Abstract
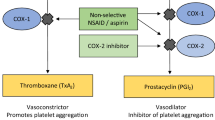
Celecoxib (Celebrex®) was the first cyclo-oxygenase (COX)-2 selective inhibitor (coxib) to be introduced into clinical practice. Coxibs were developed to provide anti-inflammatory/analgesic activity similar to that of nonselective NSAIDs, but without their upper gastrointestinal (GI) toxicity, which is thought to result largely from COX-1 inhibition. Celecoxib is indicated in the EU for the symptomatic treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis in adults. This article reviews the clinical efficacy and tolerability of celecoxib in these EU-approved indications, as well as overviewing its pharmacological properties.
In randomized controlled trials, celecoxib, at the recommended dosages of 200 or 400 mg/day, was significantly more effective than placebo, at least as effective as or more effective than paracetamol (acetaminophen) and as effective as non-selective NSAIDs and the coxibs etoricoxib and lumiracoxib for the symptomatic treatment of patients with active osteoarthritis, rheumatoid arthritis or ankylosing spondylitis.
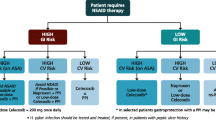
Celecoxib was generally well tolerated, with mild to moderate upper GI complaints being the most common body system adverse events. In meta-analyses and large safety studies, the incidence of upper GI ulcer complications with recommended dosages of celecoxib was significantly lower than that with non-selective NSAIDs and similar to that with paracetamol and other coxibs. However, concomitant administration of celecoxib with low-dose cardioprotective aspirin often appeared to negate the GI-sparing advantages of celecoxib over NSAIDs.
Although one polyp prevention trial noted a dose-related increase in cardiovascular risk with celecoxib 400 and 800 mg/day, other trials have not found any significant difference in cardiovascular risk between celecoxib and placebo or non-selective NSAIDs. Meta-analyses and database-derived analyses are inconsistent regarding cardiovascular risk. At recommended dosages, the risks of increased thrombotic cardiovascular events, or renovascular, hepatic or hypersensitivity reactions with celecoxib would appear to be small and similar to those with NSAIDs.
Celecoxib would appear to be a useful option for therapy in patients at high risk for NSAID-induced GI toxicity, or in those responding suboptimally to or intolerant of NSAIDs. To minimize any risk, particularly the cardiovascular risk, celecoxib, like all coxibs and NSAIDs, should be used at the lowest effective dosage for the shortest possible duration after a careful evaluation of the GI, cardiovascular and renal risks of the individual patient.






Similar content being viewed by others
References
Vane JR, Botting RM. Mechanism of action of aspirin-like drugs. Semin Arthritis Rheum 1997 Jun; 26 (6 Suppl. 1): 2–10
Zimmermann KC, Sarbia M, Schrör K, et al. Constitutive cyclooxygenase-2 expression in healthy human and rabbit gastric mucosa. Mol Pharmacol 1998 Sep; 54(3): 536–40
Zarraga IGE, Schwarz ER. Coxibs and heart disease: what we have learned and what else we need to know. J Am Coll Cardiol 2007 Jan; 49(1): 1–14
European Medicines Agency. EMEA public statement on the suspension of the marketing authorisation for Bextra (valdecoxib) in the European Union [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/12/WC500018391.pdf [Accessed 2011 Nov 16]
European Medicines Agency. Questions and answers on the recommendation to withdraw the marketing authorisations for lumiracoxib-containing medicines [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/lumiracoxib_107/WC500094235.pdf [Accessed 2011 Nov 16]
Pfizer Ltd. Celebrex (celecoxib): summary of product characteristics [online]. Available from URL: http://www.medicines.org.uk/EMC/medicine/14534/SPC/Celebrex+100mg+%26+200mg+Capsules/ [Accessed 2011 Nov 16]
G.D. Searle. Celebrex (celecoxib) capsules: US prescribing information [online]. Available from URL: http://www.pfizer.com/files/products/uspi_celebrex.pdf [Accessed 2011 Nov 16]
Clemett D, Goa KL. Celecoxib: a review of its use in osteoarthritis, rheumatoid arthritis and acute pain. Drugs 2000 Apr; 59(4): 957–80
Davies NM, McLachlan AJ, Day RO, et al. Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclooxygenase-2 inhibitor. Clin Pharmacokinet 2000 Mar; 38(3): 225–42
Patrono C, Patrignani P, García Rodríguez LA. Cyclo-oxygenase-selective inhibition of prostanoid formation: transducing biochemical selectivity into clinical read-outs. J Clin Invest 2001 Jul; 108(1): 7–13
Frampton JE, Keating GM. Celecoxib: a review of its use in the management of arthritis and acute pain. Drugs 2007; 67(16): 2433–72
Reilly IAG, FitzGerald GA. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood 1987 Jan; 69(1): 180–6
McAdam BF, Catella-Lawson F, Mardini IA, et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A 1999 Jan 5; 96(1): 272–7
Leese PT, Hubbard RC, Karim A, et al. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol 2000 Feb; 40(2): 124–32
Renda G, Tacconelli S, Capone ML, et al. Celecoxib, ibuprofen, and the antiplatelet effect of aspirin in patients with osteoarthritis and ischemic heart disease. Clin Pharmacol Ther 2006 Sep; 80(3): 264–74
Wilner KD, Rushing M, Walden C, et al. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J Clin Pharmacol 2002 Sep; 42(9): 1027–30
Sowers JR, White WB, Pitt B, et al. The effects of cyclooxygenase-2 inhibitors and nonsteroidal anti-inflammatory therapy on 24-hour blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Arch Intern Med 2005 Jan 24; 165: 161–8
White WB, Kent J, Taylor A, et al. Effects of celecoxib on ambulatory blood pressure in hypertensive patients on ACE inhibitors. Hypertension 2002 Apr; 39(4): 929–34
Widlansky ME, Price DT, Gokce N, et al. Short- and long-term COX-2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension 2003 Sep; 42(3): 310–5
Aw T-J, Haas SJ, Liew D, et al. Meta-analysis ofcyclooxygenase-2 inhibitors and their effects on blood pressure. Arch Intern Med 2005; 165: 490–6
Werner U, Werner D, Pahl A, et al. Investigation of the pharmacokinetics of celecoxib by liquid chromatography-mass spectrometry. Biomed Chromatogr 2002 Feb; 16(1): 56–60
Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics 2002 Apr; 12(3): 251–63
Jayasagar G, Krishna Kumar M, Chandrasekhar K, et al. Influence of rifampicin pretreatment on the pharmacokinetics of celecoxib in healthy male volunteers. Drug Metab Drug Interact 2003; 19(4): 287–95
Karim A, Tolbert D, Piergies A, et al. Celecoxib does not significantly alter the pharmacokinetics or hypopro-thrombinemic effect of warfarin in healthy subjects. J Clin Pharmacol 2000 Jun; 40(6): 655–63
Birbara C, Ruoff G, Sheldon E, et al. Efficacy and safety of rofecoxib 12.5mg and celecoxib 200mg in two similarly designed osteoarthritis studies. Curr Med Res Opin 2006 Jan; 22(1): 199–210
Gibofsky A, Williams GW, McKenna F, et al. Comparing the efficacy of cyclooxygenase 2-specific inhibitors in treating osteoarthritis: appropriate trial design considerations and results of a randomized, placebo-controlled trial. Arthritis Rheum 2003 Nov; 48(11): 3102–11
McKenna F, Weaver A, Fiechtner JJ, et al. COX-2 specific inhibitors in the management of osteoarthritis of the knee: a placebo-controlled, randomized, double-blind study. J Clin Rheumatol 2001 Jun; 7(3): 151–9
Smugar SS, Schnitzer TJ, Weaver AL, et al. Rofecoxib 12.5 mg, rofecoxib 25mg, and celecoxib 200mg in the treatment of symptomatic osteoarthritis: results of two similarly designed studies. Curr Med Res Opin 2006 Jul; 22(7): 1353–67
Williams GW, Ettlinger RE, Ruderman EM, et al. Treatment of osteoarthritis with a once-daily dosing regimen of celecoxib: a randomized, controlled trial. J Clin Rheumatol 2000 Apr; 6(2): 65–74
Williams GW, Hubbard RC, Yu SS, et al. Comparison of once-daily and twice-daily administration of celecoxib for the treatment of osteoarthritis of the knee. Clin Ther 2001 Feb; 23(2): 213–27
Stengaard-Pedersen K, Ekesbo R, Karvonen AL, et al. Celecoxib 200mg q.d. is efficacious in the management of osteoarthritis of the knee or hip regardless of the time of dosing. Rheumatology (Oxford) 2004 May; 43(5): 592–5
Luyten FP, Geusens P, Malaise M, et al. A prospective randomised multicentre study comparing continuous and intermittent treatment with celecoxib in patients with osteoarthritis of the knee or hip. Ann Rheum Dis 2007 Jan; 66(1): 99–106
Pincus T, Koch G, Lei H, et al. Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Ann Rheum Dis 2004 Aug;63(8):931–9
Geba GP, Weaver AL, Polis AB, et al. Efficacy of rofecoxib, celecoxib, and acetaminophen in osteoarthritis of the knee: a randomized trial. JAMA 2002 Jan 2; 287(1): 64–71
Schnitzer TJ, Weaver AL, Polis AB, et al. Efficacy of rofecoxib, celecoxib, and acetaminophen in patients with osteoarthritis of the knee: a combined analysis of the VACT studies. J Rheumatol 2005 Jun; 32(6): 1093–105
Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006 Feb 23; 354(8): 795–808
Singh G, Fort JG, Goldstein JL, et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUC-CESS-I Study. Am J Med 2006 Mar; 119(3): 255–66
Bensen WG, Fiechtner JJ, McMillen JI, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999 Nov; 74(11): 1095–105
Emery P, Koncz T, Pan S, et al. Analgesic effectiveness of celecoxib and diclofenac in patients with osteoarthritis of the hip requiring joint replacement surgery: a 12-week, multicenter, randomized, double-blind, parallel-group, double-dummy, noninferiority study. Clin Ther 2008 Jan; 30(1): 70–83
Hawel R, Klein G, Singer F, et al. Comparison of the efficacy and tolerability of dexibuprofen and celecoxib in the treatment of osteoarthritis of the hip. Int J Clin Pharmacol Ther 2003 Apr; 41(4): 153–64
Hochberg MC, Fort JG, Svensson O, et al. Fixed-dose combination of enteric-coated naproxen and immediate-release esomeprazole has comparable efficacy to celecoxib for knee osteoarthritis: two randomized trials. Curr Med Res Opin 2011; 27(6): 1243–53
Kivitz AJ, Moskowitz RW, Woods E, et al. Comparative efficacy and safety of celecoxib and naproxen in the treatment of osteoarthritis of the hip. J Int Med Res 2001 Nov; 29(6): 467–79
McKenna F, Borenstein D, Wendt H, et al. Celecoxib versus diclofenac in the management of osteoarthritis of the knee. Scand J Rheumatol 2001; 30(1): 11–8
Rother M, Lavins BJ, Kneer W, et al. Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial. Ann Rheum Dis 2007 Sep; 66(9): 1178–83
Suárez-Otero R, Robles-San Román M, Jaimes-Hernández J, et al. Efficacy and safety of diclofenac-cholestyramine and celecoxib in osteoarthritis. Proc West Pharmacol Soc 2002; 45: 26–8
Bingham 3rd CO, Sebba AI, Rubin BR, et al. Efficacy and safety of etoricoxib 30mg and celecoxib 200mg in the treatment of osteoarthritis in two identically designed, randomized, placebo-controlled, non-inferiority studies. Rheumatology (Oxford) 2007 Mar; 46(3): 496–507
Fleischmann R, Sheldon E, Maldonado-Cocco J, et al. Lumiracoxib is effective in the treatment of osteoarthritis of the knee: a prospective randomized 13-week study versus placebo and celecoxib. Clin Rheumatol 2005 Feb; 25(1): 42–53
Lehmann R, Brzosko M, Kopsa P, et al. Efficacy and tolerability of lumiracoxib 100mg once daily in knee osteoarthritis: a 13-week, randomized, double-blind study vs. placebo and celecoxib. Curr Med Res Opin 2005 Apr; 21(4): 517–26
Sheldon E, Beaulieu A, Paster Z, et al. Efficacy and tolerability of lumiracoxib in the treatment of osteoarthritis of the knee: a 13-week, randomized, double-blind comparison with celecoxib and placebo. Clin Ther 2005 Jan; 27(1): 64–77
Tannenbaum H, Berenbaum F, Reginster JY, et al. Lumiracoxib is effective in the treatment of osteoarthritis of the knee: a 13 week, randomised, double blind study versus placebo and celecoxib. Ann Rheum Dis 2004 Nov; 63(11): 1419–26
Fleischmann R, Tannenbaum H, Patel NP, et al. Long-term retention on treatment with lumiracoxib 100mg once or twice daily compared with celecoxib 200 mg once daily: a randomised controlled trial in patients with osteoarthritis. BMC Musculoskelet Disord 2008; 9: 32
Wittenberg RH, Schell E, Krehan G, et al. First-dose analgesic effect of the cyclo-oxygenase-2 selective inhibitor lumiracoxib in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled comparison with celecoxib [NCT00267215]. Arthritis Res Ther 2006; 8(2): R35
Emery P, Zeidler H, Kvien TK, et al. Celecoxib versus diclofenac in long-term management of rheumatoid arthritis: randomised double-blind comparison. Lancet 1999 Dec; 354(9196): 2106–11
Moskowitz RW, Sunshine A, Brugger A, et al. American Pain Society pain questionnaire and other pain measures in the assessment of osteoarthritis pain: a pooled analysis of three celecoxib pivotal studies. Am J Ther 2003 Jan; 10(1): 12–20
Zhao SZ, McMillen JI, Markenson JA, et al. Evaluation of the functional status aspects of health-related quality of life of patients with osteoarthritis treated with celecoxib. Pharmacotherapy 1999 Nov; 19(11): 1269–78
Bianchi M, Broggini M. A randomised, double-blind, clinical trial comparing the efficacy of nimesulide, celecoxib and rofecoxib in osteoarthritis of the knee. Drugs 2003; 63 Suppl. 1: 37–46
Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis 2010 Aug; 69(8): 1459–64
Lisse J, Espinoza L, Zhao SZ, et al. Functional status and health-related quality of life of elderly osteoarthritic patients treated with celecoxib. J Gerontol A Biol Sci Med Sci 2001 Mar; 56(3): M167–75
Shi W, Wang YM, Li LS, et al. Safety and efficacy of oral nonsteroidal anti-inflammatory drugs in patients with rheumatoid arthritis: a six-month randomised study. Clin Drug Invest 2004; 24(2): 89–101
Song YW, Lee EY, Koh EM, et al. Assessment of comparative pain relief and tolerability of SKI306X compared with celecoxib in patients with rheumatoid arthritis: a 6-week, multicenter, randomized, double-blind, double-dummy, phase III, noninferiority clinical trial. Clin Ther 2007 May; 29(5): 862–73
Zhao SZ, Fiechtner JI, Tindall EA, et al. Evaluation of health-related quality of life of rheumatoid arthritis patients treated with celecoxib. Arthritis Care Res 2000 Apr; 13(2): 112–21
Simon LS, Weaver AL, Graham DY, et al. Antiinflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA 1999 Nov 24; 282(20): 1921–8
Barkhuizen A, Steinfeld S, Robbins J, et al. Celecoxib is efficacious and well tolerated in treating signs and symptoms of ankylosing spondylitis. J Rheumatol 2006 Sep; 33(9): 1805–12
Dougados M, Behier JM, Jolchine I, et al. Efficacy of celecoxib, a cyclooxygenase 2-specific inhibitor, in the treatment of ankylosing spondylitis: a six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum 2001 Jan; 44(1): 180–5
Kvien TK, Bjørneboe O, Gran JT, et al. Celecoxib and diclofenac have comparable efficacy in ankylosing spondylitis (AS): results from a Norwegian multicenter, 12-week, double-blind, randomized trial [abstract no. SAT0266]. Ann Rheum Dis 2006; 65 (Suppl. 2): 531
Sieper J, Klopsch T, Richter M, et al. Comparison of two different dosages of celecoxib with diclofenac for the treatment of active ankylosing spondylitis: results of a 12-week randomised, double-blind, controlled study. Ann Rheum Dis 2008 Mar; 67(3): 323–9
Wanders A, van der Heijde D, Landewé R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 2005 Jun; 52(6): 1756–65
Pfizer Inc. A 12-week, randomized, double-blind, parallel-group study of 2 doses of celecoxib compared to diclofenac in patients with ankylosing spondylitis [online]. Available from URL: http://www.clinicalstudyresults.org/documents/company-study_1112_0.pdf [Accessed 2011 Nov 16]
Moore RA, Derry S, Makinson GT, et al. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports. Arthritis Res Ther 2005; 7(3): R644–65
Dahlberg LE, Holme I, Hoye K, et al. A randomized, multicentre, double-blind, parallel-group study to assess the adverse event-related discontinuation rate with celecoxib and diclofenac in elderly patients with osteoarthritis. Scand J Rheumatol 2009 Mar; 38(2): 133–43
Cryer B, Luo X, Assaf AR, et al. Persistence with non-selective NSAIDs and celecoxib among patients with gastroesophageal reflux disease and osteoarthritis or rheumatoid arthritis. Curr Med Res Opin 2011 Feb; 27(2): 295–302
Hawkey CJ, Svoboda P, Fiedorowicz-Fabrycy IF, et al. Gastroduodenal safety and tolerability of lumiracoxib compared with ibuprofen and celecoxib in patients with osteoarthritis. J Rheumatol 2004 Sep; 31(9): 1804–10
Goldstein JL, Correa P, Zhao WW, et al. Reduced incidence of gastroduodenal ulcers with celecoxib, a novel cyclooxygenase-2 inhibitor, compared to naproxen in patients with arthritis. Am J Gastroenterol 2001 Apr; 96(4): 1019–27
Jajić Z, Malaise M, Nekam K, et al. Gastrointestinal safety of amtolmetin guacyl in comparison with celecoxib in patients with rheumatoid arthritis. Clin Exp Rheumatol 2005 Nov; 23(6): 809–18
Cryer B, Li C, Simon L, et al. GI-REASONS: a novel 6-month, prospective, randomized, open-label, blinded end point (PROBE) trial [abstract no. 52]. American College of Gastroenterology 2011 Annual Scientific Meeting; 2011 Oct 28–Nov 2; Washington (DC)
Chan FKL, Lanas A, Scheiman J, et al. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet 2010 Jul 17; 376(9736): 173–9
Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal antiinflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000 Sep 13; 284(10): 1247–55
Lanas A, García-Rodríguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin, and combinations. Gut 2006; 55(12): 1731–8
Mamdani M, Rochon PA, Juurlink DN, et al. Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. BMJ 2002 Sep 21; 325(7365): 624
Rahme E, Barkun AN, Toubouti Y, et al. Do proton-pump inhibitors confer additional gastrointestinal protection in patients given celecoxib? Arthritis Rheum 2007 Jun 15; 57(5): 748–55
Hippisley-Cox J, Coupland C, Logan R, et al. Risk of adverse gastrointestinal outcomes in patients taking cyclooxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005 Dec 3; 331(7528): 1310–6
Lai K-C, Chu K-M, Hui W-M, et al. Celecoxib compared with lansoprazole and naproxen to prevent gastrointestinal ulcer complications. Am J Med 2005 Nov; 118(11): 1271–8
Chan FKL, Hung LCT, Suen BY, et al. Celecoxib versus diclofenac plus omeprazole in high-risk arthritis patients: results of a randomized double-blind trial. Gastroenterology 2004 Oct; 127(4): 1038–43
Chan FKL, Hung LCT, Suen BY, et al. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med 2002 Dec 26; 347(26): 2104–10
Chan FKL, Wong VWS, Suen BY, et al. Combination of a cyclo-oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomised trial. Lancet 2007 May 12; 369(9573): 1621–6
Goldstein JL, Lowry SC, Lanza FL, et al. The impact of low-dose aspirin on endoscopic gastric and duodenal ulcer rates in users of a non-selective non-steroidal antiinflammatory drug or a cyclo-oxygenase-2-selective inhibitor. Aliment Pharmacol Ther 2006 May 15; 23(10): 1489–98
Goldstein JL, Cryer B, Amer F, et al. Celecoxib plus aspirin versus naproxen and lansoprazole plus aspirin: a randomized, double-blind, endoscopic trial. Clin Gastroenterol Hepatol 2007 Oct; 5(10): 1167–74
White WB, Faich G, Borer JS, et al. Cardiovascular thrombotic events in arthritis trials of the cyclooxygenase-2 inhibitor celecoxib. Am J Cardiol 2003 Aug 15; 92(4): 411–8
García Rodríguez LA, Tacconelli S, Patrignani P. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J Am Coll Cardiol 2008 Nov 11; 52(20): 1628–36
Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005 Mar 17; 352(11): 1092–102
Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med 2000; 343(21): 1520–8
Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med 2006 Aug 31; 355(9): 873–84
Solomon SD, McMurray JJV, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 2005 Mar 17; 352(11): 1071–80
Solomon SD, Pfeffer MA, McMurray JJV, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation 2006 Sep 5; 114(10): 1028–35
Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 2006 Aug 31; 355(9): 885–95
White WB, Faich G, Whelton A, et al. Comparison of thromboembolic events in patients treated with celecoxib, a cyclooxygenase-2 specific inhibitor, versus ibuprofen or diclofenac. Am J Cardiol 2002 Feb 15; 89(4): 425–30
ADAPT Research Group. Cardiovascular and cerebro-vascular events in the randomized, controlled Alzheimer’s disease anti-inflammatory prevention trial (ADAPT). PLoS Clin Trials 2006 Nov 17; 1(7): e33
Chen LC, Ashcroft DM. Risk of myocardial infarction associated with selective COX-2 inhibitors: meta-analysis of randomised controlled trials. Pharmacoepidemiol Drug Saf 2007 Jul; 16(7): 762–72
Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of athero-thrombosis? Meta-analysis of randomised trials. BMJ 2006 Jun 3; 332: 1302–8
Solomon SD, Wittes J, Finn PV, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation 2008 Apr 22; 117(16): 2104–13
White WB, West CR, Borer JS, et al. Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol 2007 Jan 1; 99(1): 91–8
Gislason GH, Jacobsen S, Rasmussen JN, et al. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective non-steroidal antiinflammatory drugs after acute myocardial infarction. Circulation 2006 Jun 27; 113(25): 2906–13
Andersohn F, Suissa S, Garbe E. Use of first- and second-generation cyclooxygenase-2-selective nonsteroidal anti-inflammatory drugs and risk of acute myocardial infarction. Circulation 2006 Apr 25; 113(16): 1950–7
McGettigan P, Henry D. Cardiovascular risk with nonsteroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med 2011 Sep; 8(9): e1001098
Becker MC, Wang TH, Wisniewski L, et al. Rationale, design, and governance of Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen Or Naproxen (PRECISION), a cardiovascular end point trial of nonsteroidal antiinflammatory agents in patients with arthritis. Am Heart J 2009 Apr; 157(4): 606–12
Whelton A, Lefkowith JL, West CR, et al. Cardiorenal effects of celecoxib as compared with the nonsteroidal anti-inflammatory drugs diclofenac and ibuprofen. Kidney Int 2006 Oct; 70(8): 1495–502
Hegazy R, Alashhab M, Amin M. Cardiorenal effects of newer NSAIDs (celecoxib) versus classic NSAIDs (ibuprofen) in patients with arthritis. J Toxicol 2011; 2011: 862153
Whelton A, Fort JG, Puma JA. Cyclooxygenase-2-specific inhibitors and cardiorenal function: a randomized, controlled trial of celecoxib and rofecoxib in older hypertensive osteoarthritis patients. Am J Ther 2001 Mar; 8(2): 85–95
Whelton A, White WB, Bello AE, et al. Effects of celecoxib and rofecoxib on blood pressure and edema in patients ≥65 years of age with systemic hypertension and osteoarthritis. Am J Cardiol 2002 Nov 1; 90(9): 959–63
Wang J, Mullins CD, Mamdani M, et al. New diagnosis of hypertension among celecoxib and nonselective NSAID users: a population-based cohort study. Ann Pharmacother 2007 Jun; 41(6): 937–43
Solomon DH, Schneeweiss S, Levin R, et al. Relationship between COX-2 specific inhibitors and hypertension. Hypertension 2004 Aug; 44(2): 140–5
Wolfe F, Michaud K, Zhao SZ. Patient perception of the burden of weight gain and blood pressure increase among RA patients using celecoxib, rofecoxib, and non-specific NSAIDs. J Clin Rheumatol 2003 Dec; 9(6): 344–53
Wolfe F, Zhao S, Reynolds M, et al. Blood pressure destabilization and edema among 8538 users of celecoxib, rofecoxib, and nonselective nonsteroidal antiinflammatory drugs (NSAID) and nonusers of NSAID receiving ordinary clinical care. J Rheumatol 2004 Jun; 31(6): 1143–51
Hudson M, Rahme E, Richard H, et al. Risk of congestive heart failure with nonsteroidal antiinflammatory drugs and selective cyclooxygenase 2 inhibitors: a class effect? Arthritis Rheum 2007 Apr 15; 57(3): 516–23
Mamdani M, Juurlink DN, Lee DS, et al. Cyclooxygenase-2 inhibitors versus non-selective non-steroidal anti-inflammatory drugs and congestive heart failure outcomes in elderly patients: a population-based cohort study. Lancet 2004 May 29; 363(9423): 1751–6
Ray WA, Stein CM, Daugherty JR, et al. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet 2002 Oct 5; 360(9339): 1071–3
Spalding WM, Reeves MJ, Whelton A. Thromboembolic cardiovascular risk among arthritis patients using cyclooxygenase-2-selective inhibitor or nonselective cyclooxygenase inhibitor nonsteroidal anti-inflammatory drugs. Am J Ther 2007 Jan; 14(1): 3–12
Whelton A, Maurath CJ, Verburg KM, et al. Renal safety and tolerability of celecoxib, a novel cyclooxygenase-2 inhibitor. Am J Ther 2000 May; 7(3): 159–75
Soni P, Shell B, Cawkwell G, et al. The hepatic safety and tolerability of the cyclooxygenase-2 selective NSAID celecoxib: pooled analysis of 41 randomized controlled trials. Curr Med Res Opin 2009; 25(8): 1841–51
Roll A, Wüthrich B, Schmid-Grendelmeier P, et al. Tolerance to celecoxib in patients with a history of adverse reactions to nonsteroidal anti-inflammatory drugs. Swiss Med Wkly 2006 Oct 28; 136(43–44): 684–90
Sánchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A, et al. Safety of etoricoxib, a new cyclooxygenase 2 inhibitor, in patients with nonsteroidal anti-inflammatory drug-induced urticaria and angioedema. Ann Allergy Asthma Immunol 2005 Aug; 95: 154–8
Woessner KM, Simon RA, Stevenson DD. The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum 2002 Aug; 46(8): 2201–6
Quiralte J, Delgado J, Sáenz de San Pedro B, et al. Safety of the new selective cyclooxygenase type 2 inhibitors rofecoxib and celecoxib in patients with anaphylactoid reactions to nonsteroidal anti-inflammatory drugs. Ann Allergy Asthma Immunol 2004 Oct; 93: 360–4
Andri L, Falagiani P. Safety of celecoxib in patients with cutaneous reactions due to ASA-NSAIDs intolerance. Allergol Immunopathol 2007; 35(4): 126–9
Shapiro LE, Knowles SR, Weber E, et al. Safety of celecoxib in individuals allergic to sulfonamide: a pilot study. Drug Saf 2003; 26(3): 187–95
Knowles S, Shapiro L, Shear NH. Should celecoxib be contraindicated in patients who are allergic to sulfonamides? Revisiting the meaning of ‘sulfa’ allergy. Drug Saf 2001; 24(4): 239–47
Bessette L, Risebrough N, Mittmann N, et al. Cost-utility of celecoxib use in different treatment strategies for osteoarthritis and rheumatoid arthritis from the Quebec healthcare system perspective. J Med Econ 2009 Sep; 12(3): 246–58
Maetzel A, Krahn M, Naglie G. The cost effectiveness of rofecoxib and celecoxib in patients with osteoarthritis or rheumatoid arthritis. Arthritis Rheum 2003 Jun 15; 49(3): 283–92
Loyd M, Rublee D, Jacobs P. An economic model of long-term use of celecoxib in patients with osteoarthritis. BMC Gastroenterol 2007; 7: 25
Pettitt D, Goldstein JL, McGuire A, et al. Overview of the arthritis Cost Consequence Evaluation System (ACCES): a pharmacoeconomic model for celecoxib. Rheumatology (Oxford) 2000 Dec; 39 Suppl. 2: 33–42; discussion 57-9
Haglund U, Svarvar P. The Swedish ACCES model: predicting the health economic impact of celecoxib in patients with osteoarthritis or rheumatoid arthritis. Rheumatology (Oxford) 2000 Dec; 39 Suppl. 2: 51–6
Svarvar P, Aly A. Use of the ACCES model to predict the health economic impact of celecoxib in patients with osteoarthritis or rheumatoid arthritis in Norway. Rheumatology (Oxford) 2000 Dec; 39 Suppl 2: 43–50
Chancellor JVM, Hunsche E, de Cruz E, et al. Economic evaluation of celecoxib, a new cyclo-oxygenase 2 specific inhibitor, in Switzerland. Pharmacoeconomics 2001; 19 Suppl. 1: 59–75
Louder AM, Joshi AV, Ball AT, et al. Impact of celecoxib restrictions in Medicare beneficiaries with arthritis. Am J Manag Care 2011 Jul; 17(7): 503–12
Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011 Jun; 70(6): 896–904
Jordan KM, Arden NK, Doherty M, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003; 62: 1145–55
Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010 Jun; 69(6): 964–75
Combe B, Landewe R, Lukas C, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2007; 66: 34–45
National Institute for Health and Clinical Excellence. NICE clinical guide 79. Rheumatoid arthritis: the management of rheumatoid arthritis in adults [online]. Available from URL: http://guidance.nice.org.uk/CG79 [Accessed 2011 Nov 16]
Zhao SZ, Wentworth C, Burke TA, et al. Drug switching patterns among patients with rheumatoid arthritis and osteoarthritis using COX-2 specific inhibitors and nonspecific NSAIDs. Pharmacoepidemiol Drug Saf 2004 May; 13(5): 277–87
Schnitzer TJ, Burmester GR, Mysler E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004 Aug 21; 364(9435): 665–74
Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 2005 Mar 17; 352(11): 1081–91
Baraf HS, Fuentealba C, Greenwald M, et al. Gastrointestinal side effects of etoricoxib in patients with osteoarthritis: results of the Etoricoxib versus Diclofenac Sodium Gastrointestinal Tolerability and Effectiveness (EDGE) trial. J Rheumatol 2007 Feb; 34(2): 408–20
Howes LG. Selective COX-2 inhibitors, NSAIDs and cardiovascular events: is celecoxib the safest choice? Ther Clin Risk Manage 2007; 3(5): 831–45
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: A. Lanas, University of Zaragoza. IIS Aragón, Zaragoza, Spain; F. McKenna, Rheumatic Diseases Unit, Trafford General Hospital, Manchester, UK; P. Patrignani, Department of Neuroscience and Imaging, “G. d’Annunzio” University and Center of Excellence on Aging (CeSI), Chieti, Italy; L.S. Simon, SDG LLC, Cambridge, MA, USA.
Data Selection
Sources: Medical literature (including published and unpublished data) on ‘celecoxib’ was identified by searching databases since 1996 (including MEDLINE and EMBASE and in-house AdisBase), bibliographies from published literature, clinical trial registries/databases and websites (including those of regional regulatory agencies and the manufacturer). Additional information (including contributory unpublished data) was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘celecoxib’ and (‘arthritis’ or ‘osteoarthritis’ or ‘rheumatoid arthritis’ or ‘arthritis, rheumatoid’ or ‘ankylosing spondylitis’ or ‘spondylitis, ankylosing’). Searches were last updated 16 November 2011.
Selection: Studies in patients with osteoarthritis, rheumatoid arthritis or ankylosing spondylitis who received celecoxib. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Celecoxib, osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability, pharmacoeconomics.
Rights and permissions
About this article
Cite this article
McCormack, P.L. Celecoxib. Drugs 71, 2457–2489 (2011). https://doi.org/10.2165/11208240-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11208240-000000000-00000