Key Points
-
DNA-damaging agents are among the most effective anticancer agents in clinical use; however, they have significant limitations. Many patients with cancer either do not respond, or develop resistance to them, they are also toxic, and have only a limited therapeutic window.
-
The DNA-damage-response network regulates not only cell-cycle checkpoints, but also DNA repair, genome maintenance, senescence and apoptosis. Modulation of the DNA-damage response, depending on where in the network this modulation occurs, could have different consequences including chemosensitization and chemoprotection.
-
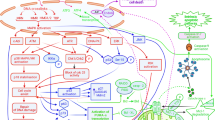
CHK1 and CHK2 were originally discovered as checkpoint kinases. However, further studies have indicated that they are actually DNA-damage-response kinases, regulating more than just cell-cycle checkpoints.
-
CHK1 regulates numerous checkpoint pathways, including S-phase and G2–M checkpoints. Other potential CHK1 functions include replication, chromatin remodelling and DNA repair. CHK1 inhibition is expected to sensitize cells to a broad spectrum of DNA-damaging agents.
-
CHK2 might have a redundant and supportive role in checkpoints, but its role in ionizing-radiation-induced apoptosis is more prominent. However, its role in other DNA-damage-induced apoptosis mechanisms is less well established. CHK2 inhibition is expected to protect normal cells from the side effects of ionizing radiation, but its role in chemoprotection still needs to be clarified.
Abstract
An important part of the cellular response to DNA damage is checkpoint activation — checkpoint kinases CHK1 and CHK2 phosphorylate key proteins to elicit cell-cycle blocks. Inhibiting these kinases was believed to sensitize tumour cells to cancer treatments that damage DNA, because in the absence of checkpoints and efficient DNA repair, the response would switch to cell death or senescence. Recent discoveries have, however, highlighted different and expanded roles for CHK1 and CHK2, so should the therapeutic hypothesis that is concerned with targeting so-called checkpoint kinases be modified?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hurley, L. H. DNA and its associated processes as targets for cancer therapy. Nature Rev. Cancer 2, 188–200 (2002).
Lea, D. E. Actions of Radiations on Living Cells (University Press, Cambridge, 1947).
Tobey, R. A. Different drugs arrest cells at a number of distinct stages in G2. Nature 254, 245–247 (1975).
Painter, R. B. & Young, B. R. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc. Natl Acad. Sci. USA 77, 7315–7317 (1980).
Weinert, T. A. & Hartwell, L. H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science 241, 317–322 (1988).
Hartwell, L. H. & Weinert, T. A. Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629–634 (1989).
Zhou, B. -B. & Elledge, S. J. The DNA damage response: putting checkpoints in perspective. Nature 408, 433–439 (2000).
Khanna, K. K. & Jackson, S. P. DNA double-strand breaks: signaling, repair and the cancer connection. Nature Genet. 27, 247–254 (2001).
Chang, B. D. et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 59, 3761–3767 (1999).
Hartwell, L. H. & Kastan, M. B. Cell cycle control and cancer. Science 266, 1821–1828 (1994).
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nature Rev. Cancer 3, 155–168 (2003).
Abraham, R. T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15, 2177–2196 (2001).
Kaneko, Y. et al. Cell cycle-dependent and ATM-independent expression of human Chk1 kinase. Oncogene 18, 3673–3681 (1999).
Lukas, C. et al. DNA damage-activated kinase Chk2 is independent of proliferation or differentiation yet correlates with tissue biology. Cancer Res. 61, 4990–4993 (2001).
Sanchez, Y. et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497–1501 (1997). This paper describes the cloning, and preliminary biochemical characterization of CHK1, particularly its role in the G2–M transition
Liu, Q. et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14, 1448–1459 (2000).
Zhao, H. & Piwnica-Worms, H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21, 4129–4139 (2001).
Zhao, H., Watkins, J. L. & Piwnica-Worms, H. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc. Natl Acad. Sci. USA 99, 14795–14800 (2002).
Sorensen, C. S. et al. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell 3, 247–258 (2003).
Gatei, M. et al. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J. Biol. Chem. 278, 14806–14811 (2003).
Xiao, Z. et al. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J. Biol. Chem. 278, 21767–21773 (2003).
Kumagai, A. & Dunphy, W. G. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol. Cell 6, 839–849 (2000).
Chini, C. C. & Chen, J. Human claspin is required for replication checkpoint control. J. Biol. Chem. 278, 30057–30062. (2003).
Mailand, N. et al. Rapid destruction of human Cdc25A in response to DNA damage. Science 288, 1425–1429 (2000).
Mailand, N. et al. Regulation of G(2)/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 21, 5911–5920 (2002).
Peng, C. Y. et al. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277, 1501–1505 (1997).
Dalal, S. N., Schweitzer, C. M., Gan, J. & DeCaprio, J. A. Cytoplasmic localization of human cdc25C during interphase requires an intact 14-3-3 binding site. Mol. Cell. Biol. 19, 4465–4479 (1999).
Yang, J., Winkler, K., Yoshida, M. & Kornbluth, S. Maintenance of G2 arrest in the Xenopus oocyte: a role for 14-3-3-mediated inhibition of Cdc25 nuclear import. EMBO J. 18, 2174–2183 (1999).
Zachos, G., Rainey, M. D. & Gillespie, D. A. Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects. EMBO J. 22, 713–723 (2003).
Akiyama, T. et al. G1 Phase accumulation induced by UCN-01 is associated with dephosphorylation of Rb and CDK2 proteins as well as induction of CDK inhibitor p21/Cip1/WAF1/sdil in p53-mutated human epidermoid carcinoma A431 cells. Cancer Res. 57, 1495–1501 (1997).
Graves, P. R. et al. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J. Biol. Chem. 275, 5600–5605 (2000).
Busby, E. C., Leistritz, D. F., Abraham, R. T., Karnitz, L. M. & Sarkaria, J. N. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res. 60, 2108–2112 (2000).
Zhao, B. et al. Structural basis for Chk1 inhibition by UCN-01. J. Biol. Chem. 277, 46609–46615 (2002).
Sato, S., Fujita, N. & Tsuruo, T. Interference with PDK1–Akt survival signaling pathway by UCN-01 (7-hydroxystaurosporine). Oncogene 21, 1727–1738 (2002).
Brown, E. J. & Baltimore, D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14, 397–402 (2000).
de Klein, A. et al. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 10, 479–482 (2000).
Takai, H. et al. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes Dev. 14, 1439–1447 (2000). Work described in this reference and reference 16 indicates that Chk1 in mammals might have an essential role during the normal cell cycle.
Nghiem, P., Park, P. K., Kim, Y., Vaziri, C. & Schreiber, S. L. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc. Natl Acad. Sci. USA 98, 9092–9097 (2001).
Feijoo, C. et al. Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J. Cell Biol. 154, 913–923 (2001).
Fogarty, P. et al. The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol. 7, 418–426 (1997).
Chen, Z. et al. Human Chk1 expression is dispensable for somatic cell death and critical for sustaining G2 DNA damage checkpoint. Mol. Cancer Ther. 2, 543–548 (2003).
Koniaras, K., Cuddihy, A. R., Christopoulos, H., Hogg, A. & O'Connell, M. J. Inhibition of Chk1-dependent G2 DNA damage checkpoint radiosensitizes p53 mutant human cells. Oncogene 20, 7453–7463 (2001).
Wang, H. et al. Ku affects the ataxia and Rad 3-related/CHK1-dependent S phase checkpoint response after camptothecin treatment. Cancer Res. 62, 2483–2487 (2002).
Akinaga, S., Nomura, K., Gomi, K., and Okabe, M. Enhancement of antitumor activity of mitomycin C in vitro and in vivo by UCN-01, a selective inhibitor of protein kinase C. Cancer Chemother. Pharmacol. 32, 183–189 (1993).
Hsueh, C., Kelsen, D., and Schwartz, G. K. UCN-01 suppresses thymidylate synthase gene expression and enhances 5-fluorouracil-induced apoptosis in sequence-dependent manner. Clin. Cancer Res. 4, 2201–2206 (1998).
Bunch, R. T. & Eastman, A. Enhancement of cisplatin-induced cytotoxicity by 7-hydroxystaurosporine (ucn-01), a new G2-checkpoint inhibitor. Clin. Cancer Res. 2, 791–797 (1996).
Shao, R. et al. Abrogation of an S-phase checkpoint and potentiation of camptothecin cytotoxicity by 7-hydroxystaurosporine (UCN–01) in human cancer cell lines, possibly influenced by p53 function. Cancer Res. 57, 4029–4035 (1997).
Russell, K. J. et al. Abrogation of the G2 checkpoint results in differential radiosensitization of G1 checkpoint-deficient and G1 checkpoint-competent cells. Cancer Res. 55, 1639–1642 (1995).
Wang, Q. et al. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J. Natl. Cancer Inst. 88, 956–965 (1996).
Jeggo, P. A., Carr, A. M. & Lehmann, A. R. Splitting the ATM: distinct repair and checkpoint defects in ataxia-telangiectasia. Trends Genet. 14, 312–316 (1998).
Xu, B., Kim, S. T., Lim, D. S. & Kastan, M. B. Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Mol. Cell. Biol. 22, 1049–1059 (2002).
Lehmann, A. R. et al. A derivative of an ataxia-telangiectasia (A-T) cell line with normal radiosensitivity but A-T-like inhibition of DNA synthesis. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 49, 639–643 (1986).
Griffiths, D. J., Barbet, N. C., McCready, S., Lehmann, A. R. & Carr, A. M. Fission yeast rad17: a homologue of budding yeast RAD24 that shares regions of sequence similarity with DNA polymerase accessory proteins. EMBO J. 14, 5812–5823 (1995).
Kanter-Smoler, G., Knudsen, K. E., Jimenez, G., Sunnerhagen, P. & Subramani, S. Separation of phenotypes in mutant alleles of the Schizosaccharomyces pombe cell-cycle checkpoint gene rad1+. Mol. Biol. Cell 6, 793–805 (1995).
al-Khodairy, F. et al. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol. Biol. Cell 5, 147–160 (1994).
Groth, A. et al. Human Tousled like kinases are targeted by an ATM- and Chk1-dependent DNA damage checkpoint. EMBO J. 22, 1676–1687 (2003).
Krause, D. R. et al. Suppression of Tousled-like kinase activity after DNA damage or replication block requires ATM, NBS1 and Chk1. Oncogene 22, 5927–5937 (2003).
Sausville, E. A. et al. Phase I trial of 72-hour continuous infusion UCN-01 in patients with refractory neoplasms. J. Clin. Oncol. 19, 2319–2333 (2001).
Matsuoka, S., Huang, M. & Elledge, S. J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282, 1893–1897 (1998). Work in this reference, and in references 60, 62 and 63, describes the cloning and preliminary biochemical characterization of CHK2.
Chaturvedi, P. et al. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene 18, 4047–4054 (1999).
O'Neill, T. et al. Determination of substrate motifs for human Chk1 and hCds1/Chk2 by the oriented peptide library approach. J. Biol. Chem. 277, 16102–16115 (2002).
Blasina, A. et al. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr. Biol. 9, 1–10 (1999).
Brown, A. L. et al. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc. Natl Acad. Sci. USA 96, 3745–3750 (1999).
Chehab, N. H., Malikzay, A., Appel, M. & Halazonetis, T. D. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 14, 278–288 (2000).
Falck, J., Mailand, N., Syljuasen, R. G., Bartek, J. & Lukas, J. The ATM–Chk2–Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410, 842–847 (2001).
Falck, J., Petrini, J. H., Williams, B. R., Lukas, J. & Bartek, J. The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nature Genet. 30, 290–294 (2002). References 65 and 66 describe the first signalling and effector cascade that provided a mechanistic insight into the ionizing-radiation-induced intra-S-phase checkpoint in terms of its direct impact on cell-cycle machinery.
Hirao, A. et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ATM-dependent and ATM-independent manner. Mol. Cell. Biol. 22, 6521–6532 (2002).
Takai, H. et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 21, 5195–5205 (2002).
Jack, M. T. et al. Chk2 is dispensable for p53-mediated G1 arrest but is required for a latent p53-mediated apoptotic response. Proc. Natl Acad. Sci. USA 99, 9825–9829 (2002).
Bartek, J. & Lukas, J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 3, 421–429 (2003).
Hirao, A. et al. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287, 1824–1827 (2000). Work described in this reference and references 67–69 show that CHK2 is crucial for IR-induced apoptosis and indicates that CHK2 could be a radioprotection target.
Chehab, N. H., Malikzay, A., Stavridi, E. S. & Halazonetis, T. D. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc. Natl Acad. Sci. USA 96, 13777–13782 (1999).
Shieh, S. Y., Ahn, J., Tamai, K., Taya, Y. & Prives, C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 14, 289–300 (2000).
Craig, A. et al. Allosteric effects mediate CHK2 phosphorylation of the p53 transactivation domain. EMBO Rep. 4, 787–792 (2003).
Wu, Z. et al. Mutation of mouse p53 Ser23 and the response to DNA damage. Mol. Cell. Biol. 22, 2441–2449 (2002).
Ahn, J., Urist, M. & Prives, C. Questioning the role of checkpoint kinase 2 in the p53 DNA damage response. J. Biol. Chem. 278, 20480–20489 (2003).
Jallepalli, P. V., Lengauer, C., Vogelstein, B. & Bunz, F. The Chk2 tumor suppressor is not required for p53 responses in human cancer cells. J. Biol. Chem. 278, 20475–20479 (2003).
Brown, K. D. et al. The mismatch repair system is required for S-phase checkpoint activation. Nature Genet. 33, 80–84 (2003).
Giannini, G. et al. Human MRE11 is inactivated in mismatch repair-deficient cancers. EMBO Rep. 3, 248–254 (2002).
Keramaris, E., Hirao, A., Slack, R. S., Mak, T. W. & Park, D. S. ATM can regulate p53 and neuronal death independent of Chk2 in response to DNA damage. J. Biol. Chem. 278, 37782–37789 (2003).
Yang, S., Kuo, C., Bisi, J. E. & Kim, M. K. PML-dependent apoptosis after DNA damage is regulated by the checkpoint kinase hCds1/Chk2. Nature Cell Biol. 4, 865–870 (2002).
Stevens, C., Smith, L. & La Thangue, N. B. Chk2 activates E2F-1 in response to DNA damage. Nature Cell Biol. 5, 401–409 (2003).
Komarov, P. G. et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285, 1733–1737 (1999). This reference describes the identification — by chemical library screening — and in vitro and in vivo testing of a p53 inhibitor, and indicates that inhibition of the p53 pathway could reduce IR-induced DNA damage and lead to radioprotection.
Gudkov, A. V. & Komarova, E. A. The role of p53 in determining sensitivity to radiotherapy. Nature Rev. Cancer. 3, 117–129 (2003).
Botchkarev, V. A. et al. p53 is essential for chemotherapy-induced hair loss. Cancer Res. 60, 5002–5006 (2000).
Davis, S. T. et al. Prevention of chemotherapy-induced alopecia in rats by CDK inhibitors. Science 291, 134–137 (2001).
Kim, S. T., Xu, B. & Kastan, M. B. Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev. 16, 560–570 (2002).
Komarova, E. A., Christov, K., Faerman, A. I. & Gudkov, A. V. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene 19, 3791–3798 (2000).
Lee, J. S., Collins, K. M., Brown, A. L., Lee, C. H. & Chung, J. H. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature 404, 201–204 (2000).
Zhang, J. et al. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol. Cell. Biol. 24, 708–718 (2004).
Zou, L. & Elledge, S. J. Sensing DNA damage through ATRIP recognition of RPA–ssDNA complexes. Science 300, 1542–1548 (2003).
Rouse, J. & Jackson, S. P. Interfaces between the detection, signaling, and repair of DNA damage. Science 297, 547–551 (2002).
Bakkenist, C. J. & Kastan, M. B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 (2003).
D'Amours, D. & Jackson, S. P. The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nature Rev. Mol. Cell Biol. 3, 317–327 (2002).
Petrini, H. J. & Stracker, T. H. The cellular response to DNA double strand breaks: defining the sensors and mediators. Trends Cell Biol. 13, 458–462 (2003).
Pinedo, H. M. & Peters, G. F. Fluorouracil: biochemistry and pharmacology. J. Clin. Oncol. 6, 1653–1664 (1988).
Longley, D. B., Harkin, D. P. & Johnston, P. G. 5-fluorouracil: mechanisms of action and clinical strategies. Nature Rev. Cancer 3, 330–338 (2003).
Advanced Colorectal Cancer Meta-Analysis Project. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. J. Clin. Oncol. 10, 896–903 (1992).
Peters, G. J. & van der Vijgh, W. J. Protection of normal tissues from the cytotoxic effects of chemotherapy and radiation by amifostine (WR-2721): preclinical aspects. Eur. J. Cancer. 31A, S1–S7 (1995).
Capizzi, R. Amifostine: the preclinical basis for broad-spectrum selective cytoprotection of normal tissues from cytotoxic therapies. Semin. Oncol. 23, 2–17 (1996).
Budd, G. T. et al. Randomized trial of carboplatin plus amifostine versus carboplatin alone in patients with advanced solid tumors. Cancer 80, 1134–1140 (1997).
Santini, V. & Giles, F. J. The potential of amifostine: from cytoprotectant to therapeutic agent. Haematologica 84, 1035–1042 (1999).
Schiller, J. H. et al. Amifostine, cisplatin, and vinblastine in metastatic non-small-cell lung cancer: a report of high response rates and prolonged survival. J. Clin. Oncol. 14, 1913–1921 (1996).
Andreassen, C. N., Grau, C. & Lindegaard, J. C. Chemical radioprotection: a critical review of amifostine as a cytoprotector in radiotherapy. Semin. Radiat. Oncol. 13, 62–72 (2003).
Li, Y., Sun, X., LaMont, J. T., Pardee, A. B. & Li, C. J. Selective killing of cancer cells by β-lapachone: direct checkpoint activation as a strategy against cancer. Proc. Natl Acad. Sci. USA 100, 2674–2678 (2003).
Acknowledgements
B.Z. would like to acknowledge helpful discussions with S. Davis and E. Rowinsky during the course of writing this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- THERAPEUTIC WINDOW
-
The ratio between the toxic dose and the therapeutic dose of a drug, used as a measure of the relative safety of the drug for a particular treatment. Also called therapeutic index.
- ATAXIA TELANGIECTASIA
-
(AT). AT belongs to a group of human diseases collectively known as 'genomic instability syndromes'. It is characterized by cerebellar degeneration — which leads to severe, progressive neuromotor dysfunction — immunodeficiency, genomic instability, thymic and gonadal atrophy, and a striking predisposition to lymphoreticular malignancies. It is associated with defects in the ATM gene, a member of the phosphatidylinositol 3-kinase superfamily.
- CELL-CYCLE CHECKPOINTS
-
Regulatory mechanisms that do not allow the initiation of a new phase of the cell cycle before the previous one is completed, or temporarily arrest cell-cycle progression in response to stress. DNA damage activates specific checkpoints at the G1–S and G2–M boundaries, and in S phase, with each one based on a different mechanism.
- MITOTIC CATASTROPHE
-
A series of cellular events that occur after premature and aberrant mitosis and that usually result in cell death. Such a mitosis does not produce proper chromosome segregation and cell division, but leads to the formation of large non-viable cells with several nuclei, which contain fractions of broken chromosomes.
- RADIORESISTANT DNA SYNTHESIS
-
Characteristic inability to arrest DNA synthesis after irradiation, commonly seen in cells from patients with ataxia telangiectasia and those who are defective in other components of the intra-S-phase checkpoint.
- XEROSTOMIA
-
Dryness of the mouth resulting from diminished or arrested salivary secretion
Rights and permissions
About this article
Cite this article
Zhou, BB., Bartek, J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer 4, 216–225 (2004). https://doi.org/10.1038/nrc1296
Issue Date:
DOI: https://doi.org/10.1038/nrc1296
This article is cited by
-
Knockdown of MRPL35 promotes cell apoptosis and inhibits cell proliferation in non-small-cell lung cancer
BMC Pulmonary Medicine (2023)
-
Inhibition of checkpoint kinase 1 potentiates anticancer activity of gemcitabine in bladder cancer cells
Scientific Reports (2021)
-
Functional genomics identifies new synergistic therapies for retinoblastoma
Oncogene (2020)
-
Combination of a novel microtubule inhibitor MBRI-001 and gemcitabine synergistically induces cell apoptosis by increasing DNA damage in pancreatic cancer cell lines
Investigational New Drugs (2020)
-
SOX17 overexpression sensitizes chemoradiation response in esophageal cancer by transcriptional down-regulation of DNA repair and damage response genes
Journal of Biomedical Science (2019)