Abstract
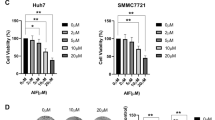
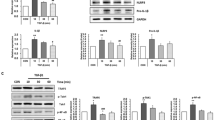
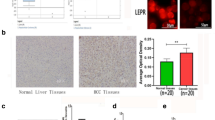
Leptin, an adipose tissue-derived hormone, exhibits potent tumor promoting effects through various mechanisms. Cathepsin B, a member of the lysosomal cysteine proteases, has been shown to modulate the growth of cancer cells. In this study, we have investigated the role of cathepsin B signaling in leptin-induced hepatic cancer growth. Leptin treatment caused significant increase in the levels of active cathepsin B through the axis of endoplasmic reticulum stress and autophagy induction without significant effects on pre- and pro-forms of cathepsin B. Interestingly, inhibition of cathepsin B signaling by gene silencing or treatment with a selective pharmacological inhibitor (CA-074) prevented leptin-enhanced viability of hepatic cancer cell and suppressed progression of cell cycle, indicating the critical role of cathepsin B in leptin-induced hepatic cancer growth. We have further observed that maturation of cathepsin B is required for NLRP3 inflammasomes activation, which is implicated in the growth of hepatic cancer cell. The crucial roles of cathepsin B maturation in leptin-induced hepatic cancer growth and NLRP3 inflammasomes activation were confirmed in an in vivo HepG2 tumor xenograft model. Taken together, these results demonstrate that cathepsin B signaling plays a pivotal role in leptin-induced hepatic cancer cell growth by activating NLRP3 inflammasomes.







Similar content being viewed by others
References
Allen IC, Tekippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP (2010) The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med 207:1045–1056. https://doi.org/10.1084/jem.20100050
Araujo TF, Cordeiro AV, DaA V, Vitzel KF, Silva VRR (2018) The role of cathepsin B in autophagy during obesity: a systematic review. Life Sci 209:274–281. https://doi.org/10.1016/j.lfs.2018.08.024
Banks AS, Davis SM, Bates SH, Myers MG Jr (2000) Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275:14563–14572. https://doi.org/10.1074/jbc.275.19.14563
Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M (2010) Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59:1192–1199. https://doi.org/10.1136/gut.2009.197822
Bengsch F, Buck A, Gunther SC, Seiz JR, Tacke M, Pfeifer D, Von Elverfeldt D, Sevenich L, Hillebrand LE, Kern U, Sameni M, Peters C, Sloane BF, Reinheckel T (2014) Cell type-dependent pathogenic functions of overexpressed human cathepsin B in murine breast cancer progression. Oncogene 33:4474–4484. https://doi.org/10.1038/onc.2013.395
Bhoopathi P, Chetty C, Gujrati M, Dinh DH, Rao JS, Lakka S (2010) Cathepsin B facilitates autophagy-mediated apoptosis in SPARC overexpressed primitive neuroectodermal tumor cells. Cell Death Differ 17:1529–1539. https://doi.org/10.1038/cdd.2010.28
Biasizzo M, Kopitar-Jerala N (2020) Interplay between NLRP3 inflammasome and autophagy. Front Immunol 11:591803. https://doi.org/10.3389/fimmu.2020.591803
Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, Kanellopoulos J, Martin F, Rebe C, Apetoh L, Ghiringhelli F (2013) Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 19:57–64. https://doi.org/10.1038/nm.2999
Chen X, Guo X, Ge Q, Zhao Y, Mu H, Zhang J (2019) ER stress activates the NLRP3 inflammasome: a novel mechanism of atherosclerosis. Oxid Med Cell Longev 2019:3462530. https://doi.org/10.1155/2019/3462530
Chevriaux A, Pilot T, Derangere V, Simonin H, Martine P, Chalmin F, Ghiringhelli F, Rebe C (2020) Cathepsin B is required for NLRP3 inflammasome activation in macrophages, through NLRP3 interaction. Front Cell Dev Biol 8:167. https://doi.org/10.3389/fcell.2020.00167
Droga-Mazovec G, Bojic L, Petelin A, Ivanova S, Romih R, Repnik U, Salvesen GS, Stoka V, Turk V, Turk B (2008) Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J Biol Chem 283:19140–19150. https://doi.org/10.1074/jbc.M802513200
Ebert MP, Kruger S, Fogeron ML, Lamer S, Chen J, Pross M, Schulz HU, Lage H, Heim S, Roessner A, Malfertheiner P, Rocken C (2005) Overexpression of cathepsin B in gastric cancer identified by proteome analysis. Proteomics 5:1693–1704. https://doi.org/10.1002/pmic.200401030
Ershaid N, Sharon Y, Doron H, Raz Y, Shani O, Cohen N, Monteran L, Leider-Trejo L, Ben-Shmuel A, Yassin M, Gerlic M, Ben-Baruch A, Pasmanik-Chor M, Apte R, Erez N (2019) NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat Commun 10:4375. https://doi.org/10.1038/s41467-019-12370-8
Farr OM, Gavrieli A, Mantzoros CS (2015) Leptin applications in 2015: what have we learned about leptin and obesity? Curr Opin Endocrinol Diabetes Obes 22:353–359. https://doi.org/10.1097/MED.0000000000000184
Fasshauer M, Bluher M (2015) Adipokines in health and disease. Trends Pharmacol Sci 36:461–470. https://doi.org/10.1016/j.tips.2015.04.014
Fernandez PL, Farre X, Nadal A, Fernandez E, Peiro N, Sloane BF, Shi GP, Chapman HA, Campo E, Cardesa A (2001) Expression of cathepsins B and S in the progression of prostate carcinoma. Int J Cancer 95:51–55. https://doi.org/10.1002/1097-0215(20010120)95:1%3c51::aid-ijc1009%3e3.0.co;2-j
Fruhbeck G (2006) Intracellular signalling pathways activated by leptin. Biochem J 393:7–20. https://doi.org/10.1042/BJ20051578
Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E (2006) Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res 12:1447–1453. https://doi.org/10.1158/1078-0432.CCR-05-1913
Grant RW, Dixit VD (2013) Mechanisms of disease: inflammasome activation and the development of type 2 diabetes. Front Immunol 4:50. https://doi.org/10.3389/fimmu.2013.00050
Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ (2000) Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest 106:1127–1137. https://doi.org/10.1172/JCI9914
Guicciardi ME, Miyoshi H, Bronk SF, Gores GJ (2001) Cathepsin B knockout mice are resistant to tumor necrosis factor-alpha-mediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. Am J Pathol 159:2045–2054. https://doi.org/10.1016/s0002-9440(10)63056-8
Hu Y, Shi Y, Chen H, Tao M, Zhou X, Li J, Ma X, Wang Y, Liu N (2022) Blockade of autophagy prevents the progression of hyperuricemic nephropathy through inhibiting NLRP3 inflammasome-mediated pyroptosis. Front Immunol 13:8494. https://doi.org/10.3389/fimmu.2022.858494
Ishikawa M, Kitayama J, Nagawa H (2004) Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res 10:4325–4331. https://doi.org/10.1158/1078-0432.CCR-03-0749
Iwama H, Mehanna S, Imasaka M, Hashidume S, Nishiura H, Yamamura KI, Suzuki C, Uchiyama Y, Hatano E, Ohmuraya M (2021) Cathepsin B and D deficiency in the mouse pancreas induces impaired autophagy and chronic pancreatitis. Sci Rep 11:6596. https://doi.org/10.1038/s41598-021-85898-9
Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, Pham CT, Van Der Meer JW, Dinarello CA, Van Den Berg WB (2009) Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum 60:3651–3662. https://doi.org/10.1002/art.25006
Joy B, Sivadasan R, Abraham TE, John M, Sobhan PK, Seervi M, T RS, (2010) Lysosomal destabilization and cathepsin B contributes for cytochrome c release and caspase activation in embelin-induced apoptosis. Mol Carcinog 49:324–336. https://doi.org/10.1002/mc.20599
Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS, Myers MG Jr (2002) Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem 277:41547–41555. https://doi.org/10.1074/jbc.M205148200
Kolb R, Phan L, Borcherding N, Liu Y, Yuan F, Janowski AM, Xie Q, Markan KR, Li W, Potthoff MJ, Fuentes-Mattei E, Ellies LG, Knudson CM, Lee MH, Yeung SJ, Cassel SL, Sutterwala FS, Zhang W (2016) Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat Commun 7:13007. https://doi.org/10.1038/ncomms13007
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13:397–411. https://doi.org/10.1038/nri3452
Lee S, Pham DV, Park PH (2022) Sestrin2 induction contributes to anti-inflammatory responses and cell survival by globular adiponectin in macrophages. Arch Pharm Res 45:38–50. https://doi.org/10.1007/s12272-021-01364-0
Lian D, Zhu L, Yu Y, Zhang X, Lin Y, Liu J, Han R, Guo Y, Cai D, Xiao W, Chen Y, He H, Xu D, Zheng C, Wang X, Huang Y, Chen Y (2022) Kakonein restores hyperglycemia-induced macrophage digestion dysfunction through regulation of cathepsin B-dependent NLRP3 inflammasome activation. J Leukoc Biol 112:143–155. https://doi.org/10.1002/JLB.3MA0821-418R
Malik BR, Maddison DC, Smith GA, Peters OM (2019) Autophagic and endo-lysosomal dysfunction in neurodegenerative disease. Mol Brain 12:100. https://doi.org/10.1186/s13041-019-0504-x
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10:417–426. https://doi.org/10.1016/s1097-2765(02)00599-3
Moosavi MA, Haghi A, Rahmati M, Taniguchi H, Mocan A, Echeverria J, Gupta VK, Tzvetkov NT, Atanasov AG (2018) Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett 424:46–69. https://doi.org/10.1016/j.canlet.2018.02.030
Nagaraj NS, Vigneswaran N, Zacharias W (2006) Cathepsin B mediates TRAIL-induced apoptosis in oral cancer cells. J Cancer Res Clin Oncol 132:171–183. https://doi.org/10.1007/s00432-005-0053-9
Olson OC, Joyce JA (2015) Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat Rev Cancer 15:712–729. https://doi.org/10.1038/nrc4027
Patel S, Homaei A, El-Seedi HR, Akhtar N (2018) Cathepsins: proteases that are vital for survival but can also be fatal. Biomed Pharmacother 105:526–532. https://doi.org/10.1016/j.biopha.2018.05.148
Pham DV, Park PH (2020) Recent insights on modulation of inflammasomes by adipokines: a critical event for the pathogenesis of obesity and metabolism-associated diseases. Arch Pharm Res 43:997–1016. https://doi.org/10.1007/s12272-020-01274-7
Pham DV, Tilija Pun N, Park PH (2021) Autophagy activation and SREBP-1 induction contribute to fatty acid metabolic reprogramming by leptin in breast cancer cells. Mol Oncol 15:657–678. https://doi.org/10.1002/1878-0261.12860
Rao Malla R, Gopinath S, Alapati K, Gorantla B, Gondi CS, Rao JS (2013) Knockdown of cathepsin B and uPAR inhibits CD151 and alpha3beta1 integrin-mediated cell adhesion and invasion in glioma. Mol Carcinog 52:777–790. https://doi.org/10.1002/mc.21915
Raut PK, Kim SH, Choi DY, Jeong GS, Park PH (2019) Growth of breast cancer cells by leptin is mediated via activation of the inflammasome: critical roles of estrogen receptor signaling and reactive oxygen species production. Biochem Pharmacol 161:73–88. https://doi.org/10.1016/j.bcp.2019.01.006
Reilly MP, Iqbal N, Schutta M, Wolfe ML, Scally M, Localio AR, Rader DJ, Kimmel SE (2004) Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J Clin Endocrinol Metab 89:3872–3878. https://doi.org/10.1210/jc.2003-031676
Reiser J, Adair B, Reinheckel T (2010) Specialized roles for cysteine cathepsins in health and disease. J Clin Invest 120:3421–3431. https://doi.org/10.1172/JCI42918
Rempel SA, Rosenblum ML, Mikkelsen T, Yan PS, Ellis KD, Golembieski WA, Sameni M, Rozhin J, Ziegler G, Sloane BF (1994) Cathepsin B expression and localization in glioma progression and invasion. Cancer Res 54:6027–6031
Rouet-Benzineb P, Aparicio T, Guilmeau S, Pouzet C, Descatoire V, Buyse M, Bado A (2004) Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-kappaB signaling. J Biol Chem 279:16495–16502. https://doi.org/10.1074/jbc.M312999200
Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P, Hiller S (2016) GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J 35:1766–78. https://doi.org/10.15252/embj.201694696
Shankar A, Xiao J (2010) Positive relationship between plasma leptin level and hypertension. Hypertension 56:623–628. https://doi.org/10.1161/HYPERTENSIONAHA.109.148213
Shanker J, Rao VS, Ravindran V, Dhanalakshmi B, Hebbagodi S, Kakkar VV (2012) Relationship of adiponectin and leptin to coronary artery disease, classical cardiovascular risk factors and atherothrombotic biomarkers in the IARS cohort. Thromb Haemost 108:769–780. https://doi.org/10.1160/TH12-04-0263
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. https://doi.org/10.1038/nature15514
Shrestha M, Park PH (2016) p53 signaling is involved in leptin-induced growth of hepatic and breast cancer cells. Korean J Physiol Pharmacol 20:487–498. https://doi.org/10.4196/kjpp.2016.20.5.487
Turk V, Turk B, Turk D (2001) Lysosomal cysteine proteases: facts and opportunities. EMBO J 20:4629–4633. https://doi.org/10.1093/emboj/20.17.4629
Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, Peters C, Reinheckel T (2006) Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res 66:5242–5250. https://doi.org/10.1158/0008-5472.CAN-05-4463
Yadati T, Houben T, Bitorina A, Shiri-Sverdlov R (2020) The ins and outs of cathepsins: physiological function and role in disease management. Cells. https://doi.org/10.3390/cells9071679
Yim WW, Mizushima N (2020) Lysosome biology in autophagy. Cell Discov 6:6. https://doi.org/10.1038/s41421-020-0141-7
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432. https://doi.org/10.1038/372425a0
Acknowledgements
This work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2021R1A2C1013132) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03044512). The authors thank the Ministry of Education and the Basic Science Research Program of the National Research Foundation of Korea (NRF) for financial support; the Core Research Support Center for Natural Products and Medical Materials (CRCNM) for the technical support regarding the confocal microscopic analysis.
Funding
This work was supported by National research foundation of Korea (Grant Nos. NRF-2021R1A2C1013132, 2020R1A6A1A03044512).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, T., Kumar, R.P. & Park, PH. Cathepsin B maturation plays a critical role in leptin-induced hepatic cancer cell growth through activation of NLRP3 inflammasomes. Arch. Pharm. Res. 46, 160–176 (2023). https://doi.org/10.1007/s12272-023-01437-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-023-01437-2