Abstract
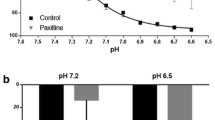
This study investigated the effects of changing the extracellular [Mg2+] ([Mg2+]o) on endothelin-1 (ET-1)-induced contraction of rabbit coronary artery smooth muscle and the involvement of non-selective cation (NSC) channels in this response. Increased [Mg2+]o shifted the concentration/contraction relationship curve of ET-1 to the right. In whole-cell patch clamp recordings, ET-1 (10−7 M) induced a long-lasting inwards current (94.7±7.2 pA) that was inhibited by 8 mM [Mg2+]o (45.3±4.4%) and NSC channel blockers (10−3 M streptomycin and 10−3 M La3+), but not by the voltage-dependent Ca2+ channel blocker nicardipine. The current/voltage (I/V) curve was linear. Furthermore, in pressurized arteries, the ET-1-induced contraction was also inhibited by La3+ and streptomycin, but not by nicardipine. U-73122, a selective phospholipase C (PLC) inhibitor and staurosporine and GF 109203X, which block protein kinase C (PKC), reduced ET-1-activated NSC currents by 54.2±5.1%, 60.3±5.5% and 48.5±2.9%, respectively. The inwards current was increased by 1-oleoyl-2-acetyl-sn-glycerol (OAG) and phorbol 12,13-dibutyrate (PDBu), which activate PKC selectively. Like transient receptor potential channel (TRPC3) currents, ET-1-activated NSC currents had a linear I/V relationship, were blocked by flufenamate and activated by a diacylglycerol analogue. These results suggest that [Mg2+]o blocks ET-1-induced contraction of coronary arteries by inhibiting NSC channels. Activation of PLC and PKC might be involved in activation of NSC channels.






Similar content being viewed by others
References
Albert AP, Large WA (2001) Comparison of spontaneous and noradrenaline-evoked non-selective cation channels in rabbit portal vein myocytes. J Physiol (Lond) 530:457–468
Albert AP, Piper AS, Large WA (2003) Properties of a constitutively active Ca2+-permeable non-selective cation channel in rabbit ear artery myocytes. J Physiol (Lond) 549:143–156
Altura BT, Altura BM (1980) Withdrawal of magnesium causes vasospasm while elevated magnesium produces relaxation of tone in cerebral arteries. Neurosci Lett 20:323–327
Altura BM, Turlapaty PD (1982) Withdrawal of magnesium enhances coronary arterial spasms produced by vasoactive agents. Br J Pharmacol 77:649–659
Bae YM, Park MK, Lee SH, Ho WK, Earm YE (1999) Contribution of Ca2+-activated K+ channels and non-selective cation channels to membrane potential of pulmonary arterial smooth muscle cells of the rabbit. J Physiol (Lond) 514:747–758
Bae M, Kim S, Ko E, Lee SH, Ho WK, Earm YE (2002) Intracellular Mg2+ hyperpolarizes rabbit coronary artery smooth muscle cells by differential modulation of Ca2+i-dependent ion channels. Pflugers Arch 444:523–531
Chen C, Wagoner PK (1991) Endothelin induces a nonselective cation current in vascular smooth muscle cells. Circ Res 69:447–454
Chen S, Inoue R, Ito Y (1993) Pharmacological characterization of muscarinic receptor-activated cation channels in guinea-pig ileum. Br J Pharmacol 109:793–801
D’Angelo EK, Singer HA, Rembold CM (1992) Magnesium relaxes arterial smooth muscle by decreasing intracellular Ca2+ without changing intracellular Mg2+. J Clin Invest 89:1988–1994
Dettmann ES, Luscher TF, Flammer J, Haefliger IO (1998) Modulation of endothelin-1-induced contractions by magnesium/calcium in porcine ciliary arteries. Graefes Arch Clin Exp Ophthalmol 236:47–51
Enoki T, Miwa S, Sakamoto A, Minowa T, Komuro T, Kobayashi S, Ninomiya H, Masaki T (1995) Long-lasting activation of cation current by low concentration of endothelin-1 in mouse fibroblasts and smooth muscle cells of rabbit aorta. Br J Pharmacol 115:479–485
Goto K, Kasuya Y, Matsuki N, Takuwa Y, Kurihara H, Ishikawa T, Kimura S, Yanagisawa M, Masaki T (1989) Endothelin activates the dihydropyridine-sensitive, voltage-dependent Ca2+ channel in vascular smooth muscle. Proc Natl Acad Sci USA 86:3915–3918
Görlach C, Benyo Z, Wahl M (1998) Endothelin-1-induced contraction in cerebral vessels mediated by phospholipase C/protein kinase C cascade. Kidney Int 54 (Suppl. 67):S224–S225
Helliwell RM, Large WA (1997) Alpha 1-adrenoceptor activation of a non-selective cation current in rabbit portal vein by 1,2-diacyl-sn-glycerol. J Physiol (Lond) 499:417–428
Hidaka H, Kobayashi R (1992) Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol 32:377–397
Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397:259–263
Howell RE, Carrier GO (1986) Influence of magnesium on norepinephrine-and histamine-induced contractions of pulmonary vascular smooth muscle. Pharmacology 33:27–33
Hu SL, Kim HS, Jeng AY (1991) Dual action of endothelin-1 on the Ca2+-activated K+ channel in smooth muscle cells of porcine coronary artery. Eur J Pharmacol 194:31–36
Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y (2001) The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88:325–332
Inoue Y, Oike M, Nakao K, Kitamura K, Kuriyama H (1990) Endothelin augments unitary calcium channel currents on the smooth muscle cell membrane of guinea-pig portal vein. J Physiol (Lond) 423:171–191
Jung S, Strotmann R, Schultz G, Plant TD (2002) TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol 282:C347–C359
Kamouchi M, Philipp S, Flockerzi V, Wissenbach U, Mamin A, Raeymaekers L, Eggermont J, Droogmans G, Nilius B (1999) Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells. J Physiol (Lond) 518:345–358
Kemp PA, Gardiner SM, Bennett T, Rubin PC (1993) Magnesium sulphate reverses the carotid vasoconstriction caused by endothelin-I, angiotensin II and neuropeptide-Y, but not that caused by NG-nitro-L-arginine methyl ester, in conscious rats. Clin Sci (Lond) 85:175–181
Kurihara H, Yoshizumi M, Sugiyama T, Yamaoki K, Nagai R, Takaku F, Satoh H, Inui J, Yanagisawa M, Masaki T (1989) The possible role of endothelin-1 in the pathogenesis of coronary vasospasm. J Cardiovasc Pharmacol 13:S132–S137; discussion S142
Large WA (2002) Receptor-operated Ca2+-permeable nonselective cation channels in vascular smooth muscle: a physiologic perspective. J Cardiovasc Electrophysiol 13:493–501
Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, Groschner K (2000) Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J Biol Chem 275:27799–27805
Luscher TF, Oemar BS, Boulanger CM, Hahn AW (1993) Molecular and cellular biology of endothelin and its receptors—Part II. J Hypertens 11:121–126
Meggio F, Donella Deana A, Ruzzene M, Brunati AM, Cesaro L, Guerra B, Meyer T, Mett H, Fabbro D, Furet P (1995) Different susceptibility of protein kinases to staurosporine inhibition. Kinetic studies and molecular bases for the resistance of protein kinase CK2. Eur J Biochem 234:317–322
Miyoshi Y, Nakaya Y, Wakatsuki T, Nakaya S, Fujino K, Saito K, Inoue I (1992) Endothelin blocks ATP-sensitive K+ channels and depolarizes smooth muscle cells of porcine coronary artery. Circ Res 70:612–616
Minami K, Hirata Y, Tokumura A, Nakaya Y, Fukuzawa K (1995) Protein kinase C-independent inhibition of the Ca2+-activated K+ channel by angiotensin II and endothelin-1. Biochem Pharmacol 49:1051–1056
Mordes JP, Wacker WE (1977) Excess magnesium. Pharmacol Rev 29:273–300
Nakajima T, Hazama H, Hamada E, Wu SN, Igarashi K, Yamashita T, Seyama Y, Omata M, Kurachi Y (1996) Endothelin-1 and vasopressin activate Ca2+-permeable non-selective cation channels in aortic smooth muscle cells: mechanism of receptor-mediated Ca2+ influx. J Mol Cell Cardiol 28:707–722
Nakajima T, Iwasawa K, Hazama H, Asano M, Okuda Y, Omata M (1997) Extracellular Mg2+ inhibits receptor-mediated Ca2+-permeable non-selective cation currents in aortic smooth muscle cells. Eur J Pharmacol 320:81–86
Ohya Y, Terada K, Yamaguchi K, Inoue R, Okabe K, Kitamura K, Hirata M, Kuriyama H (1988) Effects of inositol phosphates on the membrane activity of smooth muscle cells of the rabbit portal vein. Pflugers Arch 412:382–389
Ruegg UT, Burgess GM (1989) Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci 10:218–20
Schiffrin EL (2001) Role of endothelin-1 in hypertension and vascular disease. Am J Hypertens 14:83S–89S
Shimoda LA, Sylvester JT, Sham JS (1998) Inhibition of voltage-gated K+ current in rat intrapulmonary arterial myocytes by endothelin-1. Am J Physiol 274:L842–L853
Slish DF, Welsh DG, Brayden JE (2002) Diacylglycerol and protein kinase C activate cation channels involved in myogenic tone. Am J Physiol 283:H2196–H2201
Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE (1990) Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther 253:688–697
Sonna LA, Hirshman CA, Croxton TL (1996) Role of calcium channel blockade in relaxation of tracheal smooth muscle by extracellular Mg2+. Am J Physiol 271:L251–L257
Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE (2001) TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29:645–655
Thompson AK, Mostafapour SP, Denlinger LC, Bleasdale JE, Fisher SK (1991) The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells. A role for Gp in receptor compartmentation. J Biol Chem 266:23856–23862
Tolsa JF, Gao Y, Raj JU (1999) Developmental change in magnesium sulfate-induced relaxation of rabbit pulmonary arteries. J Appl Physiol 87:1589–1594
Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F (1991) The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266:15771–15781
Trebak M, Vazquez G, Bird GS, Putney JW Jr (2003) The TRPC3/6/7 subfamily of cation channels. Cell Calcium 33:451–461
Trepakova ES, Gericke M, Hirakawa Y, Weisbrod RM, Cohen RA, Bolotina VM (2001) Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J Biol Chem 276:7782–7790
Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332:411–415
Yanagihara N, Tachikawa E, Izumi F, Yasugawa S, Yamamoto H, Miyamoto E (1991) Staurosporine: an effective inhibitor for Ca2+/calmodulin-dependent protein kinase II. J Neurochem 56:294–298
Yang ZW, Gebrewold A, Nowakowski M, Altura BT, Altura BM (2000) Mg2+-induced endothelium-dependent relaxation of blood vessels and blood pressure lowering: role of NO. Am J Physiol 278:R628–R639
Zhang A, Cheng TP, Altura BT, Altura BM (1992) Extracellular magnesium regulates intracellular free Mg2+ in vascular smooth muscle cells. Pflugers Arch 421:391–393
Zhang A, Cheng TP, Wu XY, Altura BT, Altura BM (1997) Extracellular Mg2+ regulates intracellular Mg2+ and its subcellular compartmentation in fission yeast, Schizosaccharomyces pombe. Cell Mol Life Sci 53:69–72
Zubkov AY, Rollins KS, Parent AD, Zhang J, Bryan RM Jr (2000) Mechanism of endothelin-1-induced contraction in rabbit basilar artery. Stroke 31:526–533
Acknowledgements
This work was supported by BK21 Human Life Sciences of Ministry of Education and by National Research Laboratory Grant from Ministry of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ko, E.A., Park, W.S. & Earm, Y.E. Extracellular Mg2+ blocks endothelin-1-induced contraction through the inhibition of non-selective cation channels in coronary smooth muscle. Pflugers Arch - Eur J Physiol 449, 195–204 (2004). https://doi.org/10.1007/s00424-004-1319-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-004-1319-9