Abstract
Purpose
The lymphatic system is an essential but often understudied component of the circulatory system in comparison with its cardiovascular counterpart. Such disparity could often be explained by the difficulty in imaging lymphatics and the specialized microsurgical skills that are often required for lymphatic injury models. Recently, it has been shown that verteporfin, a photosensitive drug used for photodynamic therapy (PDT) to ablate the blood vessels, provides a similar effect on lymphatic vessels. Here, we seek to administer verteporfin and perform a modified form of PDT on collecting lymphatics in the mouse tail, a commonly used location for the study of lymphatic disorders, and examine lymphatic remodeling, contractility, and transport in response to the procedure.
Methods
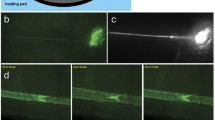
Mice collecting lymphatics in the tail were injured by PDT through an intradermal injection of verteporfin in the distal tip of the tail followed by light activation on the proximal portion of the tail downstream of the injection site. Lymphatic function was evaluated using a near-infrared (NIR) imaging system weekly for up to 28 days after injury.
Results
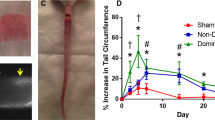
PDT resulted in a loss in lymphatic function contractile frequency that persisted for up to 7 days after injury. Packet transport and packet amplitude, measurements reflective of the strength of contraction, were significantly reduced 14 days after injury. The lymphatics showed a delayed increase in lymphatic leakage at 7 days that persisted until the study endpoint on day 28.
Conclusion
This technique provides an easy-to-use method for injuring lymphatics to understand their remodeling response to injury by PDT as well as potentially for screening therapeutics that seek to normalize lymphatic permeability or contractile function after injury.








Similar content being viewed by others
References
Abrahamse, H., and M. R. Hamblin. New photosensitizers for photodynamic therapy. Biochem. J. 473:347–364, 2016. https://doi.org/10.1042/BJ20150942.
Baluk, P., J. Fuxe, H. Hashizume, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204:2349–2362, 2007. https://doi.org/10.1084/jem.20062596.
Bausch & Lomb Incorporated. VISUDYNE (Package Insert). Bausch & Lomb Incorporated, 2000. https://www.bausch.com/globalassets/pdf/packageinserts/pharma/visudyne-prescribing-information.pdf.
Bernard, F. C., J. Kaiser, S. K. Raval, et al. Multichromatic near-infrared imaging to assess interstitial lymphatic and venous uptake in vivo. J. Biomed. Opt. 2021. https://doi.org/10.1117/1.jbo.26.12.126001.
Bouta, E. M., C. Blatter, T. A. Ruggieri, et al. Lymphatic function measurements influenced by contrast agent volume and body position. JCI Insights. 2018. https://doi.org/10.1172/jci.insight.96591.
Breslin, J. W., Y. Yang, J. P. Scallan, et al. Lymphatic vessel network structure and physiology. Compr. Physiol. 9:207–299, 2019. https://doi.org/10.1002/cphy.c180015.
Brodowska, K., A. Al-Moujahed, A. Marmalidou, et al. The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp. Eye Res. 124:67–73, 2014. https://doi.org/10.1016/j.exer.2014.04.011.
Cho, H., J. Kim, J. H. Ahn, et al. YAP and TAZ negatively regulate Prox1 during developmental and pathologic lymphangiogenesis. Circ. Res. 124:225–242, 2018. https://doi.org/10.1161/CIRCRESAHA.118.313707.
Chong, C., F. Scholkmann, S. B. Bachmann, et al. In vivo visualization and quantification of collecting lymphatic vessel contractility using near-infrared imaging. Sci. Rep. 6:22930, 2016. https://doi.org/10.1038/srep22930.
Cribb, M. T., L. F. Sestito, S. G. Rockson, et al. The kinetics of lymphatic dysfunction and leukocyte expansion in the draining lymph node during LTB4 antagonism in a mouse model of lymphedema. Int. J. Mol. Sci. 22:4455, 2021. https://doi.org/10.3390/ijms22094455.
Dixon, J. B. Lymphatic lipid transport: sewer or subway? Trends Endocrinol. Metab. 21:480–487, 2010. https://doi.org/10.1016/j.tem.2010.04.003.
Doan, T. N., F. C. Bernard, J. M. McKinney, et al. Endothelin-1 inhibits size dependent lymphatic clearance of PEG-based conjugates after intra-articular injection into the rat knee. Acta Biomater. 93:270–281, 2019. https://doi.org/10.1016/j.actbio.2019.04.025.
Dolmans, D. E. J. G. J., D. Fukumura, and R. K. Jain. Photodynamic therapy for cancer. Nat. Rev. Cancer. 3:380–387, 2003. https://doi.org/10.1038/nrc1071.
Du, H.-T., L.-L. Du, X.-L. Tang, et al. Blockade of MMP-2 and MMP-9 inhibits corneal lymphangiogenesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 255:1573–1579, 2017. https://doi.org/10.1007/s00417-017-3651-8.
Dylke, E. S., M. F. McEntee, G. P. Schembri, et al. Reliability of a radiological grading system for dermal backflow in lymphoscintigraphy imaging. Acad Radiol. 20:758–763, 2013. https://doi.org/10.1016/j.acra.2013.01.018.
Gashev, A. A. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J. Physiol. 540:1023–1037, 2002. https://doi.org/10.1113/jphysiol.2002.016642.
Gashev, A. A. Physiologic aspects of lymphatic contractile function. Ann. N. Y. Acad. Sci. 979:178–187, 2002. https://doi.org/10.1111/j.1749-6632.2002.tb04878.x.
Karaçavuş, S., Y. K. Yılmaz, and H. Ekim. Clinical significance of lymphoscintigraphy findings in the evaluation of lower extremity lymphedema. Mol. Imaging Radionucl. Ther. 24:80–84, 2015. https://doi.org/10.4274/mirt.58077.
Kawashima, Y., M. Sugimura, Y.-C. Hwang, and N. Kudo. The lymph system in mice. Jpn. J. Vet. Res. 1964. https://doi.org/10.14943/jjvr.12.4.69.
Kilarski, W. W., A. Muchowicz, M. Wachowska, et al. Optimization and regeneration kinetics of lymphatic-specific photodynamic therapy in the mouse dermis. Angiogenesis. 17:347–357, 2014. https://doi.org/10.1007/s10456-013-9365-6.
Koller, A., R. Mizuno, and G. Kaley. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: role of endothelial prostanoids. Am. J. Physiol. Regul. Integr. Comp. Physiol. 277:R1683–R1689, 1999. https://doi.org/10.1152/ajpregu.1999.277.6.R1683.
Kornuta, J. A., Z. Nepiyushchikh, O. Y. Gasheva, et al. Effects of dynamic shear and transmural pressure on wall shear stress sensitivity in collecting lymphatic vessels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309:R1122–R1134, 2015. https://doi.org/10.1152/ajpregu.00342.2014.
Kraft, J. C., P. M. Treuting, and R. J. Y. Ho. Indocyanine green nanoparticles undergo selective lymphatic uptake, distribution and retention and enable detailed mapping of lymph vessels, nodes and abnormalities. J. Drug Target. 26:494–504, 2018. https://doi.org/10.1080/1061186X.2018.1433681.
Leman, J. A., and C. A. Morton. Photodynamic therapy: applications in dermatology. Expert Opin. Biol. Ther. 2:45–53, 2002. https://doi.org/10.1517/14712598.2.1.45.
Liao, S., G. Cheng, D. A. Conner, et al. Impaired lymphatic contraction associated with immunosuppression. Proc. Natl Acad. Sci. USA. 113:E5992, 2016. https://doi.org/10.1073/pnas.1614689113.
Lim, H. Y., C. H. Thiam, K. P. Yeo, et al. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 17:671–684, 2013. https://doi.org/10.1016/j.cmet.2013.04.002.
Liu-Chittenden, Y., B. Huang, J. S. Shim, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26:1300–1305, 2012. https://doi.org/10.1101/gad.192856.112.
Mendez, U., E. M. Stroup, L. L. Lynch, et al. A chronic and latent lymphatic insufficiency follows recovery from acute lymphedema in the rat foreleg. Am. J. Physiol. Heart Circ. Physiol. 303:H1107–H1113, 2012. https://doi.org/10.1152/ajpheart.00522.2012.
Muchowicz, A., M. Wachowska, J. Stachura, et al. Inhibition of lymphangiogenesis impairs antitumour effects of photodynamic therapy and checkpoint inhibitors in mice. Eur. J. Cancer. 83:19–27, 2017. https://doi.org/10.1016/j.ejca.2017.06.004.
Mukherjee, A., J. Hooks, Z. Nepiyushchikh, and J. B. Dixon. Entrainment of lymphatic contraction to oscillatory flow. Sci. Rep. 9:5840, 2019. https://doi.org/10.1038/s41598-019-42142-9.
Nelson, T. S., R. E. Akin, M. J. Weiler, et al. Minimally invasive method for determining the effective lymphatic pumping pressure in rats using near-infrared imaging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306:R281–R290, 2014. https://doi.org/10.1152/ajpregu.00369.2013.
Nelson, T. S. The functional and remodeling response of collecting lymphatic vessels to disruption of lymphatic drainage pathways. Doctoral Dissertation, Georgia Institute of Technology, Georgia Tech Theses and Dissertations, 2018.
Novartis Ophthalmics. Visudyne (Verteporfin for Injection). Professional Product Labeling. NDA 21-119/S-001. Novartis Ophthalmics, 2001, pp. 3–12.
Proulx, S. T., P. Luciani, A. Christiansen, et al. Use of a PEG-conjugated bright near-infrared dye for functional imaging of rerouting of tumor lymphatic drainage after sentinel lymph node metastasis. Biomaterials. 34:5128–5137, 2013. https://doi.org/10.1016/j.biomaterials.2013.03.034.
Randolph, G. J., V. Angeli, and M. A. Swartz. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5:617–628, 2005. https://doi.org/10.1038/nri1670.
Rutkowski, J. M., M. Moya, J. Johannes, et al. Secondary lymphedema in the mouse tail: lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc. Res. 72:161–171, 2006. https://doi.org/10.1016/j.mvr.2006.05.009.
Schmidt-Erfurth, U., and T. Hasan. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv. Ophthalmol. 45:195–214, 2000. https://doi.org/10.1016/S0039-6257(00)00158-2.
Swartz, M. A. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 50:3–20, 2001. https://doi.org/10.1016/S0169-409X(01)00150-8.
Swartz, M. A., A. Kaipainen, P. A. Netti, et al. Mechanics of interstitial-lymphatic fluid transport: theoretical foundation and experimental validation. J. Biomech. 32:1297–1307, 1999. https://doi.org/10.1016/S0021-9290(99)00125-6.
Szuba, A., M. Skobe, M. J. Karkkainen, et al. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 16:1985–1987, 2002. https://doi.org/10.1096/fj.02-0401fje.
Tammela, T., A. Saaristo, T. Holopainen, et al. Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci. Transl. Med. 3:1–8, 2011. https://doi.org/10.1126/scitranslmed.3001699.
Tobbia, D., J. Semple, A. Baker, et al. Lymphedema development and lymphatic function following lymph node excision in sheep. J. Vasc. Res. 46:426–434, 2009. https://doi.org/10.1159/000194273.
Wei, H., F. Wang, Y. Wang, et al. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. 108:478–487, 2017. https://doi.org/10.1111/cas.13138.
Weiler, M. J., M. T. Cribb, Z. Nepiyushchikh, et al. A novel mouse tail lymphedema model for observing lymphatic pump failure during lymphedema development. Sci. Rep. 9:10405, 2019. https://doi.org/10.1038/s41598-019-46797-2.
Weiler, M., T. Kassis, and J. B. Dixon. Sensitivity analysis of near-infrared functional lymphatic imaging. J. Biomed. Opt.17:066019, 2012. https://doi.org/10.1117/1.JBO.17.6.066019.
Zawieja, S. D., W. Wang, X. Wu, et al. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 302:H643–H653, 2012. https://doi.org/10.1152/ajpheart.00606.2011.
Zheng, W., H. Nurmi, S. Appak, et al. Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev. 28:1592–1603, 2014. https://doi.org/10.1101/gad.237677.114.
Acknowledgments
This work was funded by The National Institutes of Health (Grant No. R01-HL113061) and Naumann-Etienne Foundation (Kuzminich).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate Institutional Committees.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Christine P. Hendon oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 297 kb)
Supplementary file2 (MP4 691 kb)
Supplementary file3 (MP4 679 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuzminich, Y., Dixon, J.B. Evaluation of Longitudinal Lymphatic Function Changes upon Injury in the Mouse Tail with Photodynamic Therapy. Cardiovasc Eng Tech 14, 204–216 (2023). https://doi.org/10.1007/s13239-022-00645-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-022-00645-z