Abstract
Breast cancer is the most prominent cause of cancer death in women worldwide. The highlights of this review are to provide an overview of the targeted therapeutic agents, challenges with metastatic breast cancer (MBCa), mechanisms of action through Hedgehog/Gli 1 signaling pathway and future prospective. Over a decade of success, several drugs have been approved and are in the advanced stages of clinical trials that target the receptors such as estrogen receptor, growth factor receptor, receptor activator of nuclear factor kappa-B, etc. Currently, several monoclonal antibodies are also used for the treatment of breast cancer. Advances in understanding tumor biology, particularly signaling pathways such as Notch signaling pathway, Hedgehog/Gli 1 signaling pathway, and inhibitors are considered to be important for bone metastasis. These studies may provide vital information for the design and development of new strategies with respect to efficacy, reduction of the side effects, and treatment strategies.


Similar content being viewed by others
References
Bhandari PR. Crocus sativus L. (saffron) for cancer chemoprevention: a mini review. JTCM. 2015;5:81–7.
Elancheran R, Maruthanila VL, Ramanathan M, et al. Recent discoveries and developments of androgen receptor based therapy for prostate cancer. Med Chem Commun. 2015;6:746–68.
Chen J, Duan Y, Zhang X, et al. Genistein induces apoptosis by the inactivation of the IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cells. Food Funct. 2015;6:995–1000.
Carvalho AF, Hyphantis T, Sales PMG, et al. Major depressive disorder in breast cancer: a critical systematic review of pharmacological and psychotherapeutic clinical trials. Cancer Treat Rev. 2014;40:349–55.
Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. Bethesda. MD, http://seer.cancer.gov/csr/1975_2013/. Based on November 2015 SEER data submission, posted to the SEER web site, April 2016.
Di Leo A, Curigliano G, Dieras V, et al. New approaches for improving outcomes in breast cancer in Europe. Breast. 2015;24:321–30.
Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet. 1896;2:104–7.
Kumar R, Zakharov MN, Khan SH, et al. The dynamic structure of the estrogen receptor. J Amino Acids. doi:10.4061/2011/812540.
Roy SS, Vadlamudi RK. Role of estrogen receptor signaling in breast cancer metastasis. Int J Breast Cancer. 2012;. doi:10.1155/2012/654698.
Platet N, Cathiard A, Gleizes M, et al. Estrogen and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51:55–67.
Nagaraj G, Ma C. Revisiting the estrogen receptor pathway and its role in endocrine therapy for postmenopausal women with estrogen receptor-positive metastatic breast cancer. Breast Cancer Res Treat. 2015;150(2):231–42.
Kampa M, Pelekanou V, Notas G, et al. The estrogen receptor: two or more molecules, multiple variants, diverse localizations, signaling and functions. Are we undergoing a paradigm-shift as regards their significance in breast cancer? Hormones. 2013;12(1):69–85.
Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol. 2011;22:1736–47.
Lee SM, Moon J, Redman BG, et al. A phase II study of RO4929097 gamma-secretase inhibitor in metastatic melanoma: SWOG 0933. Cancer. 2015;121(3):432–40.
Yuan X, Wu H, Xu H, et al. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett. 2015. doi:10.1016/j.canlet.2015.07.048.
Al-Hussaini H, Subramanyam D, Reedijk M, et al. notch signaling pathway as a therapeutic target in breast cancer. Mol Cancer Ther. 2011;10(1):9–15.
Li L, Zhang J, Xiong N, et al. Notch-1 signaling activates NF-jB in human breast carcinoma MDA-MB-231 cells via PP2A-dependent AKT pathway. Med Oncol. 2016;33:1–11.
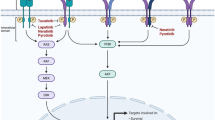
Ramaswamy B, Lu Y, Teng KY, et al. Hedgehog signaling is a novel therapeutic target in tamoxifen-resistant breast cancer aberrantly activated by PI3K/AKT pathway. Cancer Res. 2012;72(19):5048–59.
Kubo M, Nakamura M, Tasaki A, et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004;64:6071–4.
Souzaki M, Kubo M, Kai M, et al. Hedgehog signaling pathway mediates the progression of non-invasive breast cancer to invasive breast cancer. Cancer Sci. 2011;102:373–81.
Kwon YJ, Hurst DR, Steg AD, et al. Gli1 enhances migration and invasion via upregulation of MMP-11 and promotes metastasis in ER alpha negative breast cancer cell lines. ClinExp Metastasis. 2011;28:437–49.
Benvenuto M, Masuelli L, Smaele ED, et al. In vitro and in vivo inhibition of breast cancer cell growth by targeting the Hedgehog/GLI pathway with SMO (GDC-0449) or GLI (GANT-61) inhibitors. Oncotarget. 2016;7:9250–70.
Gonnissen A, Isebaert S, Haustermans K. Targeting the Hedgehog signaling pathway in cancer: beyond Smoothened. Oncotarget. 2015;6:13899–913.
Li YH, Gao HF, Wang Y, et al. Overexpression of Gli1 in cancer interstitial tissues predicts early relapse after radical operation of breast cancer. Chin J Cancer Res. 2012;24(4):263–74.
Wang X, Yang KH, Wanyan P, et al. Comparison of the efficacy and safety of denosumab versus bisphosphonates in breast cancer and bone metastases treatment: a meta–analysis of randomized controlled trials. Oncol Lett. 2014;7:1997–2002.
Coleman R, Aksnes AK, Naume B, et al. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Canc Res Treat. 2014;145:411–8.
Clézardin P. Therapeutic targets for bone metastases in breast cancer. Breast Cancer Res. 2011;13:207.
Bäuerle T, Komljenovic D, Merz M, et al. Cilengitide inhibits progression of experimental breast cancer bone metastases as imaged noninvasively using VCT, MRI and DCE-MRI in a longitudinal in vivo study. Int J Cancer. 2011;128(10):2453–62.
Baselga J, Cervantes A, Martinelli E, et al. Phase I safety, pharmacokinetics, and inhibition of SRC activity study of saracatinib in patients with solid tumors. Clin Cancer Res. 2010;16:4876–83.
Park BJ, Whichard ZL, Corey SJ. Dasatinib synergizes with both cytotoxic and signal transduction inhibitors in heterogeneous breast cancer cell lines—lessons for design of combination targeted therapy. Cancer Lett. 2012;320:104–10.
Neves H, Kwok HF. Recent advances in the field of anti-cancer immunotherapy. BBA Clin. 2015;3:280–8.
Miles D, Roché H, Martin M. Phase III multicenter clinical trial of the Sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist. 2011;16:1092–100.
Berman D, Korman A, Peck R, et al. The development of immunomodulatory monoclonal antibodies as a new therapeutic modality for cancer: the Bristol-Myers Squibb experience. Pharmacol Ther. 2015;148:132–53.
Khoja L, Butler MO, Kang SP, et al. Pembrolizumab. J Immunother Cancer. 2015;3:36.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62.
Montero AJ, Avancha K, Glück S, et al. A cost-benefit analysis of bevacizumab in combination with paclitaxel in the first-line treatment of patients with metastatic breast cancer. Breast Cancer Res Treat. 2012;132:747–51.
Mackey JR, Ramos-Vazquez M, Lipatov O, et al. Primary results of ROSE/TRIO-12, a randomized placebo-controlled phase III Trial evaluating the addition of ramucirumab to first-line docetaxel chemotherapy in metastatic breast cancer. J ClinOncol. 2014;32:1–8.
Luu T, Frankel P, Chung C, et al. Phase I/II trial of vinorelbine and sorafenib in metastatic breast cancer. Clin Breast Cancer. 2014;14:94–100.
Burstein HJ, Elias AD, Rugo HS, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–6.
Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8.
Stalker L, Pemberton J, Moorehead RA. Inhibition of proliferation and migration of luminal and claudin-low breast cancer cells by PDGFR inhibitors. Cancer Cell Int. 2014;14:89.
Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272–83.
Sharma T, Dhingra R, Singh S, et al. Aflibercept: a novel VEGF targeted agent to explore the future perspectives of anti-angiogenic therapy for the treatment of multiple tumors. Mini Rev Med Chem. 2013;13(4):530–40.
Kort A, Durmus S, Sparidans RW, et al. Brain and testis accumulation of regorafenib is restricted by breast cancer resistance protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1). Pharm Res. 2015;32:2205–16.
Lu J. Palbociclib: a first-in-class CDK4/CDK6 inhibitor for the treatment of hormone-receptor positive advanced breast cancer. J Hematol Oncol. 2015;8:98. doi:10.1186/s13045-015-0194-5.
Peddi PF, Hurvitz SA. Ado-trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer: latest evidence and clinical potential. Ther Adv Med Oncol. 2014;6(5):202–9.
Amiri-Kordestani L, Wedam S, Zhang L, et al. First FDA approval of neoadjuvant therapy for breast cancer: pertuzumab for the treatment of patients with HER2-positive breast cancer. Clin Cancer Res. 2014;20:5359–64.
Patel TA, Dave B, Rodriguez AA, et al. Dual HER2 blockade: preclinical and clinical data. Breast Cancer Res. 2014;16:419. doi:10.1186/s13058-014-0419-5.
Geuna E, Montemurro F, Aglietta M, et al. Potential of afatinib in the treatment of patients with HER2-positive breast cancer. Breast Cancer Targets Ther. 2012;4:131–7.
Nabholtz JM, Abrial C, Mouret-Reynier MA, et al. Multicentricneoadjuvant phase II study of panitumumab combined with an anthracycline/taxane-based chemotherapy in operable triple-negative breast cancer: identification of biologically defined signatures predicting treatment impact. Ann Oncol. 2014;25:1570–7.
Baselga J, Gomez P, Greil R, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:2586–92.
Ryan PD, Neven P, Blackwell KL, et al. P1-17-01: figitumumab plus exemestane versus exemestane as first-line treatment of postmenopausal hormone receptor- positive advanced breast cancer: a randomized, open-label phase II trial. Cancer Res. doi:10.1158/0008-5472.SABCS11-P1-17-01.
Gao J, Chesebrough JW, Cartlidge SA, et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011;71:1029–40.
Sachdev D, Zhang X, Matise I, et al. The type I insulin-like growth factor receptor regulates cancer metastasis independently of primary tumor growth by promoting invasion and survival. Oncogene. 2010;29:251–62.
Robertson JF, Ferrero JM, Bourgeois H, et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol. 2013;14:228–35.
Fagan DH, Uselman RR, Sachdev D, et al. Acquired resistance to tamoxifen is associated with loss of the type I insulin-like growth factor receptor (IGF1R): implications for breast cancer treatment. Cancer Res. 2012;72:3372–80. doi:10.1158/0008-5472.CAN-12-0684.
Wolff AC, Lazar AA, Bondarenko I, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. 2013;31:195–202.
Donald P, McDonnell Wardell SE, et al. Oral selective estrogen receptor downregulators (SERDs), a breakthrough endocrine therapy for breast cancer. J Med Chem. 2015;58:4883–7.
Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM Phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor positive advanced breast cancer. J Clin Oncol. 2010;28:4594–600.
Lai A, Kahraman M, Govek S, et al. Identification of GDC-0810 (ARN-810), an orally bioavailable selective estrogen receptor degrader (SERD) that demonstrates robust activity in tamoxifen-resistant breast cancer xenografts. J Med Chem. 2015;58:4888–904.
Degorce SL, Bailey A, Callis R, et al. Investigation of (E)–3-[4-(2-Oxo-3-aryl-chromen-4-yl)oxyphenyl]acrylic acids as oral selective estrogen Receptor down-regulators. J Med Chem. 2015;58:3522–33.
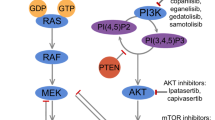
Safdari Y, Khalili M, Ebrahimzadehb MA, et al. Natural inhibitors of PI3K/AKT signaling in breast cancer: emphasis on newly-discovered molecular mechanisms of action. Pharmacol Res. 2015;93:1–10.
Lim W, Park J, Lee YH, et al. Subglutinol A, an immunosuppressive a-pyrone diterpenoid from Fusarium subglutinans, acts as an estrogen receptor antagonist. BiochemBiophys Res Commun. 2015;461:507–12.
Maruthanila VL, Poornima J, Mirunalini S. Attenuation of carcinogenesis and the mechanism underlying by the influence of indole-3-carbinol and its metabolite 3,3′-diindolylmethane: a therapeutic marvel. Adv Pharmacol Sci. 2014;. doi:10.1155/2014/832161.
Suzuki N, Liu X, Laxmi YR, et al. Anti-breast cancer potential of SS5020, a novel benzopyran antiestrogen. Int J Cancer. 2011;128:974–82.
Laxmi YR, Liu X, Suzuki N, et al. Anti-breast cancer potential of SS1020, a novel antiestrogen lacking estrogenic and genotoxic actions. Int J Cancer. 2010;127:1718–26.
Buzdar A, Vogel C, Schwartzberg L, et al. Randomized double-blind phase 2 trial of 3 doses of TAS-108 in patients with advanced or metastatic postmenopausal breast cancer. Cancer. 2012;118:3244–53.
Nishino T, Yamanouchi H, Ishibashi K, et al. Antiovulatory effect of a single injection of pure antiestrogen ZK 191703 at early stage of rat estrus cycle. J Steroid Biochem Mol Biol. 2009;114:152–60.
Van de Velde P, Nique F, Bouchoux F, et al. RU 58,668, a new pure antiestrogen inducing a regression of human mammary carcinoma implanted in nude mice. J Steroid Biochem Mol Biol. 1994;48:187–96.
Garner F, Shomali M, Paquin D, et al. RAD1901: a novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs. 2015;26:948–56.
Acknowledgments
Thanks are attributable to DST &DBT, Govt. of India for financial support and Bioinformatics Infrastructure facility, IASST.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
About this article
Cite this article
Maruthanila, V.L., Elancheran, R., Kunnumakkara, A.B. et al. Recent development of targeted approaches for the treatment of breast cancer. Breast Cancer 24, 191–219 (2017). https://doi.org/10.1007/s12282-016-0732-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-016-0732-1