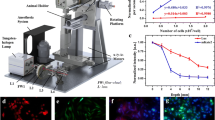
Abstract
Purpose
Preclinical in vivo analyses of treatment responses are an important prerequisite to evaluate new therapeutics. Molecular in vivo imaging in the far red (FR)/near infra red (NIR) is a promising method, as it enables measurements at different time points in individual animals, thereby reducing the number of animals required, while increasing statistical significance. Here, we show the establishment of a method to monitor response to treatment using fluorescent cells, expressing the epidermal growth factor receptor (EGFR), a target already used in therapy.
Methods
We transfected A-431 tumour cells with the far red–emitting protein Katushka (Kat2), resulting in strong fluorescence allowing for the monitoring of tumour growth when implanted in BALB/c nu/nu mice with a CRi Maestro in vivo imager. We targeted A-431 cells with a previously reported immunotoxin (IT), consisting of the anti-EGFR antibody single-chain variable fragment (scFv) 425, fused to Pseudomonas aeruginosa Exotoxin A’ (ETA’). In addition, EGFR expression was verified using the 425(scFv) conjugated to a NIR dye BG-747 through a SNAP-tag linker.
Results
The results show the feasibility to evaluate response to treatment in vivo by FR imaging, while at the same location detecting EGFR expression. Treatment with 425(scFv)-ETA’ resulted in decelerated tumour growth, while not affecting the overall health of the animals. This is in contrast to treatment with Doxorubicin, which, although decreasing the tumour size, resulted in poor health.
Conclusions
We developed a novel method to non-invasively determine treatment responses by in vivo imaging of multiple parameters which showed the efficacy of 425(scFv)-ETA’.




Similar content being viewed by others
References
Contag PR (2002) Whole-animal cellular and molecular imaging to accelerate drug development. Drug Discov Today 7:555–562
Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC (1994) Green fluorescent protein as a marker for gene expression. Science 263:802–805
Alford R, Ogawa M, Choyke PL, Kobayashi H (2009) Molecular probes for the in vivo imaging of cancer. Mol Biosyst 5:1279–1291
Gong H, Kovar J, Little G, Chen H, Olive DM (2010) In vivo imaging of xenograft tumors using an epidermal growth factor receptor-specific affibody molecule labeled with a near-infrared fluorophore. Neoplasia 12:139–149
Weissleder R (2001) A clearer vision for in vivo imaging. Progress continues in the development of smaller, more penetrable probes for biological imaging. Nat Biotechnol 19:316–317
Shcherbo D, Merzlyak EM, Chepurnykh TV, Fradkov AF, Ermakova GV, Solovieva EA et al (2007) Bright far-red fluorescent protein for whole-body imaging. Nat Methods 4:741–746
Haigler H, Ash JF, Singer SJ, Cohen S (1978) Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc Natl Acad Sci USA 75:3317–3321
Tolmachev V, Rosik D, Wållberg H, Sjöberg A, Sandström M, Hansson M et al (2010) Imaging of EGFR expression in murine xenografts using site-specifically labelled anti-EGFR 111In-DOTA-Z EGFR:2377 Affibody molecule: aspect of the injected tracer amount. Eur J Nucl Med Mol Imag 37:613–622
Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, Saffrich R et al (2005) Inhibition of alpha(v)beta3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res 11:6270–6279
Shao W, Zhao S, Liu Z, Zhang J, Ma S, Sato JD et al (2006) Inhibition of human tumor xenograft growth in nude mice by a conjugate of monoclonal antibody LA22 to epidermal growth factor receptor with anti-tumor antibiotics mitomycin C. Biochem Biophys Res Commun 349:816–824
Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R (2003) FDA drug approval summary: Gefitinib (ZD1839)(Iressa (R)) tablets. Oncologist 8:303–306
Cohen MH, Johnson JR, Chen YF, Sridhara R, Pazdur R (2005) FDA drug approval summary: erlotinib (Tarceva (R)) tablets. Oncologist 10:461–466
Vincenzi B, Zoccoli A, Pantano F, Venditti O, Galluzzo S (2010) Cetuximab: from bench to bedside. Curr Cancer Drug Targets 10:80–95
Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R (2007) FDA drug approval summary: panitumumab (Vectibix). Oncologist 12:577–583
Camp ER, Summy J, Bauer TW, Liu W, Gallick GE, Ellis LM (2005) Molecular mechanisms of resistance to therapies targeting the epidermal growth factor receptor. Clin Cancer Res 11:397–405
Bruell D, Stöcker M, Huhn M, Redding N, Küpper M, Schumacher P et al (2003) The recombinant anti-EGF receptor immunotoxin 425(scFv)-ETA’ suppresses growth of a highly metastatic pancreatic carcinoma cell line. Int J Oncol 23:1179–1186
Bruell D, Bruns CJ, Yezhelyev M, Huhn M, Müller J, Ischenko I et al (2005) Recombinant anti-EGFR immunotoxin 425 (scFv)-ETA’ demonstrates anti-tumor activity against disseminated human pancreatic cancer in nude mice. Int J Mol Med 15:305–313
Barth S, Huhn M, Matthey B, Tawadros S, Schnell R, Schinköthe T et al (2000) Ki-4(scFv)-ETA’, a new recombinant anti-CD30 immunotoxin with highly specific cytotoxic activity against disseminated Hodgkin tumors in SCID mice. Blood 95:3909–3914
Huhn M, Sasse S, Tur MK, Matthey B, Schinköthe T, Rybak SM, et al. (2001) Human angiogenin fused to human CD30 Ligand (Ang-CD30L) exhibits specific cytotoxicity against CD30-positive lymphoma. Cancer Res 61:8737–8742
Tur M, Huhn M, Thepen T, Stöcker M, Krohn R, Vogel S et al (2003) Recombinant CD64-specific single chain immunotoxin exhibits specific cytotoxicity against acute myeloid leukemia cells. Cancer Res 63:8414–8419
Stahnke B, Thepen T, Stöcker M, Rosinke R, Jost E, Fischer R et al (2008) Granzyme B-H22(scFv), a human immunotoxin targeting CD64 in acute myeloid leukemia of monocytic subtypes. Mol Cancer Ther 7:2924–2932
Tur MK, Neef I, Jost E, Galm O, Jäger G, Stöcker M et al (2009) Targeted restoration of down-regulated DAPK2 tumor suppressor activity induces apoptosis in Hodgkin lymphoma cells. J Immunother 32:431–441
Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ (2006) Immunotoxin therapy of cancer. Nat Rev Cancer 6:559–565
Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ (2007) Immunotoxin treatment of cancer. Annu Rev Med 58:221–237
Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, Fitzgerald DJ et al (2001) Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med 345:241–247
Wayne AS, Kreitman RJ, Findley HW, Lew G, Delbrook C, Steinberg SM et al (2010) Anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: preclinical studies and phase I clinical trial. Clin Cancer Res 16:1894–1903
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Luo J, Solimini NL, Elledge SJ (2009) Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136:823–837
Li C, Iida M, Dunn EF, Ghia AJ, Wheeler DL (2009) Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene 28:3801–3813
Arteaga CL (2003) EGF receptor as a therapeutic target: patient selection and mechanisms of resistance to receptor-targeted drugs. J Clin Oncol 21:289s–291s
Kampmeier F, Ribbert M, Nachreiner T, Dembski S, Beaufils F, Brecht A et al (2009) Site-specific, covalent labeling of recombinant antibody fragments via fusion to an engineered version of 6-O-alkylguanine DNA alkyltransferase. Bioconjug Chem 20:1010–1015
Kampmeier F, Niesen J, Koers A, Ribbert M, Brecht A, Fischer R et al (2010) Rapid optical imaging of EGF receptor expression with a single-chain antibody SNAP-tag fusion protein. Eur J Nucl Med Mol Imag 37:1926–1934
Matthey B, Engert A, Klimka A, Diehl V, Barth S (1999) A new series of pET-derived vectors for high efficiency expression of Pseudomonas exotoxin-based fusion proteins. Gene 229:145–153
Ribbert T, Thepen T, Tur MK, Fischer R, Huhn M, Barth S (2010) Improved efficacy by increased valency, both in vitro, as well as in vivo in a chronic cutaneous inflammation model in hCD64 transgenic mice. Br J Dermatol 162:1–3
Kapp U, Wolf J, Von Kalle C, Tawadros S, Röttgen A, Engert A et al (1992) Preliminary report: growth of Hodgkin’s lymphoma derived cells in immune compromised mice. Ann Oncol 3:S21–S23
Stocker M, Tur MK, Sasse S, Krüßmann A, Barth S, Engert A (2003) Secretion of functional anti-CD30-angiogenin immunotoxins into the supernatant of transfected 293T-cells. Protein Expr Purif 28:211–219
Johansen PB (1981) Doxorubicin pharmacokinetics after intravenous and intraperitoneal administration in the nude mouse. Cancer Chemother Pharmacol 5:267–270
Mizutani H, Tada-Oikawa S, Hiraku Y, Kojima M, Kawanishi S (2005) Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci 76:1439–1453
Ajaj KA, Graeser R, Fichtner I, Kratz F (2009) In vitro and in vivo study of an albumin-binding prodrug of doxorubicin that is cleaved by cathepsin B. Cancer Chemother Pharmacol 64:413–418
Jensen MM, Jørgensen JT, Binderup T, Kjaer A (2008) Tumor volume in subcutaneous mouse xenografts measured by microCT is more accurate and reproducible than determined by 18F-FDG-microPET or external caliper. BMC Med Imag 8:16
He X, Nie H, Wang K, Tan W, Wu X, Zhang P (2008) In vivo study of biodistribution and urinary excretion of surface-modified silica nanoparticles. Anal Chem 80:9597–9603
Kobayashi H, Longmire MR, Ogawa M, Choyke PL (2011) Rational chemical design of the next generation of molecular imaging probes based on physics and biology: mixing modalities, colors and signals. Chem Soc Rev 40:4626–4648
Barrett T, Koyama Y, Hama Y, Ravizzini G, Shin IS, Jang BS et al (2007) In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res 13:6639–6648
Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M et al (2009) Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med 15:104–109
Acknowledgments
We thank Dirk Scheffler, Reinhard Rosinke and Peggy Jirak for excellent technical assistance. This publication is based upon work that was in part financed by the Bundesministerium für Bildung und Forschung (0315254A/B). The responsibility for the content of this publication lies with the author.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Theo Thepen and Stefan Barth contributed equally to the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pardo, A., Stöcker, M., Kampmeier, F. et al. In vivo imaging of immunotoxin treatment using Katushka-transfected A-431 cells in a murine xenograft tumour model. Cancer Immunol Immunother 61, 1617–1626 (2012). https://doi.org/10.1007/s00262-012-1219-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1219-3