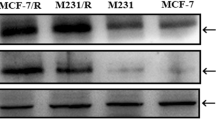
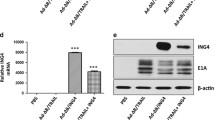
Abstract
Purpose
Targeted oncolytic adenoviruses capable of replication selectively in cancer cells are an appealing approach for the treatment of various cancer types refractory to conventional therapies. The aim of this study was to evaluate the effect of Ad5/3MDR1E1, a multidrug resistance gene 1 (MDR1)-targeted fiber-modified replication-competent adenovirus for the therapy of platinum-pretreated ovarian cancer in combination with cytostatic agents.
Methods
MDR1-specific tumor cell killing of Ad5/3MDR1E1 was systematically evaluated in chemotherapy naïve and pretreated ovarian cancer cells in vitro. Combinations of Ad5/3MDR1E1 and cytostatic agents were studied in vivo and in vitro. An in vivo hepatotoxicity model was used to evaluate liver toxicity.
Results
We demonstrate efficient oncolysis of Ad5/3MDR1E1 in chemotherapy-resistant ovarian cancer cells as well as therapeutic efficacy in an orthotopic mouse model. Further, combining Ad5/3MDR1E1 with paclitaxel resulted in greater therapeutic benefit than either agent alone.
Conclusion
These preclinical data suggest that a fiber-modified adenovirus vector under the control of the MDR1 promoter represents a promising treatment strategy for platinum-pretreated ovarian cancer as a single agent or in combination with conventional anticancer drugs.





Similar content being viewed by others
References
Annereau JP, Szakacs G, Tucker CJ, Arciello A, Cardarelli C, Collins J, Grissom S, Zeeberg BR, Reinhold W, Weinstein JN, Pommier Y, Paules RS, Gottesman MM (2004) Analysis of ATP-binding cassette transporter expression in drug-selected cell lines by a microarray dedicated to multidrug resistance. Mol Pharmacol 66(6):1397–1405
Bapat SA, Mali AM, Koppikar CB, Kurrey NK (2005) Stem and progenitor-like cells contribute to the aggressive behaviour of human epithelial ovarian cancer. Cancer Res 65(8):3025–3029. doi:10.1158/0008-5472.CAN-04-3931
Bauerschmitz GJ, Lam JT, Kanerva A, Suzuki K, Nettelbeck DM, Dmitriev I, Krasnykh V, Mikheeva GV, Barnes MN, Alvarez RD, Dall P, Alemany R, Curiel DT, Hemminki A (2002) Treatment of ovarian cancer with a tropism modified oncolytic adenovirus. Cancer Res 62(5):1266–1270
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Davydova J, Le LP, Gavrikova T, Wang M, Krasnykh V, Yamamoto M (2004) Infectivity-enhanced cyclooxygenase-2-based conditionally replicative adenoviruses for esophageal adenocarcinoma treatment. Cancer Res 64(12):4319–4327
Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT (1998) An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol 72(12):9706–9713
Douglas JT, Kim M, Sumerel LA, Carey DE, Curiel DT (2001) Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res 61(3):813–817
Gomez-Manzano C, Alonso MM, Yung WK, McCormick F, Curiel DT, Lang FF, Jiang H, Bekele BN, Zhou X, Alemany R, Fueyo J (2006) Delta-24 increases the expression and activity of topoisomerase I and enhances the antiglioma effect of irinotecan. Clin Cancer Res 12(2):556–562. doi:10.1158/1078-0432.CCR-05-1892
Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM (2003) A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res 9(2):555–561
Hoffmann D, Bangen JM, Bayer W, Wildner O (2006) Synergy between expression of fusogenic membrane proteins, chemotherapy and facultative virotherapy in colorectal cancer. Gene Ther 13(21):1534–1544. doi:10.1038/sj.gt.3302806
Huebner RJ, Rowe WP, Schatten WE, Smith RR, Thomas LB (1956) Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer 9(6):1211–1218
Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, Langford G, Murray N, Yla-Herttuala S (2004) AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther 10(5):967–972. doi:10.1016/j.ymthe.2004.08.002
Ingemarsdotter CK, Baird SK, Connell CM, Oberg D, Hallden G, McNeish IA (2010) Low-dose paclitaxel synergizes with oncolytic adenoviruses via mitotic slippage and apoptosis in ovarian cancer. Oncogene 29(45):6051–6063. doi:10.1038/onc.2010.335
Jakubczak JL, Ryan P, Gorziglia M, Clarke L, Hawkins LK, Hay C, Huang Y, Kaloss M, Marinov A, Phipps S, Pinkstaff A, Shirley P, Skripchenko Y, Stewart D, Forry-Schaudies S, Hallenbeck PL (2003) An oncolytic adenovirus selective for retinoblastoma tumor suppressor protein pathway-defective tumors: dependence on E1A, the E2F–1 promoter, and viral replication for selectivity and efficacy. Cancer Res 63(7):1490–1499
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics 2010. CA Cancer J Clin 60 (5):277–300. doi:10.3322/caac.20073
Jessen BA, Mullins JS, De Peyster A, Stevens GJ (2003) Assessment of hepatocytes and liver slices as in vitro test systems to predict in vivo gene expression. Toxicol Sci 75(1):208–222
Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, Barker SD, Straughn M, Barnes MN, Alvarez RD, Hemminki A, Curiel DT (2002a) Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res 8(1):275–280
Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM, Barnes MN, Alvarez RD, Siegal GP, Curiel DT, Hemminki A (2002b) Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther 5(6):695–704
Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG, Hakkarainen T, Bauerschmitz GJ, Wang M, Liu B, Cao Z, Alvarez RD, Curiel DT, Hemminki A (2003) Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther 8(3):449–458
Kanerva A, Raki M, Hemminki A (2007) Gene therapy of gynaecological diseases. Expert Opin Biol Ther 7(9):1347–1361. doi:10.1517/14712598.7.9.1347
Kaye SB (2008) Reversal of drug resistance in ovarian cancer: where do we go from here? J Clin Oncol 26(16):2616–2618. doi:10.1200/JCO.2008.16.2123
Lee WP, Tai DI, Tsai SL, Yeh CT, Chao Y, Lee SD, Hung MC (2003) Adenovirus type 5 E1A sensitizes hepatocellular carcinoma cells to gemcitabine. Cancer Res 63(19):6229–6236
Lu W, Zheng S, Li XF, Huang JJ, Zheng X, Li Z (2004) Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: a pilot phase II clinical trial. World J Gastroenterol 10(24):3634–3638
Lu S, Wang X, Xiao L, Cai L, Zhang Y, Wang H, Wang Z (2007) Gene therapy for ovarian cancer using adenovirus-mediated transfer of cytosine deaminase gene and uracil phosphoribosyltransferase gene directed by MDR1 promoter. Cancer Biol Ther 6(3):397–404
Mantwill K, Kohler-Vargas N, Bernshausen A, Bieler A, Lage H, Kaszubiak A, Surowiak P, Dravits T, Treiber U, Hartung R, Gansbacher B, Holm PS (2006) Inhibition of the multidrug-resistant phenotype by targeting YB-1 with a conditionally oncolytic adenovirus: implications for combinatorial treatment regimen with chemotherapeutic agents. Cancer Res 66(14):7195–7202. doi:10.1158/0008-5472.CAN-05-2339
Nelson AR, Davydova J, Curiel DT, Yamamoto M (2009) Combination of conditionally replicative adenovirus and standard chemotherapies shows synergistic antitumor effect in pancreatic cancer. Cancer Sci 100(11):2181–2187. doi:10.1111/j.1349-7006.2009.01289.x
Puumalainen AM, Vapalahti M, Agrawal RS, Kossila M, Laukkanen J, Lehtolainen P, Viita H, Paljarvi L, Vanninen R, Yla-Herttuala S (1998) Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther 9(12):1769–1774
Raki M, Kanerva A, Ristimaki A, Desmond RA, Chen DT, Ranki T, Sarkioja M, Kangasniemi L, Hemminki A (2005) Combination of gemcitabine and Ad5/3-Delta24, a tropism modified conditionally replicating adenovirus, for the treatment of ovarian cancer. Gene Ther 12(15):1198–1205. doi:10.1038/sj.gt.3302517
Raki M, Rein DT, Kanerva A, Hemminki A (2006) Gene transfer approaches for gynecological diseases. Mol Ther 14(2):154–163. doi:10.1016/j.ymthe.2006.02.019
Raki M, Sarkioja M, Desmond RA, Chen DT, Butzow R, Hemminki A, Kanerva A (2008) Oncolytic adenovirus Ad5/3-delta24 and chemotherapy for treatment of orthotopic ovarian cancer. Gynecol Oncol 108(1):166–172. doi:10.1016/j.ygyno.2007.09.013
Rein DT, Breidenbach M, Curiel DT (2006) Current developments in adenovirus-based cancer gene therapy. Future Oncol 2(1):137–143. doi:10.2217/14796694.2.1.137
Rein DT, Volkmer A, Beyer IM, Curiel DT, Janni W, Dragoi A, Hess AP, Maass N, Baldus SE, Bauerschmitz G, Breidenbach M (2011) Treatment of chemotherapy resistant ovarian cancer with a MDR1 targeted oncolytic adenovirus. Gynecol Oncol 123(1):138–146. doi:10.1016/j.ygyno.2011.06.007
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5(3):219–234. doi:10.1038/nrd1984
Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK (2006) Ovarian cancer side population defines cells with stem cell-like characteristics and mullerian inhibiting substance responsiveness. Proc Natl Acad Sci USA 103(30):11154–11159. doi:10.1073/pnas.0603672103
Vanderkwaak TJ, Wang M, Gomez-Navarro J, Rancourt C, Dmitriev I, Krasnykh V, Barnes M, Siegal GP, Alvarez R, Curiel DT (1999) An advanced generation of adenoviral vectors selectively enhances gene transfer for ovarian cancer gene therapy approaches. Gynecol Oncol 74(2):227–234
Wrighton SA, Ring BJ, VandenBranden M (1995) The use of in vitro metabolism techniques in the planning and interpretation of drug safety studies. Toxicol Pathol 23(2):199–208
Xia ZJ, Chang JH, Zhang L, Jiang WQ, Guan ZZ, Liu JW, Zhang Y, Hu XH, Wu GH, Wang HQ, Chen ZC, Chen JC, Zhou QH, Lu JW, Fan QX, Huang JJ, Zheng X (2004a) Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng 23(12):1666–1670. doi:1000467X2004121666
Xia ZJ, Chang JH, Zhang L, Jiang WQ, Guan ZZ, Liu JW, Zhang Y, Hu XH, Wu GH, Wang HQ, Chen ZC, Chen JC, Zhou QH, Lu JW, Fan QX, Huang JJ, Zheng X (2004b) Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng 23(12):1666–1670. doi:1000467X2004121666
Yadav S, van Vlerken LE, Little SR, Amiji MM (2009) Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother Pharmacol 63(4):711–722. doi:10.1007/s00280-008-0790-y
Yamamoto M, Davydova J, Wang M, Siegal GP, Krasnykh V, Vickers SM, Curiel DT (2003) Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology 125(4):1203–1218
You L, Yang CT, Jablons DM (2000) ONYX-015 works synergistically with chemotherapy in lung cancer cell lines and primary cultures freshly made from lung cancer patients. Cancer Res 60(4):1009–1013
Yu DC, Chen Y, Dilley J, Li Y, Embry M, Zhang H, Nguyen N, Amin P, Oh J, Henderson DR (2001) Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. Cancer Res 61(2):517–525
Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV (2003) Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets 3(1):1–19
Zeimet AG, Muller-Holzner E, Schuler A, Hartung G, Berger J, Hermann M, Widschwendter M, Bergelson JM, Marth C (2002) Determination of molecules regulating gene delivery using adenoviral vectors in ovarian carcinomas. Gene Ther 9(16):1093–1100
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rein, D.T., Volkmer, A., Bauerschmitz, G. et al. Combination of a MDR1-targeted replicative adenovirus and chemotherapy for the therapy of pretreated ovarian cancer. J Cancer Res Clin Oncol 138, 603–610 (2012). https://doi.org/10.1007/s00432-011-1135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-011-1135-5