Abstract
Hypoxia-inducible factors (HIFs) are transcription factors that activate the transcription of target genes involved in crucial aspects of cancer development. This study investigated the expression of HIFs and their contribution to the regulation of target genes related to angiogenesis and glucose metabolism in gastric cancer. The data showed that HIFs were over-expressed in gastric cancer and that activation of the target genes was observed mainly in the early stages. Moreover, the results of the present study revealed that only HIF-1α, but not HIF-2α dimerizes with HIF-1β and then regulates expression of target genes in response to hypoxia. The results of the present study demonstrate that HIF-1α and HIF-1β enhances expression of VEGF and glucose metabolism-related genes in response to hypoxia in gastric cancer. These data offer important information regarding HIF pathways in the development of gastric cancer.
Similar content being viewed by others
Introduction
Hypoxia is a common feature of many cancers (Ryan et al., 2000). Hypoxia-inducible factors (HIFs) are essential components in regulating transcription in tumor cells in response to hypoxia. HIF-1α and HIF-2α dimerize with a constitutively expressed β-subunit, and exhibit distinct roles in hypoxic expression of target genes (Hu et al., 2003; Park et al., 2003). Although HIF-1 is induced by hypoxia in almost all cell types, the expression of HIF-1 target genes induced by hypoxia is cell type specific manner (Yu et al., 1999; Semenza, 2003). The cell type specificity is thought to be due to the functional interactions of HIFs with other transcription factors or to activation of a subgroup of HIFs in a particular cell type during hypoxia (Ema et al., 1997; Tian et al., 1997; Brusselmans et al., 2001).
Gastric cancer is one of the leading causes of cancer related motality in East Asia, including Korea (Neugut et al., 1996). Several reports have shown that HIFs play important roles in gastric tumorigenesis (Zhong et al., 1999; Park et al., 2003). A previous study by our group indicated that genes related to glucose metabolism, such as PKM2 (pyruvate kinase), PGK1 (phosphoglycerate kinase 1), GLUT 1 (glucose transporter 1), LDHA (lactate dehydrogenase A), and ENO1 (enolase 1), were up-regulated in gastric cancer cell lines (Kim et al., 2004). So far, the roles of HIF-1α and HIF-2α in glucose metabolism and other HIF-regulated genes under hypoxic conditions in gastric cancer remain unclear. To identify the roles of HIFs in gastric cancer, gastric cancer tissue specimens from human patients as well as cancer cell lines, were used to assess the contributions of HIF-1α and HIF-2α to the expression of glucose metabolism-related genes and VEGF during specific cancer developmental stages and in response to hypoxia.
Results
HIFs were over-expressed in gastric cancer cells
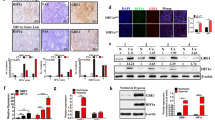
To confirm the relationship of the HIF pathway to the development of gastric cancer, the expression of HIFs at the level of transcription was examined in the gastric cancer cell lines and tissues. The results of semiquantitative (Sq) RT-PCR in the gastric cancer cell lines showed that the expression of HIF-1α and HIF-2α did not differ between gastric cancer cell lines and normal Hs 667.st cells, while HIF-1β expression was significantly increased in the gastric cancer cell lines (Figure 1A). In contrast, Sq RT-PCR data for HIFs expression in the human gastric tissue specimens revealed up-regulation of HIF genes expression compared to adjacent normal tissues (Figure 1B, a-c). In particular, these over-expressions were observed in the early stage of IA, although HIF-1α and HIF-2α were over-expressed in the IIIA/B stage. In addition, immunohistochemical analysis of the human gastric tissues showed that 22 of 38 (58%) specimens showed positive staining for HIF-1α, 17 of 38 (45%) were positive for HIF-1β and 18 of 38 (47%) were positive for HIF-2α in the cancerous regions compared with adjacent normal tissues (Table 1). Interestingly, in the early stage of IA/B, these positives were mainly observed as an over-expression of HIF-1α compared with those of HIF-1β and HIF-2α (Table 1). And the expression levels of HIFs were observed to increase in proportion to tumor grades. The HIF-positive cells were clustered within gastric adenocarcinoma tissue, but were not detected or were expressed at very low levels in nonmalignant epithelial and stromal cells (Figure 2). HIF-1α and HIF-2α was located in the cytosol and nucleus, while HIF-1β was limited to the nucleus. These data indicate that HIFs are commonly over-expressed in gastric cancer, and that HIF-1α plays a particularly important role in its early stages.
Transcriptional levels of target genes in gastric cancer cell lines (A) and gastric tumor tissues (B) using Sq RT-PCR. The transcriptional levels of the target genes were calculated relative to the amount of β-2-microglobulin (B2M) mRNA. Data of (B) represent the mean ± SD of transcriptional levels of the target genes in 5 pairs of gastric tumor patients of each stage group. Two independent experiments were performed, both of which showed similar results. Asterisks denote a significant difference in target genes between the sample and control by the t test (★, P < 0.05; ★★, P < 0.01; ★★★, P < 0.001).
Immunohistochemical expression of HIF-1α, HIF-1β, and HIF-2α in gastric tumor tissues of stage III. The HIF-1α (A) and HIF-2α (C) staining were present in the nucleus and cytoplasm of tumor cells. HIF-1β (B) was observed in nuclear of malignant cells. HIF-1α was predominantly present compared with HIF-2α in the nucleus of tumor cells. N, peri-tumoral normal tissue; T, tumor tissue.
Over-expression of glucose metabolism-related genes and VEGF is common in gastric cancer cells, especially in the early stages
To further confirm the activation of HIF pathways, the expression of glucose metabolism-related genes and VEGF also was investigated in gastric cancer cell lines and tissues. The results shown in Figure 1A reveal that glucose metabolism-related target genes, as well as the VEGF, were over-expressed in gastric cancer cell lines compared with normal Hs 677.st cells. In addition, results of the Sq RT-PCR in gastric tumor tissues show that VEGF was over-expressed only during stage IA (Figure 1B, d). Interestingly, over-expression of glucose metabolism related target genes was mainly observed during stage I, although GLUT1 and ENO1 also were over-expressed during stage II (Figure 1B, e-h). These results indicate that the effective activation of HIF related angiogenesis and glucose metabolism pathways may play important roles in the progression of gastric cancer.
The heterodimer of HIF-1α and HIF-1β, but not of HIF-2α directly regulates the expression of glucose metabolism-related target genes and VEGF
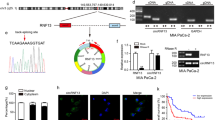
To examine the contribution of HIF members to the expression of genes related to glucose metabolism and VEGF, expression of these genes in response to hypoxia was examined in SNU-638 cells. As shown in Figure 3A and B, expression of HIFs at both the transcriptional and translational levels was increased under hypoxic conditions compared with normoxia. In particular, an HIF-1α expression in gastric cancer cell line under hypoxic conditions was revealed to be regulated more in the translational level than in the transcriptional level. In addition, the increased transcriptional levels of glucose metabolism-related genes, as well as of the VEGF, were confirmed under hypoxic conditions (Figure 3A). Among them, immunoblot analysis of GLUT1 and ENO1 protein levels also revealed over-expression at the translational level (Figure 3B). However, when SNU-638 cells were transfected with siRNA to HIFs, HIF-1α and HIF-1β but not HIF-2α resulted in the down regulation of target genes related to glucose metabolism and of VEGF at both the transcriptional and translational levels (Figure 3A and B). On the other hands, when the transcriptional activity of HIFs in SNU-638 cells under hypoxic condition was examined using a HRE (hypoxic response element)-luciferase reporter construct, the siRNA of HIF-1α and HIF-1β were shown to reduce the relative luciferase activity induced by CoCl2 stimulation whereas siRNA of HIF-2α had no effect (Figure 3C). These data indicate that HIF-2α was not required to the expression of glucose metabolism-related genes in gastric cancer cells under hypoxic conditions.
Transcriptional levels (A) and translational levels (B) of target genes in SNU-638 cells under hypoxic condition after siRNA transfection against HIFs. Vertical bars in A-(b) represent mean ± SD of transcriptional levels of the target genes in three independent experiments, all of which showed similar results. ★, P < 0.05; ★★, P < 0.01; ★★★, P < 0.001. (C) HIF-dependent luciferase reporter assays were performed in HIFs knocked down SNU-638 cells. The SNU-638 cells were cotransfected with the HIF dependent luciferase reporter and siRNA of HIF-1α, HIF-1β or HIF-2α for 48 h. And then the cells were treated with or without CoCl2 for 12 h. The relative luciferase activity was measured using an enhanced luciferase assay kit (Promega) and normalized to the β-galactosidase activity. Data represent the mean ± SD of duplicate determinations from one of three independent sets of experiments, all of which showed similar results. (D) Immunoprecipitatation of HIF-1α and HIF-2α using monoclonal antibodies against HIF-1β or HIF-1α. N, normoxia; H, hypoxia. The proteins of (B) and (D) were detected using immunoblot analysis.
To confirm which heterodimer of HIF-1α and -2α directly regulates these genes, an immunoprecipitation assay with HIF-1β antibody was performed. As shown in Figure 3D, HIF-1β interacted only with HIF-1α in SNU-638 cells under hypoxic conditions. This interaction was also confirmed in immunoprecipitation result with the antibody against HIF-1α. This result explains why HIF-2α had no effect on regulation of expression of target genes related to glucose metabolism and of VEGF, although HIF-2α was over-expressed in SNU-638 cells under hypoxic conditions. Taken together, these data strongly suggest that not only HIF-1α but also HIF-1β play significant roles in the regulation of glucose metabolism and angiogenesis related genes in gastric cancer.
Discussion
Over-expression of HIFs is thought to correlate with cancer progression (Semenza, 2003; Carroll and Ashcroft, 2005). However, the roles of HIF-1α and HIF-2α in regulating expression of HIF target genes, such as glucose metabolism-related genes and VEGF, in gastric cancer under hypoxic conditions remain unclear. The present study, we investigated HIF expression as well as their relationship to expression of glucose metabolism-related genes and VEGF in gastric cancer cells. The resulting data provide new clues to understanding the molecular mechanisms of the HIF pathway in gastric cancer development.
The HIF expression data showed that mRNA and protein of HIFs, HIF-1α, HIF-1β and -2α were over-expressed in gastric cancer tissues. This result was coincident with others observation in which HIF-1α was over-expressed, as assessed by immunochemical histological analysis, in 2 gastric cancer tissue specimens (Zhong et al., 1999). In addition, it was partially supported by a previous study that the combination of a HIF-1α protein over-expression with nonfunctional p53 tends to indicate a dismal prognosis in gastric cancer (Sumiyoshi et al., 2006). Furthermore, the results of the present study revealed that the activation of glucose metabolism-related genes and VEGF was mainly observed in the early stages of gastric cancer, especially in stage I. These data offer valuable evidence that therapeutic strategies targeting angiogenesis and glucose metabolism pathways should be implemented during the early stages of gastric cancer.
Moreover, it was determined that HIF-1α but not HIF-2α primarily dimerizes with HIF-1β and regulates expression of VEGF and glucose metabolism-related genes in response to hypoxia. It is well-known that HIF-1α but not HIF-1β plays important roles in the HIF-1 pathway. It also has been reported that HIF target genes are regulated by HIF-1α in prostate, ovarian, breast, and lung cancers (Volm and Koomagi, 2000; Schindl et al., 2002). Taking these reports into account, the data from the present study offer strong evidence that, at least in gastric cancer, not only HIF-1α but also HIF-1β play crucial roles in HIF pathway. In other words, under the hypoxic condition, HIF-1α heterodimerizes with HIF-1β in nucleus, the heterodimmer binds to an enhancer element called the HRE in target genes involved in glucose metabolism and angiogenesis and then regulates expression of these genes. In addition, HIF-2α has been reported to contribute to HIF target gene expression in renal carcinoma cells (Carroll and Ashcroft, 2006), suggesting that the contributions of HIF-1α and HIF-2α to HIF target gene expression induced by hypoxia are regulated in a cell type specific manner in which dimerization with HIF-1β is an important factor. The results of the present study demonstrate that HIF-1α and HIF-1β but not HIF-2α enhances expression of glucose metabolism-related genes and VEGF in response to hypoxia in gastric cancer. These data offer important information relevant to therapies that target angiogenesis and glucose metabolism using the HIF pathway in gastric cancer.
Methods
Patient tissue samples
Twenty-seven tissues with pairs of gastric tumors and adjacent non-tumor tissue for gene expression examination and 38 gastric cancer blocks for immunohistochemical staining analysis were obtained from the College of Medicine, Chungnam National University, and from the Department of Pathology, Eulji University School of Medicine, Korea with informed consent, respectively. The gastric tumors were staged according to tumor-node-metastasis classification of Union Internationale Contre le Cancer.
Cell culture and semiquantitative (Sq) RT-PCR analysis
Human gastric cancer cell lines (SNU-216, -484, -601, -638) were obtained from the Korean Cell Line Bank (Seoul, Korea) (Park et al., 1997; Choi et al., 2007) and normal gastric cell line (Hs677.st) was obtained from ATCC. All cell lines were maintained in RPMI1640 supplemented with 10% FBS (Hyclone Laboratories) and gentamicin (10 µg/ml) at 37℃ in a 5% CO2 humidified atmosphere. Environmental hypoxic conditions (2%) were achieved in an airtight humidified chamber continuously flushed with a gas mixture containing 5% CO2 and 95% N2. The culture of human normal and gastric cancer cell lines, as well as Sq RT-PCR analysis using total RNAs from these cell lines, were performed as previously described (Kim et al., 2004). The primers used in Sq RT-PCR are indicated in Supplemental Data Table S1 and the size of PCR products ranged from 300 to 600 bp. The PCR cycle numbers were varied from 25 to 35 according to the exponential part of the amplification curve of target genes.
siRNA treatment
siRNA targeted to HIF-1α, (5'-CUGAUGACCAGCAACUUGATT-3' and 5'-UCAAGUUGCUGGUCAUCAGTT-3'), HIF-1β, (5'-CAAUGCGGAUCAGAGUAAATT-3' and 5'-UUUACUCUGAUCCGCAUUGTT-3'), and HIF-2α, (5'-CAGCAUCUUUGAUAGCAGUTT-3' and 5'-ACUGCUAUCAAAGAUGCUGTT-3') were designed using programs such as Dharmacon-siDESIGN and Invitrogen BOCK-iT RNAi designer, and synthesized from Samchully Pharm (Korea). The human gastric cancer SNU-638 cells were plated at 40% confluency (8 × 105 cells/dish) in 10 cm plates and transfected with oligonucleotide duplexes (100 nM) that had been premixed with Oligofectamine (Invitrogen) in Opti-MEM-I (Invitrogen) for 4 h. An siRNA targeted to an irrelevant mRNA of GFP served as the nonspecific control.
Immunoprecipitation and immunoblot analysis
The SNU-638 cells were treated for 4 h under hypoxic or normoxic conditions, rinsed once with cold PBS, and lysed in lysis buffer A [20 mM HEPES (pH 7.5), 150 mM NaCl, 1 mM EDTA, 2 mM EGTA, 1% Triton X-100, 10% glycerol, 1 mM AEBSF, 1 mM Na3VO4, 5 mM NaF, 10 µg/ml aprotinin and leupeptin]. The cell lysates were pre-cleared with 10 µl protein A/G agarose beads (Amersham Biosciences) for 1 h. The cleared lysates were incubated with 6 µg HIF-1β or HIF-1α antibodies (Santa Cruz Biotechnology, Inc.) overnight at 4℃. The lysates were mixed with 30 µl protein A/G agarose beads for 2 h at 4℃. The beads were washed three times with 1 ml lysis buffer A. The final protein precipitates were subjected to immunoblot analysis.
Proteins resolved by 6% SDS-PAGE were transferred to nitrocellulose membranes and nonspecific binding sites were blocked by incubation in TBS containing 0.5% Tween-20 and 5% (w/v) dry milk. Immunoblot analysis was performed with the indicated antibodies in figure. A mouse monoclonal antibody against HIF-1α antibody was purchased from BD Transduction Laboratories. Antibodies to α-tubulin, GLUT1, HIF-1β, and ENO1 were obtained from Santa Cruz Biotechnology. A mouse monoclonal antibody to HIF-2α was purchased from Abcam Inc. The bound primary antibodies were visualized with HRP-conjugated goat anti rabbit-IgG, goat anti-mouse-IgG (Pierce), or donkey anti-goat IgG (Santa Cruz Biotechnology Inc.), and enhanced with a chemiluminescence kit (AB Frontier, Korea).
Immunohistochemistry
Immunohistochemical staining for HIF-1α, HIF-2α, and HIF-1β was performed using the previously described procedures (Kim et al., 2005; Choi et al., 2007). The primary antibodies used in the immunohistochemical staining were as followings: a rabbit polyclonal antibody against HIF-1α at a 1:200 dilution; a mouse monoclonal anti-HIF-1β at a 1:200 dilution (Santa Cruz Biotechnology, Inc.); a mouse monoclonal antibody against HIF-2α at a 1:1000 dilution (Abcam Inc.).
Luciferase reporter assay
SNU-638 cells were transfected by Fugene 6 liposome technique (Boehringer Mannheim) according to protocol of the supplier. For reporter assays in HIF-1α, HIF-1β, or HIF-2α knocked-down cells, SNU-638 cells were cotransfected with siRNA of HIFs and HRE-Luciferase reporter. At 48 h post-transfection, the cells were incubated with 200 µM of CoCl2 in complete medium for 12 h. Relative luciferase activities were determined by normalizing the β-galactosidase activities of the cell lysates. The luciferase assay carried out by the method using enhanced luciferase assay kit (Promega) according to the manufacture's instruction.
Statistics
Data were analyzed using a Student's t-test on SigmaPlot 8.0 software. P-values were assessed to derive the statistical significance.
Supplemental Data
Supplemental Data include a table and can be found with this article online.
Abbreviations
- HIFs:
-
hypoxia-inducible factors
- Sq:
-
semiqauntitative
- HRE:
-
hypoxic response element
- B2M:
-
β-2-microglobulin
References
Brusselmans K, Bono F, Maxwell P, Dor Y, Dewerchin M, Collen D, Herbert JM, Carmeliet P . Hypoxia-inducible factor-2alpha (HIF-2alpha) is involved in the apoptotic response to hypoglycemia but not to hypoxia . J Biol Chem 2001 ; 276 : 39192 - 39196
Carroll VA, Ashcroft M . Targeting the molecular basis for tumour hypoxia . Expert Rev Mol Med 2005 ; 7 : 1 - 16
Carroll VA, Ashcroft M . Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway . Cancer Res 2006 ; 66 : 6264 - 6270
Choi B, Suh Y, Kim WH, Christa L, Park J, Bae CD . Downregulation of regenerating islet-derived 3 alpha (REG3A) in primary human gastric adenocarcinomas . Exp Mol Med 2007 ; 39 : 796 - 804
Choi SC, Yoon SR, Park YP, Song EY, Kim JW, Kim WH, Yang Y, Lim JS, Lee HG . Expression of NDRG2 is related to tumor progression and survival of gastric cancer patients through Fas-mediated cell death . Exp Mol Med 2007 ; 39 : 705 - 714
Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y . A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development . Proc Natl Acad Sci USA 1997 ; 94 : 4273 - 4278
Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC . Differential roles of hypoxia-inducible factor 1alpha (HIF-1α) and HIF-2α in hypoxic gene regulation . Mol Cell Biol 2003 ; 23 : 9361 - 9374
Kim JM, Sohn HY, Yoon SY, Oh JH, Yang JO, Kim JH, Song KS, Rho SM, Yoo HS, Kim YS, Kim JG, Kim NS . Identification of gastric cancer-related genes using a cDNA microarray containing novel expressed sequence tags expressed in gastric cancer cells . Clin Cancer Res 2005 ; 11 : 473 - 482
Kim NS, Hahn Y, Oh JH, Lee JY, Oh KJ, Kim JM, Park HS, Kim S, Song KS, Rho SM, Yoo HS, Kim YS . Gene cataloging and expression profiling in human gastric cancer cells by expressed sequence tags . Genomics 2004 ; 83 : 1024 - 1045
Neugut AI, Hayek M, Howe G . Epidemiology of gastric cancer . Semin Oncol 1996 ; 23 : 281 - 291
Park JG, Yang HK, Kim WH, Chung JK, Kang MS, Lee JH, Oh JH, Park HS, Yeo KS, Kang SH, Song SY, Kang YK, Bang YJ, Kim YH, Kim JP . Establishment and characterization of human gastric carcinoma cell lines . Int J Cancer 1997 ; 70 : 443 - 449
Park JH, Kim TY, Jong HS, Chun YS, Park JW, Lee CT, Jung HC, Kim NK, Bang YJ . Gastric epithelial reactive oxygen species prevent normoxic degradation of hypoxia-inducible factor-1α in gastric cancer cells . Clin Cancer Res 2003 ; 9 : 433 - 440
Park SK, Dadak AM, Haase VH, Fontana L, Giaccia AJ, Johnson RS . Hypoxia-induced gene expression occurs solely through the action of hypoxia-inducible factor 1α (HIF-1α): role of cytoplasmic trapping of HIF-2α . Mol Cell Biol 2003 ; 23 : 4959 - 4971
Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS . Hypoxia-inducible factor-1α is a positive factor in solid tumor growth . Cancer Res 2000 ; 60 : 4010 - 4015
Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P, Oberhuber G . Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer . Clin Cancer Res 2002 ; 8 : 1831 - 1837
Semenza GL . Targeting HIF-1 for cancer therapy . Nat Rev Cancer 2003 ; 3 : 721 - 732
Sumiyoshi Y, Kakeji Y, Egashira A, Mizokami K, Orita H, Maehara Y . Overexpression of hypoxia-inducible factor 1α and p53 is a marker for an unfavorable prognosis in gastric cancer . Clin Cancer Res 2006 ; 12 : 5112 - 5117
Tian H, McKnight SL, Russell DW . Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells . Genes Dev 1997 ; 11 : 72 - 82
Volm M, Koomagi R . Hypoxia-inducible factor (HIF-1) and its relationship to apoptosis and proliferation in lung cancer . Anticancer Res 2000 ; 20 : 1527 - 1533
Yu J, Zhang L, Hwang PM, Rago C, Kinzler KW, Vogelstein B . Identification and classification of p53-regulated genes . Proc Natl Acad Sci USA 1999 ; 96 : 14517 - 14522
Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW . Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases . Cancer Res 1999 ; 59 : 5830 - 5835
Acknowledgements
We thank Prof. Kyu-Sang Song, School College of Medicine, ChungNam National University, Korea, for the gift of human gastric cancer tissues. This work was supported by a grant from the 21C Frontier Functional Human Genome Project, and by the Drug Target Discovery Project from the Ministry of Science and Technology of Korea, and by the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Song, IS., Wang, AG., Yoon, S. et al. Regulation of glucose metabolism-related genes and VEGF by HIF-1α and HIF-1β, but not HIF-2α, in gastric cancer. Exp Mol Med 41, 51–58 (2009). https://doi.org/10.3858/emm.2009.41.1.007
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2009.41.1.007
Keywords
This article is cited by
-
TRIB3 Interacts with STAT3 to Promote Cancer Angiogenesis
Current Medical Science (2022)
-
Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-α/NF-κB and PI3K/AKT signaling pathway
Cell Death Discovery (2018)
-
The increased expression of DEC1 gene is related to HIF-1α protein in gastric cancer cell lines
Molecular Biology Reports (2012)
-
Effects of hypoxia inducible factor-1alpha siRNA on the invasion of human Hela cells and expression of related proteins
Frontiers of Medicine in China (2009)