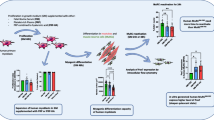
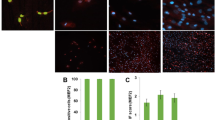
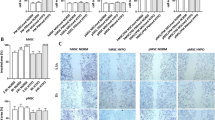
Abstract
Satellite cell-mediated skeletal muscle repair/regeneration is compromised in cases of extended damage. Bone marrow mesenchymal stromal cells (BM-MSCs) hold promise for muscle healing but some criticisms hamper their clinical application, including the need to avoid animal serum contamination for expansion and the scarce survival after transplant. In this context, platelet-rich plasma (PRP) could offer advantages. Here, we compare the effects of PRP or standard culture media on C2C12 myoblast, satellite cell and BM-MSC viability, survival, proliferation and myogenic differentiation and evaluate PRP/BM-MSC combination effects in promoting myogenic differentiation. PRP induced an increase of mitochondrial activity and Ki67 expression comparable or even greater than that elicited by standard media and promoted AKT signaling activation in myoblasts and BM-MSCs and Notch-1 pathway activation in BM-MSCs. It stimulated MyoD, myogenin, α-sarcomeric actin and MMP-2 expression in myoblasts and satellite cell activation. Notably, PRP/BM-MSC combination was more effective than PRP alone. We found that BM-MSCs influenced myoblast responses through a paracrine activation of AKT signaling, contributing to shed light on BM-MSC action mechanisms. Our results suggest that PRP represents a good serum substitute for BM-MSC manipulation in vitro and could be beneficial towards transplanted cells in vivo. Moreover, it might influence muscle resident progenitors’ fate, thus favoring the endogenous repair/regeneration mechanisms. Finally, within the limitations of an in vitro experimentation, this study provides an experimental background for considering the PRP/BM-MSC combination as a potential therapeutic tool for skeletal muscle damage, combining the beneficial effects of BM-MSCs and PRP on muscle tissue, while potentiating BM-MSC functionality.










Similar content being viewed by others
References
Alsousou J, Thompson M, Harrison P, Willett K, Franklin S (2015) Effect of platelet-rich plasma on healing tissues in acute ruptured Achilles tendon: a human immunohistochemistry study. Lancet 385(Suppl 1):S19
Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Corrêa do Amaral RJ, Granjeiro JM, Borojevic R (2013) Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. https://doi.org/10.1186/scrt218
Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R (2014) Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented media. PLoS ONE 9:e104662. https://doi.org/10.1371/journal.pone.0104662
Anderson JE (2016) Hepatocyte growth factor and satellite cell activation. Adv Exp Med Biol 900:1–25
Andia I, Abate M (2015) Platelet-rich plasma in the treatment of skeletal muscle injuries. Expert Opin Biol Ther 15:987–999
Anitua E, Pelacho B, Prado R, Aguirre JJ, Sánchez M, Padilla S, Aranguren XL, Abizanda G, Collantes M, Hernandez M, Perez-Ruiz A, Peñuelas I, Orive G, Prosper F (2015) Infiltration of plasma rich in growth factors enhances in vivo angiogenesis and improves reperfusion and tissue remodeling after severe hind limb ischemia. J Control Release 202:31–39
Balthasar S, Bergelin N, Löf C, Vainio M, Andersson S, Törnquist K (2008) Interactions between sphingosine-1-phosphate and vascular endothelial growth factor signalling in ML-1 follicular thyroid carcinoma cells. Endocr Relat Cancer 15:521–534
Bashir J, Sherman A, Lee H, Kaplan L, Hare JM (2014) Mesenchymal stem cell therapies in the treatment of musculoskeletal diseases. PM R 6:61–69
Bei Y, Wang F, Yang C, Xiao J (2015) Telocytes in regenerative medicine. J Cell Mol Med 19:1441–1454
Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA (2013) Cellular dynamics in the muscle satellite cell niche. EMBO Rep 14:1062–1072
Bojin FM, Gavriliuc O, Cristea M, Tanasie G, Tatu CS, Panaitescu C, Paunescu V (2011) Telocytes within human skeletal muscle stem cell niche. J Cell Mol Med 15:2269–2272
Cassano M, Dellavalle A, Tedesco FS, Quattrocelli M, Crippa S, Ronzoni F, Salvade A, Berardi E, Torrente Y, Cossu G, Sampaolesi M (2011) Alpha sarcoglycan is required for FGF-dependent myogenic progenitor cell proliferation in vitro and in vivo. Development 138:4523–4533
Cavallo C, Roffi A, Grigolo B, Mariani E, Pratelli L, Merli G, Kon E, Marcacci M, Filardo G (2016) Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed Res Int 2016:6591717
Chen X, Li Y (2009) Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adhes Migr 3:337–341
Chen J, Crawford R, Chen C, Xiao Y (2013) The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng B Rev 19:516–528
Chen PY, Qin L, Li G, Tellides G, Simons M (2016) Fibroblast growth factor (FGF) signaling regulates transforming growth factor beta (TGFβ)-dependent smooth muscle cell phenotype modulation. Sci Rep. https://doi.org/10.1038/srep33407
Chumanevich A, Wedman P, Oskeritzian CA (2016) Sphingosine-1-phosphate/Sphingosine-1-phosphate receptor 2 Axis can promote mouse and human primary mast cell Angiogenic potential through Upregulation of vascular endothelial growth factor-a and matrix Metalloproteinase-2. Mediat Inflamm. https://doi.org/10.1155/2016/1503206
Costamagna D, Berardi E, Ceccarelli G, Sampaolesi M (2015) Adult Stem Cells and Skeletal Muscle Regeneration. Curr Gene Ther 15:348–363
De Becker A, Riet IV (2016) Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem Cells 8:73–87
Deasy BM, Feduska JM, Payne TR, Li Y, Ambrosio F, Huard J (2009) Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther 17:1788–1798
Dimauro I, Grasso L, Fittipaldi S, Fantini C, Mercatelli N, Racca S, Geuna S, Di Gianfrancesco A, Caporossi D, Pigozzi F, Borrione P (2014) Platelet-rich plasma and skeletal muscle healing: a molecular analysis of the early phases of the regeneration process in an experimental animal model. PLoS ONE. https://doi.org/10.1371/journal.pone.0102993
Duan C, Ren H, Gao S (2010) Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol 167:344–351
Dumont NA, Wang YX, Rudnicki MA (2015) Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 142:1572–1581
Farup J, Madaro L, Puri PL, Mikkelsen UR (2015) Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis. https://doi.org/10.1038/cddis.2015.198
Ferrari G, Pintucci G, Seghezzi G, Hyman K, Galloway AC, Mignatti P (2006) VEGF, a prosurvival factor, acts in concert with TGF-beta1 to induce endothelial cell apoptosis. Proc Natl Acad Sci U S A 103:17260–17265
Fieber CB, Eldridge J, Taha TA, Obeid LM, Muise-Helmericks RC (2006) Modulation of total Akt kinase by increased expression of a single isoform: requirement of the sphingosine-1-phosphate receptor, Edg3/S1P3, for the VEGF-dependent expression of Akt3 in primary endothelial cells. Exp Cell Res 312:1164–1173
Formigli L, Benvenuti S, Mercatelli R, Quercioli F, Tani A, Mirabella C, Dama A, Saccardi R, Mazzanti B, Cellai I, Zecchi-Orlandini S (2012) Dermal matrix scaffold engineered with adult mesenchymal stem cells and platelet-rich plasma as a potential tool for tissue repair and regeneration. J Tissue Eng Regen Med 6:125–134
Formigli L, Paternostro F, Tani A, Mirabella C, Quattrini Li A, Nosi D, D’Asta F, Saccardi R, Mazzanti B, Lo Russo G, Zecchi-Orlandini S (2015) MSCs seeded on bioengineered scaffolds improve skin wound healing in rats. Wound Repair Regen 23:115–123
Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V (2017) Concise review: the use of adipose-derived Stromal vascular fraction cells and platelet rich plasma in regenerative plastic surgery. Stem Cells 35:117–134
Giannelli M, Chellini F, Sassoli C, Francini F, Pini A, Squecco R, Nosi D, Bani D, Zecchi-Orlandini S, Formigli L (2013) Photoactivation of bone marrow mesenchymal stromal cells with diode laser: effects and mechanisms of action. J Cell Physiol 228:172–181
Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M (2006) The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res 17:212–219
Gude NA, Emmanuel G, Wu W, Cottage CT, Fischer K, Quijada P, Muraski JA, Alvarez R, Rubio M, Schaefer E, Sussman MA (2008) Activation of notch-mediated protective signaling in the myocardium. Circ Res 102:1025–1035
Guevara-Alvarez A, Schmitt A, Russell RP, Imhoff AB, Buchmann S (2014) Growth factor delivery vehicles for tendon injuries: Mesenchymal stem cells and platelet rich plasma. Muscles Ligaments Tendons J 4:378–385
Guillodo Y, Madouas G, Simon T, Le Dauphin H, Saraux A (2016) Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J 5:284–288
Guo D, Ye J, Dai J, Li L, Chen F, Ma D, Ji C (2009) Notch-1 regulates Akt signaling pathway and the expression of cell cycle regulatory proteins cyclin D1, CDK2 and p21 in T-ALL cell lines. Leuk Res 33:678–685
Hamid MS, Yusof A, Mohamed Ali MR (2014) Platelet-rich plasma (PRP) for acute muscle injury: a systematic review. PLoS ONE. https://doi.org/10.1371/journal.pone.0090538
Han JH, Zhou W, Li W, Tuan PQ, Khoi NM, Thuong PT, Na M, Myung CS (2015) Pentacyclic Triterpenoids from Astilbe Rivularis that enhance glucose uptake via the activation of Akt and Erk1/2 in C2C12 Myotubes. J Nat Prod 78:1005–1014
Héron-Milhavet L, Mamaeva D, Rochat A, Lamb NJ, Fernandez A (2008) Akt2 is implicated in skeletal muscle differentiation and specifically binds Prohibitin2/REA. J Cell Physiol 214:158–165
Hoeferlin LA, Huynh QK, Mietla JA, Sell SA, Tucker J, Chalfant CE, Wijesinghe DS (2015) The lipid portion of activated platelet-rich plasma significantly contributes to its wound healing properties. Adv Wound Care 4:100–109
Hosny N, Goubran F, BadrEldin Hasan B, Kamel N (2015) Assessment of vascular endothelial growth factor in fresh versus frozen platelet rich plasma. J Blood Transfus. https://doi.org/10.1155/2015/706903
Hwang SY, Kang YJ, Sung B, Kim M, Kim DH, Lee Y, Yoo MA, Kim CM, Chung HY, Kim ND (2015) Folic acid promotes the myogenic differentiation of C2C12 murine myoblasts through the Akt signaling pathway. Int J Mol Med 36:1073–1080
Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T (2003) VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci U S A 100:10664–10669
Jalowiec JM, D’Este M, Bara JJ, Denom J, Menzel U, Alini M, Verrier S, Herrmann M (2016) An in vitro investigation of platelet-rich plasma-gel as a cell and growth factor delivery vehicle for tissue engineering. Tissue Eng C 22:49–58
Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S (2015) Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med 43:2102–2110
Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM (2010) Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12:153–163
Judson RN, Zhang RH, Rossi FM (2013) Tissue-resident mesenchymal stem/progenitor cells in skeletal muscle: collaborators or saboteurs? FEBS J 280:4100–4108
Kasten P, Vogel J, Luginbühl R, Niemeyer P, Weiss S, Schneider S, Kramer M, Leo A, Richter W (2006) Influence of platelet-rich plasma on osteogenic differentiation of mesenchymal stem cells and ectopic bone formation in calcium phosphate ceramics. Cells Tissues Organs 183:68–79
Kelc R, Trapecar M, Gradisnik L, Rupnik MS, Vogrin M (2015) Platelet-rich plasma, especially when combined with a TGF-β inhibitor promotes proliferation, viability and myogenic differentiation of myoblasts in vitro. PLoS ONE. https://doi.org/10.1371/journal.pone.0117302
Kobayashi Y, Saita Y, Nishio H, Ikeda H, Takazawa Y, Nagao M, Takaku T, Komatsu N, Kaneko K (2016) Leukocyte concentration and composition in platelet-rich plasma (PRP) influences the growth factor and protease concentrations. J Orthop Sci 21:683–689
Kobolak J, Dinnyes A, Memic A, Khademhosseini A, Mobasheri A (2016) Mesenchymal stem cells: identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 99:62–68
Konala VB, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R (2016) The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration. Cytotherapy 18:13–24
Kong Y, Wang H, Lin T, Wang S (2014) Sphingosine-1-phosphate/S1P receptors signaling modulates cell migration in human bone marrow-derived mesenchymal stem cells. Mediat Inflamm. https://doi.org/10.1155/2014/565369
Krishnan S, Szabo E, Burghardt I, Frei K, Tabatabai G, Weller M (2015) Modulation of cerebral endothelial cell function by TGF-β in glioblastoma: VEGF-dependent angiogenesis versus endothelial mesenchymal transition. Oncotarget 6:22480–22495
Kuang S, Kuroda K, Le Grand F, Rudnicki MA (2007) Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129:999–1010
Li H, Usas A, Poddar M, Chen CW, Thompson S, Ahani B, Cummins J, Lavasani M, Huard J (2013) Platelet-rich plasma promotes the proliferation of human muscle derived progenitor cells and maintains their stemness. PLoS ONE. https://doi.org/10.1371/journal.pone.0064923
Li H, Hicks JJ, Wang L, Oyster N, Philippon MJ, Hurwitz S, Hogan MV, Huard J (2016a) Customized platelet-rich plasma with transforming growth factor β1 neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials 87:147–156
Li L, Zhang J, Xiong N, Li S, Chen Y, Yang H, Wu C, Zeng H, Liu Y (2016b) Notch-1 signaling activates NF-κB in human breast carcinoma MDA-MB-231 cells via PP2A-dependent AKT pathway. Med Oncol. https://doi.org/10.1007/s12032-016-0747-7
Liu Y, Schneider MF (2014) FGF2 activates TRPC and ca (2+) signaling leading to satellite cell activation. Front Physiol. https://doi.org/10.3389/fphys.2014.00038
Liu LY, Hou YS, Chai JK, Hu Q, Duan HJ, Yu YH, Yin HN, Hao DF, Feng G, Li T, Du JD (2013) Basic fibroblast growth factor/vascular endothelial growth factor in the serum from severe burn patients stimulates the proliferation of cultured human umbilical cord mesenchymal stem cells via activation of notch signaling pathways. J Trauma Acute Care Surg 75:789–797
Liu S, Gao F, Wen L, Ouyang M, Wang Y, Wang Q, Luo L, Jian Z (2017) Osteocalcin induces proliferation via positive activation of the PI3K/Akt, P38 MAPK pathways and promotes differentiation through activation of the GPRC6A-ERK1/2 pathway in C2C12 Myoblast cells. Cell Physiol Biochem 43:1100–1112
Lluri G, Jaworski DM (2005) Regulation of TIMP-2, MT1-MMP, and MMP-2 expression during C2C12 differentiation. Muscle Nerve 32:492–499
Lu W, Xiu X, Zhao Y, Gui M (2015) Improved proliferation and differentiation of bone marrow Mesenchymal stem cells into vascular endothelial cells with Sphingosine 1-phosphate. Transplant Proc 47:2035–2040
Lubkowska A, Dolegowska B, Banfi G (2012) Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents 26:3S–22S
Manon-Jensen T, Multhaupt HA, Couchman JR (2013) Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J 280:2320–2331
Martinello T, Bronzini I, Perazzi A, Testoni S, De Benedictis GM, Negro A, Caporale G, Mascarello F, Iacopetti I, Patruno M (2013) Effects of in vivo applications of peripheral blood-derived mesenchymal stromal cells (PB-MSCs) and platlet-rich plasma (PRP) on experimentally injured deep digital flexor tendons of sheep. J Orthop Res 31:306–314
Marycz K, Śmieszek A, Jeleń M, Chrząstek K, Grzesiak J, Meissner J (2015) The effect of the bioactive sphingolipids S1P and C1P on multipotent stromal cells - new opportunities in regenerative medicine. Cell Mol Biol Lett 20:510–533
McClure MJ, Garg K, Simpson DG, Ryan JJ, Sell SA, Bowlin GL, Ericksen JJ (2016) The influence of platelet-rich plasma on myogenic differentiation. J Tissue Eng Regen Med 10:E239–E249
Miyazaki D, Nakamura A, Fukushima K, Yoshida K, Takeda S, Ikeda S (2011) Matrix metalloproteinase-2 ablation in dystrophin-deficient mdx muscles reduces angiogenesis resulting in impaired growth of regenerated muscle fibers. Hum Mol Genet 20:1787–1799
Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC (2014) Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD010071.pub3
Mosca MJ, Rodeo SA (2015) Platelet-rich plasma for muscle injuries: game over or time out? Curr Rev Musculoskelet Med 8:145–153
Nagura Y, Tsuno NH, Kano K, Inoue A, Aoki J, Hirowatari Y, Kaneko M, Kurano M, Matsuhashi M, Ohkawa R, Tozuka M, Yatomi Y, Okazaki H (2016) Regulation of the lysophosphatidylserine and sphingosine 1-phosphate levels in autologous whole blood by the pre-storage leukocyte reduction. Transfus Med 26:365–372
Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, Akimoto T, Higashi Y, Ochi M (2015) Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett 589:1257–1265
Navani A, Li G, Chrystal J (2017) Platelet rich plasma in musculoskeletal pathology: a necessary rescue or a lost cause? Pain Physician 20:E345–E356
Niemeyer P, Fechner K, Milz S, Richter W, Suedkamp NP, Mehlhorn AT, Pearce S, Kasten P (2010) Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials 31:3572–3579
Nishimura T, Nakamura K, Kishioka Y, Kato-Mori Y, Wakamatsu J, Hattori A (2008) Inhibition of matrix metalloproteinases suppresses the migration of skeletal muscle cells. J Muscle Res Cell Motil 29:37–44
Oh J, Takahashi R, Adachi E, Kondo S, Kuratomi S, Noma A, Alexander DB, Motoda H, Okada A, Seiki M, Itoh T, Itohara S, Takahashi C, Noda M (2004) Mutations in two matrix metalloproteinase genes, MMP-2 and MT1-MMP, are synthetic lethal in mice. Oncogene 23:5041–5048
Ohashi K, Nagata Y, Wada E, Zammit PS, Shiozuka M, Matsuda R (2015) Zinc promotes proliferation and activation of myogenic cells via the PI3K/Akt and ERK signaling cascade. Exp Cell Res 333:228–237
Ohtake Y, Tojo H, Seiki M (2006) Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J Cell Sci 119:3822–3832
Patruno M, Martinello T (2014) Treatments of the injured tendon in veterinary medicine: from scaffolds to adult stem cells. Histol Histopathol 29:417–422
Popescu LM, Manole E, Serboiu CS, Manole CG, Suciu LC, Gherghiceanu M, Popescu BO (2011) Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med 15:1379–1392
Reurink G, Goudswaard GJ, Moen MH, Weir A, Verhaar JA, Bierma-Zeinstra SM, Maas M, Tol JL (2014) Platelet-rich plasma injections in acute muscle injury. N Engl J Med 370:2546–2547
Rønning SB, Carlson CR, Stang E, Kolset SO, Hollung K, Pedersen ME (2015) Syndecan-4 regulates muscle differentiation and is internalized from the plasma membrane during Myogenesis. PLoS ONE. https://doi.org/10.1371/journal.pone.0129288
Rubio-Azpeitia E, Andia I (2014) Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J 4:52–62
Ryu JM, Baek YB, Shin MS, Park JH, Park SH, Lee JH, Han HJ (2014) Sphingosine-1-phosphate-induced Flk-1 transactivation stimulates mouse embryonic stem cell proliferation through S1P1/S1P3-dependent β-arrestin/c-Src pathways. Stem Cell Res 12:69–85
San Sebastian KM, Lobato I, Hernández I, Burgos-Alonso N, Gomez-Fernandez MC, López JL, Rodríguez B, March AG, Grandes G, Andia I (2014) Efficacy and safety of autologous platelet rich plasma for the treatment of vascular ulcers in primary care: phase III study. BMC Fam Pract. https://doi.org/10.1186/s12875-014-0211-8
Sartori R, Gregorevic P, Sandri M (2014) TGFβ and BMP signaling in skeletal muscle: potential significance for muscle-related disease. Trends Endocrinol Metab 25:464–471
Sassoli C, Formigli L, Bini F, Tani A, Squecco R, Battistini C, Zecchi-Orlandini S, Francini F, Meacci E (2011a) Effects of S1P on skeletal muscle repair/regeneration during eccentric contraction. J Cell Mol Med 15:2498–2511
Sassoli C, Pini A, Mazzanti B, Quercioli F, Nistri S, Saccardi R, Zecchi-Orlandini S, Bani D, Formigli L (2011b) Mesenchymal stromal cells affect cardiomyocyte growth through juxtacrine Notch-1/Jagged-1 signaling and paracrine mechanisms: clues for cardiac regeneration. J Mol Cell Cardiol 51:399–408
Sassoli C, Pini A, Chellini F, Mazzanti B, Nistri S, Nosi D, Saccardi R, Quercioli F, Zecchi-Orlandini S, Formigli L (2012a) Bone marrow mesenchymal stromal cells stimulate skeletal myoblast proliferation through the paracrine release of VEGF. PLoS ONE. https://doi.org/10.1371/journal.pone.0037512
Sassoli C, Zecchi-Orlandini S, Formigli L (2012b) Trophic actions of bone marrow-derived mesenchymal stromal cells for muscle repair/regeneration. Cell 1:832–850
Sassoli C, Frati A, Tani A, Anderloni G, Pierucci F, Matteini F, Chellini F, Zecchi Orlandini S, Formigli L, Meacci E (2014a) Mesenchymal stromal cell secreted sphingosine 1-phosphate (S1P) exerts a stimulatory effect on skeletal myoblast proliferation. PLoS ONE. https://doi.org/10.1371/journal.pone.0108662
Sassoli C, Nosi D, Tani A, Chellini F, Mazzanti B, Quercioli F, Zecchi-Orlandini S, Formigli L (2014b) Defining the role of mesenchymal stromal cells on the regulation of matrix metalloproteinases in skeletal muscle cells. Exp Cell Res 323:297–313
Sassoli C, Chellini F, Squecco R, Tani A, Idrizaj E, Nosi D, Giannelli M, Zecchi-Orlandini S (2016) Low intensity 635 nm diode laser irradiation inhibits fibroblast-myofibroblast transition reducing TRPC1 channel expression/activity: new perspectives for tissue fibrosis treatment. Lasers Surg Med 48:318–332
Sassoli C, Pierucci F, Tani A, Frati A, Chellini F, Matteini F, Vestri A, Anderloni G, Nosi D, Zecchi Orlandini S, Meacci E (2018) Sphingosine 1-phopsphate receptor 1 is required for Mmp-2 function in bone marrow-mesenchymal stromal cells: implications for cytoskeleton assembly and proliferation. Stem Cells Int. (in press)
Serrano AL, Muñoz-Cánoves P (2010) Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 316:3050–3058
Sun HY, Wei SP, Xu RC, Xu PX, Zhang WC (2010) Sphingosine-1-phosphate induces human endothelial VEGF and MMP-2 production via transcription factor ZNF580: novel insights into angiogenesis. Biochem Biophys Res Commun 395:361–366
Suthar M, Gupta S, Bukhari S, Ponemone V (2017) Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci. https://doi.org/10.1186/s12929-017-0324-1
Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB (2009) Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell 4:217–225
Tanimoto T, Jin ZG, Berk BC (2002) Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS). J Biol Chem 277:42997–43001
Terada S, Ota S, Kobayashi M, Kobayashi T, Mifune Y, Takayama K, Witt M, Vadalà G, Oyster N, Otsuka T, Fu FH, Huard J (2013) Use of an antifibrotic agent improves the effect of platelet-rich plasma on muscle healing after injury. J Bone Joint Surg Am 95:980–988
Thomas K, Engler AJ, Meyer GA (2015) Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res 56:1–8
Tierney MT, Sacco A (2016) Satellite Cell Heterogeneity in Skeletal Muscle Homeostasis. Trends Cell Biol 26:434–444
Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musarò A, Rosenthal N (2015) Monocyte/macrophage-derived IGF-1 orchestrates Murine skeletal muscle regeneration and modulates Autocrine polarization. Mol Ther 23:1189–1200
Tonti GA, Mannello F (2008) From bone marrow to therapeutic applications: different behaviour and genetic/epigenetic stability during mesenchymal stem cell expansion in autologous and foetal bovine sera? Int J Dev Biol 52:1023–1032
Tsai WC, Yu TY, Lin LP, Lin MS, Tsai TT, Pang J (2017) Platelet rich plasma promotes skeletal muscle cell migration in association with up-regulation of FAK, paxillin, and F-Actin formation. J Orthop Res. https://doi.org/10.1002/jor.23547
van den Dolder J, Mooren R, Vloon AP, Stoelinga PJ, Jansen JA (2006) Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng 12:3067–3073
Velleman SG, Song Y (2017) Development and growth of the avian Pectoralis major (breast) muscle: function of Syndecan-4 and Glypican-1 in adult Myoblast proliferation and differentiation. Front Physiol. https://doi.org/10.3389/fphys.2017.00577
von Bahr L, Sundberg B, Lönnies L, Sander B, Karbach H, Hägglund H, Ljungman P, Gustafsson B, Karlsson H, Le Blanc K, Ringdén O (2012) Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant 18:557–564
Walker N, Kahamba T, Woudberg N, Goetsch K, Niesler C (2015) Dose-dependent modulation of myogenesis by HGF: implications for c-Met expression and downstream signalling pathways. Growth Factors 33:229–241
Xu Q, Wu Z (2000) The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J Biol Chem 275:36750–36757
Yang L, Chang N, Liu X, Han Z, Zhu T, Li C, Yang L, Li L (2012) Bone marrow-derived mesenchymal stem cells differentiate to hepatic myofibroblasts by transforming growth factor-β1 via sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis. Am J Pathol 181:85–97
Zhang J, Middleton KK, Fu FH, Im HJ, Wang JH (2013) HGF mediates the anti-inflammatory effects of PRP on injured tendons. PLoS ONE. https://doi.org/10.1371/journal.pone.0067303
Zhao N, Guo Y, Zhang M, Lin L, Zheng Z (2010) Akt-mTOR signaling is involved in Notch-1-mediated glioma cell survival and proliferation. Oncol Rep 23:1443–1447
Acknowledgements
The authors are grateful to Dott. Benedetta Mazzanti (Department of Experimental and Clinical Medicine - Section of Hematology, University of Florence, Italy) for having kindly provided BM-MSCs and to Dott. Carlo Mirabella (Immunohaematology and Transfusion Medicine Unit of the University Hospital of Careggi, Florence, Italy) for PRP preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human partecipants and/or animals-ethical approval
All procedures performed in studies involving animals were in accordance with the European Community guidelines for animal care (DL 116/92, application of the European Communities Council Directive of 24 November 1986; 86/609/EEC) and approved by the Committee for Animal Care and Experimental Use of the University of Florence - No. A5278–01. The protocols were communicated to local authorities and to the Italian Ministry of the Health; according to Italian law (Art.7/D.lgs 116/92) such a procedure does not require Ministry authorization. PRP was obtained at the Immunohaematology and Transfusion Medicine Unit of the University Hospital of Careggi (Florence) from the whole blood of adult healthy volunteers after receiving an informed consent and was provided in ready-to-use aliquots classified as not suitable for transfusion-infusion purposes. Its use in experimental in vitro protocols does not require Ethical Committee’s approval.
Rights and permissions
About this article
Cite this article
Sassoli, C., Vallone, L., Tani, A. et al. Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: new therapeutic perspectives for skeletal muscle repair/regeneration. Cell Tissue Res 372, 549–570 (2018). https://doi.org/10.1007/s00441-018-2792-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2792-3