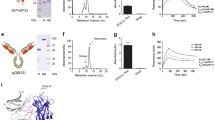
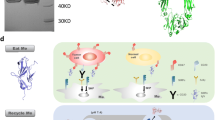
Abstract
Immunotoxins are fusion proteins that combine a targeting component such as an antibody fragment or ligand with a cytotoxic effector component that induces apoptosis in specific cell populations displaying the corresponding antigen or receptor. Human cytolytic fusion proteins (hCFPs) are less immunogenic than conventional immunotoxins because they contain human pro-apoptotic enzymes as effectors. However, one drawback of hCFPs is that target cells can protect themselves by expressing endogenous inhibitor proteins. Inhibitor-resistant enzyme mutants that maintain their cytotoxic activity are therefore promising effector domain candidates. We recently developed potent variants of the human ribonuclease angiogenin (Ang) that were either more active than the wild-type enzyme or less susceptible to inhibition because of their lower affinity for the ribonuclease inhibitor RNH1. However, combining the mutations was unsuccessful because although the enzyme retained its higher activity, its susceptibility to RNH1 reverted to wild-type levels. We therefore used molecular dynamic simulations to determine, at the atomic level, why the affinity for RNH1 reverted, and we developed strategies based on the introduction of further mutations to once again reduce the affinity of Ang for RNH1 while retaining its enhanced activity. We were able to generate a novel Ang variant with remarkable in vitro cytotoxicity against HL-60 cells and pro-inflammatory macrophages. We also demonstrated the pro-apoptotic potential of Ang-based hCFPs on cells freshly isolated from leukemia patients.






Similar content being viewed by others
Abbreviations
- AML:
-
Acute myeloid leukemia
- Ang:
-
Angiogenin
- CMML:
-
Chronic myelomonocytic leukemia
- DNA:
-
Deoxyribonucleic acid
- EC50 :
-
Half maximal effective concentration
- Gb:
-
Granzyme B
- GFP:
-
Green fluorescent protein
- hCFP:
-
Human cytolytic fusion protein
- hIFNγ:
-
Human interferon gamma
- hM1Φ:
-
Human pro-inflammatory macrophages
- HEK293T:
-
Human embryonic kidney cells
- IMAC:
-
Immobilized metal ion affinity chromatography
- K i :
-
Inhibitory constant
- MOG:
-
Myelin oligodendrocyte glycoprotein
- PBMC:
-
Peripheral blood mononuclear cell
- PCR:
-
Polymerase chain reaction
- PI:
-
Propidium iodide
- RNA:
-
Ribonucleic acid
- RNH1:
-
Ribonuclease/angiogenin inhibitor 1
- RPMI:
-
Roswell Park Memorial Institute
- SEM:
-
Standard error of the mean
- SOE:
-
Splicing by overlap extension
- tRNA:
-
Transfer RNA
- tiRNA:
-
tRNA-derived stress-induced RNA
- XTT:
-
2,3-bis-(2-Methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
References
Weidle UH, Georges G, Brinkmann U (2012) Fully human targeted cytotoxic fusion proteins: new anticancer agents on the horizon. Cancer Genomics Proteomics 9:119–133
Monti DM, D’Alessio G (2004) Cytosolic RNase inhibitor only affects RNases with intrinsic cytotoxicity. J Biol Chem 279:39195–39198. doi:10.1074/jbc.C400311200
Leland PA, Raines RT (2001) Cancer chemotherapy–ribonucleases to the rescue. Chem Biol 8:405–413
Rutkoski TJ, Raines RT (2008) Evasion of ribonuclease inhibitor as a determinant of ribonuclease cytotoxicity. Curr Pharm Biotechnol 9:185–189
Cremer C, Vierbuchen T, Hein L et al (2015) Angiogenin mutants as novel effector molecules for the generation of fusion proteins with increased cytotoxic potential. J Immunother 38:85–95. doi:10.1097/CJI.0000000000000053
Czech A, Wende S, Morl M et al (2013) Reversible and rapid transfer-RNA deactivation as a mechanism of translational repression in stress. PLoS Genet 9:e1003767. doi:10.1371/journal.pgen.1003767
Pizzo E, Sarcinelli C, Sheng J et al (2013) Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival. J Cell Sci 126:4308–4319. doi:10.1242/jcs.134551
Russo N, Shapiro R, Acharya KR et al (1994) Role of glutamine-117 in the ribonucleolytic activity of human angiogenin. Proc Natl Acad Sci USA 91:2920–2924
Chen CZ, Shapiro R (1999) Superadditive and subadditive effects of “hot spot” mutations within the interfaces of placental ribonuclease inhibitor with angiogenin and ribonuclease A. Biochemistry 38:9273–9285. doi:10.1021/bi990762a
Chen CZ, Shapiro R (1997) Site-specific mutagenesis reveals differences in the structural bases for tight binding of RNase inhibitor to angiogenin and RNase A. Proc Natl Acad Sci USA 94:1761–1766
Acharya KR, Shapiro R, Allen SC et al (1994) Crystal structure of human angiogenin reveals the structural basis for its functional divergence from ribonuclease. Proc Natl Acad Sci USA 91:2915–2919
Leonidas DD, Shapiro R, Subbarao GV et al (2002) Crystallographic studies on the role of the C-terminal segment of human angiogenin in defining enzymatic potency. Biochemistry 41:2552–2562
de Kruif J, Tijmensen M, Goldsein J et al (2000) Recombinant lipid-tagged antibody fragments as functional cell-surface receptors. Nat Med 6:223–227. doi:10.1038/72339
Graziano RF, Tempest PR, White P et al (1995) Construction and characterization of a humanized anti-gamma-Ig receptor type I (Fc gamma RI) monoclonal antibody. J Immunol 155:4996–5002
Ho SN, Hunt HD, Horton RM et al (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59
Stocker M, Tur MK, Sasse S et al (2003) Secretion of functional anti-CD30-angiogenin immunotoxins into the supernatant of transfected 293T-cells. Protein Expr Purif 28:211–219
Gallagher R, Collins S, Trujillo J et al (1979) Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 54:713–733
Diehl V, Kirchner HH, Schaadt M et al (1981) Hodgkin’s disease: establishment and characterization of four in vitro cell lies. J Cancer Res Clin Oncol 101:111–124
Schiffer S, Hristodorov D, Mladenov R et al (2013) Species-dependent functionality of the human cytolytic fusion proteins granzyme B-H22(scFv) and H22(scFv)-angiogenin in macrophages. Antibodies 2:9–18
Kampmeier F, Ribbert M, Nachreiner T et al (2009) Site-specific, covalent labeling of recombinant antibody fragments via fusion to an engineered version of 6-O-alkylguanine DNA alkyltransferase. Bioconjug Chem 20:1010–1015. doi:10.1021/bc9000257
Niesen J, Stein C, Brehm H et al (2015) Novel EGFR-specific immunotoxins based on panitumumab and cetuximab show in vitro and ex vivo activity against different tumor entities. J Cancer Res Clin Oncol. doi:10.1007/s00432-015-1975-5
Nachreiner T, Kampmeier F, Thepen T et al (2008) Depletion of autoreactive B-lymphocytes by a recombinant myelin oligodendrocyte glycoprotein-based immunotoxin. J Neuroimmunol 195:28–35. doi:10.1016/j.jneuroim.2008.01.001
Ribbert T, Thepen T, Tur MK et al (2010) Recombinant, ETA′-based CD64 immunotoxins: improved efficacy by increased valency, both in vitro and in vivo in a chronic cutaneous inflammation model in human CD64 transgenic mice. Br J Dermatol 163:279–286. doi:10.1111/j.1365-2133.2010.09824.x
Stöcker M, Pardo A, Hetzel C et al (2008) Eukaryotic expression and secretion of EGFP-labeled annexin A5. Protein Expr Purif 58:325–331. doi:10.1016/j.pep.2007.12.009
Verhoven B, Schlegel RA, Williamson P (1995) Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med 182:1597–1601
Krauss J, Arndt MA, Vu BK et al (2005) Targeting malignant B-cell lymphoma with a humanized anti-CD22 scFv-angiogenin immunoenzyme. Br J Haematol 128:602–609. doi:10.1111/j.1365-2141.2005.05356.x
Arndt MA, Krauss J, Vu BK et al (2005) A dimeric angiogenin immunofusion protein mediates selective toxicity toward CD22+ tumor cells. J Immunother 28:245–251
Hendrzak JA, Wallace PK, Morahan PS (1994) Optimizing the detection of cell surface antigens on elicited or activated mouse peritoneal macrophages. Cytometry 17:349–356. doi:10.1002/cyto.990170412
Leonidas DD, Shapiro R, Allen SC et al (1999) Refined crystal structures of native human angiogenin and two active site variants: implications for the unique functional properties of an enzyme involved in neovascularisation during tumour growth. J Mol Biol 285:1209–1233. doi:10.1006/jmbi.1998.2378
Dickson KA, Kang DK, Kwon YS et al (2009) Ribonuclease inhibitor regulates neovascularization by human angiogenin. Biochemistry 48:3804–3806. doi:10.1021/bi9005094
Stahnke B, Thepen T, Stocker M et al (2008) Granzyme B-H22(scFv), a human immunotoxin targeting CD64 in acute myeloid leukemia of monocytic subtypes. Mol Cancer Ther 7:2924–2932. doi:10.1158/1535-7163.MCT-08-0554
Schiffer S, Letzian S, Jost E et al (2013) Granzyme M as a novel effector molecule for human cytolytic fusion proteins: CD64-specific cytotoxicity of Gm-H22(scFv) against leukemic cells. Cancer Lett 341:178–185. doi:10.1016/j.canlet.2013.08.005
Dunphy CH, Tang W (2007) The value of CD64 expression in distinguishing acute myeloid leukemia with monocytic differentiation from other subtypes of acute myeloid leukemia: a flow cytometric analysis of 64 cases. Arch Pathol Lab Med 131:748–754. doi:10.1043/1543-2165(2007)131[748:TVOCEI]2.0.CO;2
Santos IM, Franzon CM, Koga AH (2012) Laboratory diagnosis of chronic myelomonocytic leukemia and progression to acute leukemia in association with chronic lymphocytic leukemia: morphological features and immunophenotypic profile. Rev Bras Hematol Hemoter 34:242–244. doi:10.5581/1516-8484.20120058
Schiffer S, Rosinke R, Jost E et al (2014) Targeted ex vivo reduction of CD64-positive monocytes in chronic myelomonocytic leukemia and acute myelomonocytic leukemia using human granzyme B-based cytolytic fusion proteins. Int J Cancer 135:1497–1508. doi:10.1002/ijc.28786
Karehed K, Dimberg A, Dahl S et al (2007) IFN-gamma-induced upregulation of Fcgamma-receptor-I during activation of monocytic cells requires the PKR and NFkappaB pathways. Mol Immunol 44:615–624. doi:10.1016/j.molimm.2006.01.013
Wei H, Bera TK, Wayne AS et al (2013) A modified form of diphthamide causes immunotoxin resistance in a lymphoma cell line with a deletion of the WDR85 gene. J Biol Chem 288:12305–12312. doi:10.1074/jbc.M113.461343
Conway O’Brien E, Prideaux S, Chevassut T (2014) The epigenetic landscape of acute myeloid leukemia. Adv Hematol. 2014:103175. doi:10.1155/2014/103175
Jankowska AM, Makishima H, Tiu RV et al (2011) Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood 118:3932–3941. doi:10.1182/blood-2010-10-311019
Schnerch D, Yalcintepe J, Schmidts A et al (2012) Cell cycle control in acute myeloid leukemia. Am J Cancer Res 2:508–528
Scholl C, Gilliland DG, Frohling S (2008) Deregulation of signaling pathways in acute myeloid leukemia. Semin Oncol 35:336–345. doi:10.1053/j.seminoncol.2008.04.004
Gao X, Xu Z (2008) Mechanisms of action of angiogenin. Acta Biochim Biophys Sin (Shanghai) 40:619–624
Lu Z, Xu S (2006) ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58:621–631. doi:10.1080/15216540600957438
Peng Y, Li L, Huang M et al (2014) Angiogenin interacts with ribonuclease inhibitor regulating PI3K/AKT/mTOR signaling pathway in bladder cancer cells. Cell Signal 26:2782–2792. doi:10.1016/j.cellsig.2014.08.021
Sadagopan S, Veettil MV, Chakraborty S et al (2012) Angiogenin functionally interacts with p53 and regulates p53-mediated apoptosis and cell survival. Oncogene 31:4835–4847. doi:10.1038/onc.2011.648
Ivanov P, Emara MM, Villen J et al (2011) Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell 43:613–623. doi:10.1016/j.molcel.2011.06.022
Acknowledgments
This project was funded by the Deutsche Forschungsgemeinschaft (DFG). The authors would like to thank Dr. Christoph Stein (Institute for Applied Medical Engineering, University Hospital RWTH Aachen/Fraunhofer Institute for Molecular Biology and Applied Ecology, Department of Pharmaceutical Product Development, Aachen) for helpful discussions on leukemia specimen handling, Judith Niesen (Fraunhofer Institute for Molecular Biology and Applied Ecology, Department of Pharmaceutical Product Development, Aachen) for providing the fusion protein 2112(scFv)-Ang GGRRmut, Anh-Tuah Pham (Institute for Applied Medical Engineering, University Hospital RWTH Aachen) for providing her2(scFv)-Ang GGRRmut and Fanny Frenzel (University Hospital RWTH Aachen, Department of Hematology and Oncology, Internal Medicine IV, Aachen, Germany) for providing patient data and specimens. Finally, we are very grateful to Dr. Richard M Twyman for critically reading this manuscript. Radoslav Mladenov was supported by a scholarship from the Jürgen Manchot Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Stefan Barth and Thomas Nachreiner contributed equally to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cremer, C., Braun, H., Mladenov, R. et al. Novel angiogenin mutants with increased cytotoxicity enhance the depletion of pro-inflammatory macrophages and leukemia cells ex vivo. Cancer Immunol Immunother 64, 1575–1586 (2015). https://doi.org/10.1007/s00262-015-1763-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1763-8