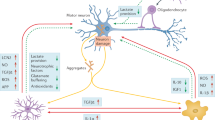
Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating progressive neurodegenerative disease affecting primarily the upper and lower motor neurons. A common feature of all ALS cases is a well-characterized neuroinflammatory reaction within the central nervous system (CNS). However, much less is known about the role of the peripheral immune system and its interplay with CNS resident immune cells in motor neuron degeneration. Here, we characterized peripheral monocytes in both temporal and spatial dimensions of ALS pathogenesis. We found the circulating monocytes to be deregulated in ALS regarding subtype constitution, function and gene expression. Moreover, we show that CNS infiltration of peripheral monocytes correlates with improved motor neuron survival in a genetic ALS mouse model. Furthermore, application of human immunoglobulins or fusion proteins containing only the human Fc, but not the Fab antibody fragment, increased CNS invasion of peripheral monocytes and delayed the disease onset. Our results underline the importance of peripheral monocytes in ALS pathogenesis and are in agreement with a protective role of monocytes in the early phase of the disease. The possibility to boost this beneficial function of peripheral monocytes by application of human immunoglobulins should be evaluated in clinical trials.






Similar content being viewed by others
References
Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW (2007) Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem 55:687–700. doi:10.1369/jhc.6A7156.2007
Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV (2007) Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10: 1538–1543. http://www.nature.com/neuro/journal/v10/n12/suppinfo/nn2014_S1.html. Accessed 19 Nov 2015
Appel SH, Zhao W, Beers DR, Henkel JS (2011) The microglial-motoneuron dialogue in ALS. Acta Myologica 30:4–8
Beers DR, Henkel JS, Zhao W, Wang J, Appel SH (2008) CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci USA 105:15558–15563. doi:10.1073/pnas.0807419105
Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G et al (2006) Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312:1389–1392. doi:10.1126/science.1123511
Bradley WG (2009) Updates on amyotrophic lateral sclerosis: improving patient care. Ann Neurol 65:S1–S2. doi:10.1002/ana.21546
Brettschneider J, Libon DJ, Toledo JB, Xie SX, McCluskey L, Elman L et al (2012) Microglial activation and TDP-43 pathology correlate with executive dysfunction in amyotrophic lateral sclerosis. Acta Neuropathol 123:395–407. doi:10.1007/s00401-011-0932-x
Brettschneider J, Toledo JB, Van Deerlin VM, Elman L, McCluskey L, Lee VMY et al (2012) Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS One 7:e39216. doi:10.1371/journal.pone.0039216
Bruhns P (2012) Properties of mouse and human IgG receptors and their contribution to disease models. Blood 119(24):5640–5649. doi:10.1182/blood-2012-01-380121
Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G et al (2012) Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest 122:3063–3087. doi:10.1172/JCI62636
Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G et al (2014) Identification of a unique TGF-β dependent molecular and functional signature in microglia. Nat Neurosci 17:131–143. doi:10.1038/nn.3599
Butovsky O, Jedrychowski MP, Cialic R, Krasemann S, Murugaiyan G, Fanek Z et al (2015) Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neurol 77:75–99. doi:10.1002/ana.24304
Chiu IM, Phatnani H, Kuligowski M, Tapia JC, Carrasco MA, Zhang M et al (2009) Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc Natl Acad Sci USA 106:20960–20965. doi:10.1073/pnas.0911405106
Chiu IM, Morimoto ETA, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP et al (2013) A Neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Reports 4:385–401. doi:10.1016/j.celrep.2013.06.018
Clay CC, Rodrigues DS, Ho YS, Fallert BA, Janatpour K, Reinhart TA et al (2007) Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J Virol 81:12040–12048. doi:10.1128/jvi.00133-07
Cleveland DW, Rothstein JD (2001) From charcot to lou gehrig: deciphering selective motor neuron death in als. Nat Rev Neurosci 2:806–819
Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B et al (2010) Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33:375–386. doi:10.1016/j.immuni.2010.08.012
D’Mello C, Le T, Swain MG (2009) Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factorα signaling during peripheral organ inflammation. J Neurosci 29:2089–2102. doi:10.1523/jneurosci.3567-08.2009
Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK et al. (2015) Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 185(10):2596–2606. doi: 10.1016/j.ajpath.2015.06.001
Davies LC, Jenkins SJ, Allen JE, Taylor PR (2013) Tissue-resident macrophages. Nat Immunol 14:986–995. doi:10.1038/ni.2705
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ et al (2011) Expanded GGGGCC hexanucleotide repeat in non-coding region of C9ORF72 causes chromosome 9p-linked frontotemporal dementia and amyotrophic lateral sclerosis. Neuron 72:245–256. doi:10.1016/j.neuron.2011.09.011
Delneste Y, Charbonnier P, Herbault N, Magistrelli G, Caron G, Bonnefoy JY et al (2003) Interferon-γ switches monocyte differentiation from dendritic cells to macrophages. Blood 101:143–150. doi:10.1182/blood-2002-04-1164
Freischmidt A, Müller K, Zondler L, Weydt P, Mayer B, von Arnim CAF et al (2015) Serum microRNAs in sporadic amyotrophic lateral sclerosis. Neurobiol Aging 36(9):2660.e15–20. doi: 10.1016/j.neurobiolaging.2015.06.003
Freischmidt A, Müller K, Zondler L, Weydt P, Volk AE, Božič AL et al (2014) Serum microRNAs in patients with genetic amyotrophic lateral sclerosis and pre-manifest mutation carriers. Brain pp 2938–2950. doi:10.1093/brain/awu249
Gao L, Brenner D, Llorens-Bobadilla E, Saiz-Castro G, Frank T, Wieghofer P et al (2015) Infiltration of circulating myeloid cells through CD95L contributes to neurodegeneration in mice. J Exp Med 212:469–480. doi:10.1084/jem.20132423
Ginhoux F, Jung S (2014) Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14:392–404. doi:10.1038/nri3671
Graber DJ, Hickey WF, Harris BT (2010) Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis. J Neuroinflammation 7:8. doi:10.1186/1742-2094-7-8
Grozdanov V, Bliederhaeuser C, Ruf WP, Roth V, Fundel-Clemens K, Zondler L et al (2014) Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol 128:651–663. doi:10.1007/s00401-014-1345-4
Gruzman A, Wood WL, Alpert E, Prasad MD, Miller RG, Rothstein JD et al (2007) Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 104:12524–12529. doi:10.1073/pnas.0705044104
Gurney M, Pu H, Chiu A, Dal Canto M, Polchow C, Alexander D et al (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264:1772–1775. doi:10.1126/science.8209258
Henkel JS, Engelhardt JI, Siklós L, Simpson EP, Kim SH, Pan T et al (2004) Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol 55:221–235. doi:10.1002/ana.10805
Hohsfield LA, Humpel C (2015) Migration of blood cells to β-amyloid plaques in Alzheimer’s disease. Exp Gerontol 65:8–15. doi:10.1016/j.exger.2015.03.002
Hübers A, Marroquin N, Schmoll B, Vielhaber S, Just M, Mayer B et al (2014) Polymerase chain reaction and Southern blot-based analysis of the C9orf72 hexanucleotide repeat in different motor neuron diseases. Neurobiol Aging 35:1214.e1211–1214.e1216. doi:10.1016/j.neurobiolaging.2013.11.034
Jefferies HBJ, Cooke FT, Jat P, Boucheron C, Koizumi T, Hayakawa M et al (2008) A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep 9:164–170. doi:10.1038/sj.embor.7401155
Jones DA, Abbassi O, McIntire LV, McEver RP, Smith CW (1993) P-selectin mediates neutrophil rolling on histamine-stimulated endothelial cells. Biophys J 65:1560–1569. doi:10.1016/s0006-3495(93)81195-0
Kawamata T, Akiyama H, Yamada T, McGeer PL (1992) Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am J Pathol 140:691–707
Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z et al (2013) Prion-like domain mutations in hnRNPs cause multisystem proteinopathy and ALS. Nature 495:467–473. doi:10.1038/nature11922
Klussmann S, Martin-Villalba A (2005) Molecular targets in spinal cord injury. J Mol Med 83:657–671. doi:10.1007/s00109-005-0663-3
Korn EL, Troendle JF, McShane LM, Simon R (2004) Controlling the number of false discoveries: application to high-dimensional genomic data. J Stat Plan Inference 124:379–398. doi:10.1016/S0378-3758(03)00211-8
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318. doi:10.1016/0166-2236(96)10049-7
Kuhle J, Lindberg RLP, Regeniter A, Mehling M, Steck AJ, Kappos L et al (2009) Increased levels of inflammatory chemokines in amyotrophic lateral sclerosis. Eur J Neurol 16:771–774. doi:10.1111/j.1468-1331.2009.02560.x
Lehnert S, Costa J, de Carvalho M, Kirby J, Kuzma-Kozakiewicz M, Morelli C et al (2014) Multicentre quality control evaluation of different biomarker candidates for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 15:344–350. doi:10.3109/21678421.2014.884592
Liao B, Zhao W, Beers DR, Henkel JS, Appel SH (2012) Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol 237:147–152. doi:10.1016/j.expneurol.2012.06.011
44. Lincecum JM, Vieira FG, Wang MZ, Thompson K, De Zutter GS, Kidd J et al. (2010) From transcriptome analysis to therapeutic anti-CD40L treatment in the SOD1 model of amyotrophic lateral sclerosis. Nat Genet 42: 392–399. http://www.nature.com/ng/journal/v42/n5/suppinfo/ng.557_S1.html
Mantovani S, Garbelli S, Pasini A, Alimonti D, Perotti C, Melazzini M et al (2009) Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. J Neuroimmunol 210:73–79. doi:10.1016/j.jneuroim.2009.02.012
Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M et al. (2007) Microglia in the adult brain arise from Ly-6ChiCCR2 + monocytes only under defined host conditions. Nat Neurosci 10:1544–1553. http://www.nature.com/neuro/journal/v10/n12/suppinfo/nn2015_S1.html
Mitchell AJ, Roediger B, Weninger W (2014) Monocyte homeostasis and the plasticity of inflammatory monocytes. Cell Immunol 291:22–31. doi:10.1016/j.cellimm.2014.05.010
Murdock BJ, Bender DE, Segal BM, Feldman EL (2015) The dual roles of immunity in ALS: injury overrides protection. Neurobiol Disease 77:1–12. doi:10.1016/j.nbd.2015.02.017
Nagelkerke SQ, Kuijpers TW (2014) Immunomodulation by IVIg and the role of Fc-gamma receptors: classic mechanisms of action after all? Front Immunol 5:674. doi:10.3389/fimmu.2014.00674
Nimmerjahn F, Ravetch JV (2008) Fc[gamma] receptors as regulators of immune responses. Nat Rev Immunol 8:34–47
Nourshargh S, Alon R (2014) Leukocyte migration into inflamed tissues. Immunity 41:694–707. doi:10.1016/j.immuni.2014.10.008
O’Neill ASG, van den Berg TK, Mullen GED (2013) Sialoadhesin: a macrophage-restricted marker of immunoregulation and inflammation. Immunology 138:198–207. doi:10.1111/imm.12042
Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JHW, Bleeker WK et al (2012) Crosstalk between human IgG isotypes and murine effector cells. J Immunol 189:3430–3438. doi:10.4049/jimmunol.1200356
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45
Randolph GJ, Jakubzick C, Qu C (2008) Antigen presentation by monocytes and monocyte-derived cells. Curr Opin Immunol 20:52–60. doi:10.1016/j.coi.2007.10.010
Rempel H, Calosing C, Sun B, Pulliam L (2008) Sialoadhesin expressed on IFN-induced monocytes binds HIV-1 and enhances infectivity. PLoS One 3:e1967. doi:10.1371/journal.pone.0001967
Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268. doi:10.1016/j.neuron.2011.09.010
Robberecht W, Philips T (2013) The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci 14: 248–264. http://www.nature.com/nrn/journal/v14/n4/suppinfo/nrn3430_S1.html
Ryberg H, An J, Darko S, Lustgarten JL, Jaffa M, Gopalakrishnan V et al (2010) Discovery and verification of amyotrophic lateral sclerosis biomarkers by proteomics. Muscle Nerve 42:104–111. doi:10.1002/mus.21683
Shi C, Pamer EG (2011) Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11:762–774. doi:10.1038/nri3070
Steiniger B, Barth P, Herbst B, Hartnell A, Crocker PR (1997) The species-specific structure of microanatomical compartments in the human spleen: strongly sialoadhesin-positive macrophages occur in the perifollicular zone, but not in the marginal zone. Immunology 92:307–316
Tanaka M, Krutzik SR, Sieling PA, Lee D, Rea TH, Modlin RL (2009) Activation of FcγR1 on monocytes triggers differentiation into immature dendritic cells that induce autoreactive T cell responses. J Immunol (Baltimore, Md : 1950) 183:2349–2355. doi:10.4049/jimmunol.0801683
Turner MR, Cagnin A, Turkheimer FE, Miller CCJ, Shaw CE, Brooks DJ et al (2004) Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Disease 15:601–609. doi:10.1016/j.nbd.2003.12.012
Weydt P, Hong SY, Kliot M, Möller T (2003) Assessing disease onset and progression in the SOD1 mouse model of ALS. NeuroReport 14:1051–1054. doi:10.1097/01.wnr.0000073685.00308.89
Wiesner D, Merdian I, Lewerenz J, Ludolph AC, Dupuis L, Witting A (2013) Fumaric Acid esters stimulate astrocytic VEGF expression through HIF-1α and Nrf2. PLoS One 8:e76670. doi:10.1371/journal.pone.0076670
Witting A, Möller T (2011) Microglia cell culture: a primer for the novice. In Vitro Neurotoxicology. Humana Press, New York, pp 49–66
Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R et al (2014) Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 211:1533–1549. doi:10.1084/jem.20132477
York MR, Nagai T, Mangini AJ, Lemaire R, van Seventer JM, Lafyatis R (2007) A macrophage marker, siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type i interferons and toll-like receptor agonists. Arthritis Rheum 56:1010–1020. doi:10.1002/art.22382
Ziegler-Heitbrock L (2014) Monocyte subsets in man and other species. Cell Immunol 289:135–139. doi:10.1016/j.cellimm.2014.03.019
Acknowledgments
We thank all blood donors, healthy control probands, as well as ALS patients and pre-symptomatic mutation carriers for participation in this study. We thank all physicians at the neurologic university clinic Ulm for recruiting and taking care of the patients who have participated in this study. We thank Antje Knehr for organizing the collection of blood from members of ALS families and we thank Birgit Linkus, Tanja Wipp, Diana Wiesner, Nadine Todt, Elena Jasovskaja, Johannes Hanselmann, and Eva Barth for technical assistance. This research was supported by the Thierry Latran Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work has been funded by the Thierry Latran Foundation, Grant Number: FTLAAP213/Weishaupt/innatetarget.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (Ethics Committee of Ulm University) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed written consent was obtained from all individual participants included in the study prior to inclusion. All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. All animal procedures were approved by the Regierungspräsidium Baden-Württemberg, Tübingen, Germany (No. 1090), and conducted according to the guidelines of the German Tierschutzgesetz.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zondler, L., Müller, K., Khalaji, S. et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol 132, 391–411 (2016). https://doi.org/10.1007/s00401-016-1548-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-016-1548-y