Abstract
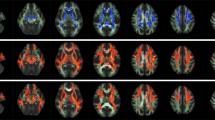
Leber’s hereditary optic neuropathy (LHON) is a mitochondrial disease characterized by retinal ganglion cell degeneration and optic nerve atrophy, leading to a loss of central vision. The aim of this study was to explore the topographical pattern of damage to the brain white matter (WM) tracts from patients with chronic LHON using diffusion tensor (DT) MRI and tract-based spatial statistics (TBSS). Brain dual-echo and DT MRI scans were acquired from 13 patients with chronic LHON and 25 matched controls using a 3.0 T scanner. TBSS analysis was performed using the FMRIB’s Diffusion Toolbox. A complete neuro-ophthalmologic examination, including standardized automated Humphrey perimetry as well as average and temporal peripapillary retinal nerve fiber layer thickness (PRNFL) measurements, was obtained in all patients. Mean average and temporal PRNFL thicknesses were decreased significantly in LHON patients. Compared to controls, TBSS analysis revealed significant diffusivity abnormalities in these patients, which were characterized by a decreased fractional anisotropy (FA) and an increased mean diffusivity and radial diffusivity, affecting exclusively the optic tracts and optic radiations (OR). In patients, a significant correlation was found between optic tract average FA and mean visual acuity (r = 0.57, p = 0.04). In LHON patients, DT MRI reveals a microstructural alteration of the WM along the entire visual pathways, with a sparing of the other main WM tracts of the brain. Damage to the OR may be secondary either to trans-synaptic degeneration, which in turn is due to neuroaxonal loss in the retina and optic nerve, or to local mitochondrial dysfunction.

Similar content being viewed by others
References
Audoin B, Fernando KT, Swanton JK, Thompson AJ, Plant GT, Miller DH (2006) Selective magnetization transfer ratio decrease in the visual cortex following optic neuritis. Brain 129:1031–1039
Barbiroli B, Montagna P, Cortelli P, Iotti S, Lodi R, Barboni P, Monari L, Lugaresi E, Frassineti C, Zaniol P (1995) Defective brain and muscle energy metabolism shown by in vivo 31P magnetic resonance spectroscopy in nonaffected carriers of 11778 mtDNA mutation. Neurology 45:1364–1369
Barcella V, Rocca MA, Bianchi-Marzoli S, Milesi J, Melzi L, Falini A, Pierro L, Filippi M (2010) Evidence for retro-chiasmatic tissue loss in Leber’s hereditary optic neuropathy. Hum Brain Mapp
Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254
Carelli V, Ross-Cisneros FN, Sadun AA (2004) Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res 23:53–89
Carelli V, Sadun AA (2001) Optic neuropathy in Lhon and Leigh syndrome. Ophthalmology 108:1172–1173
Catani M, Jones DK, Donato R, Ffytche DH (2003) Occipito-temporal connections in the human brain. Brain 126:2093–2107
Ciccarelli O, Toosy AT, Hickman SJ, Parker GJ, Wheeler-Kingshott CA, Miller DH, Thompson AJ (2005) Optic radiation changes after optic neuritis detected by tractography-based group mapping. Hum Brain Mapp 25:308–316
Concha L, Gross DW, Wheatley BM, Beaulieu C (2006) Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage 32:1090–1099
Cortelli P, Montagna P, Avoni P, Sangiorgi S, Bresolin N, Moggio M, Zaniol P, Mantovani V, Barboni P, Barbiroli B et al (1991) Leber’s hereditary optic neuropathy: genetic, biochemical, and phosphorus magnetic resonance spectroscopy study in an Italian family. Neurology 41:1211–1215
Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG (2002) Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 99:1017–1022
George R, Griffin JW (1994) Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp Neurol 129:225–236
Gupta N, Ang LC, Noel de Tilly L, Bidaisee L, Yucel YH (2006) Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol 90:674–678
Harding AE, Sweeney MG, Miller DH, Mumford CJ, Kellar-Wood H, Menard D, McDonald WI, Compston DA (1992) Occurrence of a multiple sclerosis-like illness in women who have a Leber’s hereditary optic neuropathy mitochondrial DNA mutation. Brain 115(Pt 4):979–989
Haselgrove JC, Moore JR (1996) Correction for distortion of echo-planar images used to calculate the apparent diffusion coefficient. Magn Reson Med 36:960–964
Howell N (1999) Human mitochondrial diseases: answering questions and questioning answers. Int Rev Cytol 186:49–116
Howell N (1998) Leber hereditary optic neuropathy: respiratory chain dysfunction and degeneration of the optic nerve. Vis Res 38:1495–1504
Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S (2008) Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39:336–347
Huoponen K, Vilkki J, Aula P, Nikoskelainen EK, Savontaus ML (1991) A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am J Hum Genet 48:1147–1153
Inglese M, Rovaris M, Bianchi S, Comi G, Filippi M (2001) Magnetization transfer and diffusion tensor MR imaging of the optic radiations and calcarine cortex from patients with Leber’s hereditary optic neuropathy. J Neurol Sci 188:33–36
Inglese M, Rovaris M, Bianchi S, La Mantia L, Mancardi GL, Ghezzi A, Montagna P, Salvi F, Filippi M (2001) Magnetic resonance imaging, magnetisation transfer imaging, and diffusion weighted imaging correlates of optic nerve, brain, and cervical cord damage in Leber’s hereditary optic neuropathy. J Neurol Neurosurg Psychiatry 70:444–449
Johns DR, Smith KH, Miller NR (1992) Leber’s hereditary optic neuropathy. Clinical manifestations of the 3460 mutation. Arch Ophthalmol 110:1577–1581
Kermode AG, Moseley IF, Kendall BE, Miller DH, MacManus DG, McDonald WI (1989) Magnetic resonance imaging in Leber’s optic neuropathy. J Neurol Neurosurg Psychiatry 52:671–674
Kwittken J, Barest HD (1958) The neuropathology of hereditary optic atrophy (Leber’s disease); the first complete anatomic study. Am J Pathol 34:185–207
Lodi R, Carelli V, Cortelli P, Iotti S, Valentino ML, Barboni P, Pallotti F, Montagna P, Barbiroli B (2002) Phosphorus MR spectroscopy shows a tissue specific in vivo distribution of biochemical expression of the G3460A mutation in Leber’s hereditary optic neuropathy. J Neurol Neurosurg Psychiatry 72:805–807
Mackey D, Howell N (1992) A variant of Leber hereditary optic neuropathy characterized by recovery of vision and by an unusual mitochondrial genetic etiology. Am J Hum Genet 51:1218–1228
Morrissey SP, Borruat FX, Miller DH, Moseley IF, Sweeney MG, Govan GG, Kelly MA, Francis DA, Harding AE, McDonald WI (1995) Bilateral simultaneous optic neuropathy in adults: clinical, imaging, serological, and genetic studies. J Neurol Neurosurg Psychiatry 58:70–74
Newman NJ (2002) From genotype to phenotype in Leber hereditary optic neuropathy: still more questions than answers. J Neuroophthalmol 22:257–261
Newman NJ, Lott MT, Wallace DC (1991) The clinical characteristics of pedigrees of Leber’s hereditary optic neuropathy with the 11778 mutation. Am J Ophthalmol 111:750–762
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25
Nikoskelainen EK, Marttila RJ, Huoponen K, Juvonen V, Lamminen T, Sonninen P, Savontaus ML (1995) Leber’s “plus”: neurological abnormalities in patients with Leber’s hereditary optic neuropathy. J Neurol Neurosurg Psychiatry 59:160–164
Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P (2001) Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 13:1174–1185
Sadun AA, Carelli V, Salomao SR, Berezovsky A, Quiros PA, Sadun F, DeNegri AM, Andrade R, Moraes M, Passos A, Kjaer P, Pereira J, Valentino ML, Schein S, Belfort R (2003) Extensive investigation of a large Brazilian pedigree of 11778/haplogroup J Leber hereditary optic neuropathy. Am J Ophthalmol 136:231–238
Sadun AA, Win PH, Ross-Cisneros FN, Walker SO, Carelli V (2000) Leber’s hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc 98:223–232 (discussion 232–225)
Shu N, Li J, Li K, Yu C, Jiang T (2009) Abnormal diffusion of cerebral white matter in early blindness. Hum Brain Mapp 30:220–227
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36:630–644
Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ 2nd, Nikoskelainen EK (1988) Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science 242:1427–1430
Wheeler-Kingshott CA, Cercignani M (2009) About “axial” and “radial” diffusivities. Magn Reson Med 61:1255–1260
Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N (2003) Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res 22:465–481
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milesi, J., Rocca, M.A., Bianchi-Marzoli, S. et al. Patterns of white matter diffusivity abnormalities in Leber’s hereditary optic neuropathy: a tract-based spatial statistics study. J Neurol 259, 1801–1807 (2012). https://doi.org/10.1007/s00415-011-6406-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6406-1