with extended drug release for retinal disease [online]. Available from URL: http://agn.client.shareholder.com/releasedetail.cfm?ReleaseID=390519 [Accessed 2009 Jun 23]
Cardillo JA, Souza-Filho AA, Oliveira AG. Intravitreal bioerudivel sustained-release triamcinolone microspheres system (RETAAC): preliminary report of its potential usefulness for the treatment of diabetic macular edema. Arch Soc Esp Oftalmol 2006; 81: 675–82
Ochre Media. Icon Bioscience completes enrollment in phase I study of novel ophthalmic drug candidate [press release]. 2008 Aug 5 [online]. Available from URL: http://www.pharmafocusasia.com/press_releases/pressrelease_archives.asp?PID=424 [Accessed 2009 Jun 23]
Campochiaro P, C99-PKC412-003 Study Group. Phase 2 study results of oral PKC412 in diabetic macular edema [abstract no. 4286]. Association for Research in Vision and Ophthalmology Annual Meeting; 2003 May 4–9; Fort Lauderdale (FL)
Saishin Y, Silva RL, Saishin Y, et al. Periocular injection of microspheres containing PKC412 inhibits choroidal neovascularization in a porcine model. Invest Ophthalmol Vis Sci 2003; 44(11): 4989–93
Frank RN. Diabetic retinopathy. N Engl J Med 2004; 350(1): 48–58
Frystyk J. The growth hormone hypothesis: 2005 revision. Horm Metab Res 2005; 37Suppl. 1: 44–8
Sall JW, Klisovic DD, O’Dorisio MS, et al. Somatostatin inhibits IGF-1 mediated induction of VEGF in human retinal pigment epithelial cells. Exp Eye Res 2004; 79(4): 465–76
Grant MB, Caballero Jr S. The potential role of octreotide in the treatment of diabetic retinopathy. Treat Endocrinol 2005; 4(4): 199–203
Hernaez-Ortega MC, Soto-Pedre E, Martin JJ. Sandostatin LAR for cystoid diabetic macular edema: a 1-year experience. Diabetes Res Clin Pract 2004; 64(1): 71–2
Collins W, Kiese B, Fidora H, et al. Efficacy and safety of Sandostatin LAR (octreotide), a somatostatin analog, in diabetic retinopathy: study design of an ongoing phase 3 clinical trial in North America and Brazil [abstract]. American Academy of Ophthalmology Annual Meeting; 2005 Oct 14–18; Chicago (IL)
Solomon SD, Sunness JS, Cooney MJ. Geographic atrophy. In: Lim JI, editor. Age-related macular degeneration. New York: Marcel Dekker, Inc., 2002: 83–99
Trielsch JM, Javitt JC, Coleman A, et al. The prevalence of blindness and visual impairment among nursing home residents in Baltimore. N Engl J Med 1995; 332: 1205–9
Seddon JM. Epidemiology of age-related macular degeneration. In: Ryan SJ, editor. Retina. St Louis (MO): Mosby, 2001: 1039–50
Safety and efficacy of brimonidine intravitreal implant in patients with geographic atrophy due to age-related macular degeneration (AMD) [ClinicalTrials.gov identifier NCT00658619]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2009 Jun 23]
An exploratory study to evaluate the safety of brimonidine intravitreal implant in patients with retinitis pigmentosa [ClinicalTrials.gov identifier NCT00661479]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2009 Jun 23]
Donoso LA, Kim D, Frost A, et al. The role of inflammation in the pathogenesis of age-related macular degeneration. Survey Ophthalmol 2006; 51: 137–52
Magnusson KP, Duan S, Sigurdsson H, et al. CFH Y402H confers similar risk of soft drusen and both forms of advanced AMD. PLoS Med 2006; 3: e5
Mullins RF, Russell SR, Anderson DH, et al. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J 2000; 14: 835–46
Johnson LV, Ozaki S, Staples MK, et al. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res 2000; 70: 441–9
Anderson DH, Mullins RF, Hageman GS, et al. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol 2002; 134: 411–31
Johnson LV, Leitner WP, Staples MK, et al. Complement activation and inflammatory processes in drusen formation and age related macular degeneration. Exp Eye Res 2001; 73: 887–96
Cook GP, Downing J, Rice MA, et al. Injectable PLGA-based formulations for sustained delivery of therapeutic agents for intraocular applications [abstract no. 3490]. Association for Research in Vision and Ophthalmology Annual Meeting; 2009 May 3–7; Fort Lauderdale (FL)
Campochiaro PA. Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol 1997; 115: 237–41
Pastor JC. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol 1998; 43: 3–18
Charteris DG. Proliferative vitreoretinopathy: pathobiology, surgical management, and adjunctive treatment. Br J Ophthalmol 1995; 79(10): 953–60
Moritera T, Ogura Y, Yoshimura N, et al. Biodegradable microspheres containing adriamycin in the treatment of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1992; 33(11): 3125–30
Rahimy MH, Peyman GA, Fernandes ML, et al. Effects of an intravitreal daunomycin implant on experimental proliferative vitreoretinopathy: simultaneous pharmacokinetic and pharmacodynamic evaluations. J Ocul Pharmacol 1994; 10(3): 561–70
Salah-Eldin M, Peyman GA, el-Aswad M, et al. Evaluation of toxicity and efficacy of a combination of antineoplastic agents in the prevention of PVR. Int Ophthalmol 1994; 18(2): 53–60
Einmahl S, Behar-Cohen F, D’Hermies F, et al. A new poly(ortho ester)-based drug delivery system as an adjunct treatment in filtering surgery. Invest Ophthalmol Vis Sci 2001; 42(3): 695–700
Blumenkranz M, Hernandez E, Ophir A, et al. 5-Fluorouracil: new applications in complicated retinal detachment for an established antimetabolite. Ophthalmology 1984; 91(2): 122–30
Asaria RH, Kon CH, Bunce C, et al. Adjuvant 5-fluorouracil and heparin prevents proliferative vitreoretinopathy: results from a randomized, double-blind, controlled clinical trial. Ophthalmology 2001; 108(7): 1179–83
Charteris DG, Aylward GW, Wong D, et al. A randomized controlled trial of combined 5-fluorouracil and low-molecular-weight heparin in management of established proliferative vitreoretinopathy. Ophthalmology 2004; 111(12): 2240–5
Yang CS, Khawly JA, Hainsworth DP, et al. An intravitreal sustained-release triamcinolone and 5-fluorouracil codrug in the treatment of experimental proliferative vitreoretinopathy. Arch Ophthalmol 1998; 116(1): 69–77
Berger AS, Cheng CK, Pearson PA, et al. Intravitreal sustained release corticosteroid-5-fluorouracil conjugate in the treatment of experimental proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1996; 37(11): 2318–25
Cardillo JA, Farah ME, Mitre J, et al. An intravitreal biodegradable sustained release naproxen and 5-fluorouracil system for the treatment of experimental post-traumatic proliferative vitreoretinopathy. Br J Ophthalmol 2004; 88(9): 1201–5
Weleber RG, Gregory-Evans K. Retinitis pigmentosa and allied disorders. In: Ryan SJ, editor. Retina. St Louis (MO): Mosby, 2006: 395–498
Faktorovich EG, Steinberg RH, Yasumura D, et al. Basic fibroblast growth factor and local injury protect photo-receptors from light damage in the rat. J Neurosci 1992; 12(9): 3554–67
LaVail MM, Unoki K, Yasumura D, et al. Multiple growth factors, cytokines, and neurotrophins rescue photo-receptors from the damaging effects of constant light. Proc Natl Acad Sci U S A 1992; 89(23): 11249–53
Machida S, Tanaka M, Ishii T, et al. Neuroprotective effect of hepatocyte growth factor against photoreceptor degeneration in rats. Invest Ophthalmol Vis Sci 2004; 45(11): 4174–82
Ogata N, Wang L, Jo N, et al. Pigment epithelium derived factor as a neuroprotective agent against ischemic retinal injury. Curr Eye Res 2001; 22(4): 245–52
Takita H, Yoneya S, Gehlbach PL, et al. Retinal neuroprotection against ischemic injury mediated by intraocular gene transfer of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci 2003; 44(10): 4497–504
Cayouette M, Smith SB, Becerra SP, et al. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degeneration. Neurobiol Dis 1999; 6(6): 523–32
Cao W, Tombran-Tink J, Elias R, et al. In vivo protection of photoreceptors from light damage by pigment epithelium-derived factor. Invest Ophthalmol Vis Sci 2001; 42(7): 1646–52
Sakai T, Kuno N, Takamatsu F, et al. Prolonged protective effect of basic fibroblast growth factor-impregnated nanoparticles in Royal College of Surgeons rats. Invest Ophthalmol Vis Sci 2007; 48: 3381–7
Acknowledgements
The authors are employees of Santen Pharmaceutical. No sources of funding were used to assist in the preparation of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuno, N., Fujii, S. Biodegradable Intraocular Therapies for Retinal Disorders. Drugs Aging 27, 117–134 (2010). https://doi.org/10.2165/11530970-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11530970-000000000-00000