Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Laboratory Evaluation
2.3. Nutritional Assessment
2.4. Magnetic Resonance Imaging
2.5. Intestinal Permeability Test
2.6. Glucose Hydrogen Breath Test
2.7. Measurement of Serum LPS
2.8. Synbiotic Supplementation and Dietary Intervention
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the Patients
3.2. Main Outcomes
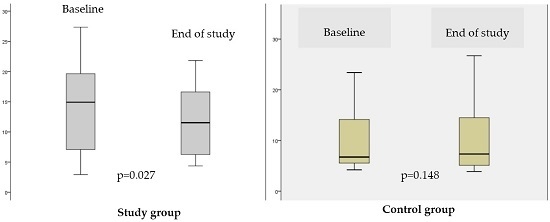
3.2.1. The Effect of Synbiotic on Fatty Liver Evaluated by MRI
3.2.2. The Effect of the Synbiotic on Metabolic Parameters and Hepatic Biochemistry
3.2.3. The Effects of the Synbiotic on Intestinal Parameters: SIBO, LPS Concentration and Intestinal Permeability
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Rozman, D. From nonalcoholic Fatty liver disease to hepatocellular carcinoma: A systems understanding. Dig. Dis. Sci. 2014, 59, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Adams, L.A.; Canbay, A.; Syn, W.K. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 2014, 59, 1174–1197. [Google Scholar] [CrossRef] [PubMed]
- Arendt, B.M.; Allard, J.P. Effect of atorvastatin, vitamin E and C on nonalcoholic fatty liver disease: Is the combination required? Am. J. Gastroenterol. 2011, 106, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.N.; Wang, J.; Muralidharan, S.; Chalasani, S.; Fullenkamp, A.M.; Wilson, L.A.; Sanyal, A.J.; Kowdley, K.V.; Neuschwander-Tetri, B.A.; Brunt, E.M.; et al. Relationship between adipose tissue insulin resistance and liver histology in nonalcoholic steatohepatitis: A pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis trial follow-up study. Hepatology 2012, 56, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Pamuk, G.E.; Sonsuz, A. N-acetylcysteine in the treatment of non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2003, 18, 1220–1221. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; de Ledinghen, V.; Oberti, F.; Mathurin, P.; Wartelle-Bladou, C.; Renou, C.; Sogni, P.; Maynard, M.; Larrey, D.; Serfaty, L.; et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J. Hepatol. 2011, 54, 1011–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shargorodsky, M.; Omelchenko, E.; Matas, Z.; Boaz, M.; Gavish, D. Relation between augmentation index and adiponectin during one-year metformin treatment for nonalcoholic steatohepatosis: Effects beyond glucose lowering? Cardiovasc. Diabetol. 2012, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Zein, C.O.; Lopez, R.; Fu, X.; Kirwan, J.P.; Yerian, L.M.; McCullough, A.J.; Hazen, S.L.; Feldstein, A.E. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: New evidence on the potential therapeutic mechanism. Hepatology 2012, 56, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Cortez-Pinto, H. Non-alcoholic fatty liver disease: What the clinician needs to know. World J. Gastroenterol. 2014, 20, 12956–12980. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P. Pathogenesis of steatohepatitis. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Sakurai, S.; Uemura, M.; Fukui, H.; Morimoto, H.; Tamagawa, Y. Mitochondrial abnormality and oxidative stress in nonalcoholic steatohepatitis. Alcohol. Clin. Exp. Res. 2007, 31, S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, B. Role of gut barrier function in the pathogenesis of nonalcoholic Fatty liver disease. Gastroenterol. Res. Pract. 2015, 2015, 287348. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Cope, K.; Risby, T.H.; Diehl, A.M. Obesity and female gender increase breath ethanol concentration: Potential implications for the pathogenesis of nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2001, 96, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Iacono, A.; Raso, G.M.; Canani, R.B.; Calignano, A.; Meli, R. Probiotics as an emerging therapeutic strategy to treat NAFLD: Focus on molecular and biochemical mechanisms. J. Nutr. Biochem. 2011, 22, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; de Luis, D.A.; Izaola, O.; Conde, R.; Gonzalez Sagrado, M.; Primo, D.; de la Fuente, B.; Gonzalez, J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1090–1095. [Google Scholar] [PubMed]
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Am. J. Clin. Nutr. 2014, 99, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; de Simone, T.; Federico, A.; Terracciano, F.; Tuccillo, C.; di Chicco, M.; Cartenì, M. Gut-liver axis: A new point of attack to treat chronic liver damage? Am. J. Gastroenterol. 2002, 97, 2144–2146. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; Federico, A.; Tuccillo, C.; Terracciano, F.; D’Auria, M.V.; de Simone, C.; del Vecchio Blanco, C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J. Clin. Gastroenterol. 2005, 39, 540–543. [Google Scholar] [PubMed]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Vajro, P.; Mandato, C.; Licenziati, M.R.; Franzese, A.; Vitale, D.F.; Lenta, S.; Caropreso, M.; Vallone, G.; Meli, R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Won, G.L.; Chim, A.M.; Chu, W.C.; Yeung, D.K.; Li, K.C.; Chan, H.L. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann. Hepatol. 2013, 12, 256–262. [Google Scholar] [PubMed]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [PubMed]
- Fak, F.; Backhed, F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe-/- mice. PLoS ONE 2012, 7, e46837. [Google Scholar] [CrossRef] [PubMed]
- Poutahidis, T.; Kleinewietfeld, M.; Smillie, C.; Levkovich, T.; Perrotta, A.; Bhela, S.; Varian, B.J.; Ibrahim, Y.M.; Lakritz, J.R.; Kearney, S.M.; et al. Microbial reprogramming inhibits Western diet-associated obesity. PLoS ONE 2013, 8, e68596. [Google Scholar] [CrossRef] [PubMed]
- Tsurumaki, M.; Kotake, M.; Iwasaki, M.; Saito, M.; Tanaka, K.; Aw, W.; Fukuda, S.; Tomita, M. The application of omics technologies in the functional evaluation of inulin and inulin-containing prebiotics dietary supplementation. Nutr. Diabetes 2015, 5, e185. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Mansi, C.; Dulbecco, P.; Savarino, V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition 2006, 22, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Group IDFETFC. The metabolic syndrome-a new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [PubMed]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Progress Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Bydder, M.; Yokoo, T.; Hamilton, G.; Middleton, M.S.; Chavez, A.D.; Schwimmer, J.B.; Lavine, J.E.; Sirlin, C.B. Relaxation effects in the quantification of fat using gradient echo imaging. Magn. Reson. Imaging 2008, 26, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Hines, C.D.; Frydrychowicz, A.; Hamilton, G.; Tudorascu, D.L.; Vigen, K.K.; Yu, H.; McKenzie, C.A.; Sirlin, C.B.; Brittain, J.H.; Reeder, S.B. T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J. Magn. Reson. Imaging 2011, 33, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Hines, C.D.; Yu, H.; Shimakawa, A.; McKenzie, C.A.; Warner, T.F.; Brittain, J.H.; McKenzie, C.A.; Sirlin, C.B.; Brittain, J.H.; Reeder, S.B. Quantification of hepatic steatosis with 3-T MR imaging: Validation in ob/ob mice. Radiology 2010, 254, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Idilman, I.S.; Aniktar, H.; Idilman, R.; Kabacam, G.; Savas, B.; Elhan, A.; Celik, A.; Bahar, K.; Karcaaltincaba, M. Hepatic steatosis: Quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 2013, 267, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Meisamy, S.; Hines, C.D.; Hamilton, G.; Sirlin, C.B.; McKenzie, C.A.; Yu, H.; Brittain, J.H.; Reeder, S.B. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: Blinded comparison with MR spectroscopy. Radiology 2011, 258, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; Cruite, I.; Hamilton, G.; Sirlin, C.B. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J. Magn. Reson. Imaging 2011, 34, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, T.; Shiehmorteza, M.; Hamilton, G.; Wolfson, T.; Schroeder, M.E.; Middleton, M.S.; Bydder, M.; Gamst, A.C.; Kono, Y.; Kuo, A.; et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology 2011, 258, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Nickel, M.D.; Kannengiesser, S.A.; Dale, B.M.; Kiefer, B.; Bashir, M.R. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn. Reson. Med. 2014, 72, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Desai, A.; Hamilton, G.; Wolfson, T.; Gamst, A.; Lam, J.; Clark, L.; Hooker, J.; Chavez, T.; Ang, B.D.; et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 2015, 274, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Koplay, M.; Sivri, M.; Erdogan, H.; Nayman, A. Importance of imaging and recent developments in diagnosis of nonalcoholic fatty liver disease. World J. Hepatol. 2015, 7, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Tan, J.; Sun, M.; Hamilton, G.; Bydder, M.; Wolfson, T.; Gamst, A.C.; Middleton, M.; Brunt, E.M.; Loomba, R.; et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013, 267, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, T.; Bydder, M.; Hamilton, G.; Middleton, M.S.; Gamst, A.C.; Wolfson, T.; Hassanein, T.; Patton, H.M.; Lavine, J.E.; Schwimmer, J.B.; et al. Nonalcoholic fatty liver disease: Diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology 2009, 251, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Talwalkar, J.A.; Yin, M.; Glaser, K.J.; Sanderson, S.O.; Ehman, R.L. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology 2011, 259, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Talwalkar, J.A.; Glaser, K.J.; Manduca, A.; Grimm, R.C.; Rossman, P.J.; Fidler, J.L.; Ehman, R.L. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin. Gastroenterol. Hepatol. 2007, 5, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Vilela, E.G.; Ferrari, M.L.A.; Torres, H.O.G.; Pinto, A.G.; Aguirre, A.C.C.; Martins, F.P.; Goulart, E.M.A.; Cunha, A.S. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol. 2008, 43, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.; Menzies, I. Intestinal permeability: Functional assessment and significance. Clin. Sci. 1992, 82, 471–488. [Google Scholar] [CrossRef] [PubMed]
- Vilela, E.G.; Ataliba, G.S.; Torres, H.O.G.; Ferrari, M.L.A.; Cunha, A.S. Intestinal permeability measurement in healthy subjects: Standartizationof technique and analysis of results. Int. J. Inflamm. Bowel Dis. 2015, 2, 57–61. [Google Scholar]
- Kerlin, P.; Wrong, I. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology 1988, 95, 982–988. [Google Scholar] [CrossRef]
- Holanda, L.B.; Filho, A.A.B. Métodosaplicadoseminquéritosalimentares. Rev. Paul. Pediatr. 2006, 24, 62–70. [Google Scholar]
- Núcleo de Estudos e PesquisaemAlimentação. TabelaBrasileira de Composição de Alimentos/NEPA-UNICAMP, 4th ed.; Book Editora: Campinas, Brazil, 2011; pp. 1–161. [Google Scholar]
- Bellentani, S.; Dalle, R.G.; Suppini, A.; Marchesini, G. Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology 2008, 47, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Departamento de AtençãoBásica, Secretaria de Atenção à Saúde, Ministério da Saúde. GuiaAlimentar Para a PopulaçãoBrasileira: PromovendoaAlimentaçãoSaudável/Ministério da Saúde, Secretaria de Saúde,Departamento de AtençãoBásica; Ministério da Saúde: Brasília, Brazil, 2005; pp. 1–210. [Google Scholar]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A.; et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1081–1093. [Google Scholar] [PubMed]
- Chen, Y.; Wang, X.; Wang, J.; Yan, Z.; Luo, J. Excess body weight and the risk of primary liver cancer: An updated meta-analysis of prospective studies. Eur. J. Cancer 2012, 48, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Nazare, J.A.; Smith, J.; Borel, A.L.; Aschner, P.; Barter, P.; van Gaal, L.; Tan, C.E.; Wittchen, H.U.; Matsuazawa, Y.; Kadowaki, T.; et al. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study). Am. J. Cardiol. 2015, 115, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Cheung, O.; Kapoor, A.; Puri, P.; Sistrun, S.; Luketic, V.A.; Sargeant, C.C.; Contos, M.J.; Shiffman, M.L.; Stravitz, R.T.; Sterling, R.K.; et al. The impact of fat distribution on the severity of nonalcoholic fatty liver disease and metabolic syndrome. Hepatology 2007, 46, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Giral, P.; Charlotte, F.; Bruckert, E.; Thibault, V.; Theodorou, I.; Khalil, L.; Turpin, G.; Opolon, P.; Poynard, T. Liver fibrosis in overweight patients. Gastroenterology 2000, 118, 1117–1123. [Google Scholar] [CrossRef]
- Ande, S.R.; Nguyen, K.H.; Grégoire Nyomba, B.L.; Mishra, S. Prohibitin-induced, obesity-associated insulin resistance and accompanying low-grade inflammation causes NASH and HCC. Sci. Rep. 2016, 23, 23608. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Caricilli, A.M.; Saad, M.J. The role of gut microbiota on insulin resistance. Nutrients 2013, 5, 829–851. [Google Scholar] [CrossRef] [PubMed]
- Duseja, A.; Chawla, Y.K. Obesity and NAFLD: The role of bacteria and microbiota. Clin. Liver Dis. 2014, 18, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, J.L.; May, T. The effect of a methanogen, Methanobrevibacter smithii, on the growth rate, organic acid production, and specific ATP activity of three predominant ruminal cellulolytic bacteria. Curr. Microbiol. 2000, 40, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Sumitani, K.; Tokunaga, M.; Ishihara, N.; Okubo, T.; Fujisawa, T. Consumption of partially hydrolysed guar gum stimulates Bifidobacteria and butyrate-producing bacteria in the human large intestine. Benef. Microbes 2015, 6, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Uzu, K.; Yoshida, T.; Hamad, E.M.; Kawakami, H.; Matsuyama, H.; Abd El-Gawad, I.A.; Imaizumi, K. Effects of milk fermented by Lactobacillus gasseri SBT2055 on adipocyte size in rats. Br. J. Nutr. 2008, 99, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Hamad, E.M.; Sato, M.; Uzu, K.; Yoshida, T.; Higashi, S.; Kawakami, H.; Kadooka, Y.; Matsuyama, H.; Abd El-Gawad, I.A.; Imaizumi, K. Milk fermented by Lactobacillus gasseri SBT2055 influences adipocyte size via inhibition of dietary fat absorption in Zucker rats. Br. J. Nutr. 2009, 101, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Anders, A.J.; van den Borne, J.J.; de Graaf, C.; Hulshof, T.; Jonathan, M.C.; Kristensen, M.; Mars, M.; Schols, H.A.; Feskens, E.J. Effects of dietary fibre on subjective appetite, energy intake and body weight: A systematic review of randomized controlled trials. Obes. Rev. 2011, 12, 724. [Google Scholar] [CrossRef] [PubMed]
- Birketvedt, G.S.; Shimshi, M.; Erling, T.; Florholmen, J. Experiences with three different fiber supplements in weight reduction. Med. Sci. Monit. 2005, 11, I5–I8. [Google Scholar]
- Pittler, M.H.; Ernst, E. Dietary supplements for body-weight reduction: A systematic review. Am. J. Clin. Nutr. 2004, 79, 529–536. [Google Scholar] [PubMed]
- Liber, A.; Szajewska, H. Effect of oligofructose supplementation on body weight in overweight and obese children: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2014, 112, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.; Wood, S.; Pelletier, X.; Donazzolo, Y.; Gahler, R.; Bellisle, F. Effects of a 3-month supplementation with a novel soluble highly viscous polysaccharide on anthropometry and blood lipids in nondieting overweight or obese adults. J. Hum. Nutr. Diet. 2011, 24, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Brockman, D.A.; Chen, X.; Gallaher, D.D. High-viscosity dietary fibers reduce adiposity and decrease hepatic steatosis in rats fed a high-fat diet. J. Nutr. 2014, 144, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Guess, N.D.; Dornhorst, A.; Oliver, N.; Bell, J.D.; Thomas, E.L.; Frost, G.S. A randomized controlled trial: The effect of inulin on weight management and ectopic fat in subjects with prediabetes. Nutr. Metab. 2015, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, H.R.; Lee, J.H.; Shin, Y.H.; Shim, J.Y. Association between serum uric acid and non-alcoholic fatty liver disease in Korean adults. Clin. Chem. Lab. Med. 2010, 48, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.C.; Suh, S.Y.; Suh, A.R.; Ahn, H.Y. The relationship between normal serum uric acid and nonalcoholic fatty liver disease. J. Korean Med. Sci. 2011, 26, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Sun, J.; He, J.; Chen, F.; Chen, R.; Chen, H. Effect of Probiotics on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. PLoS ONE 2015, 10, e0132121. [Google Scholar] [CrossRef] [PubMed]
- Abd El-KaderEman, S.M.; El-Den Ashmawy, S.M. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol. 2015, 7, 846–858. [Google Scholar]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Volynets, V.; Küper, M.A.; Strahl, S.; Maier, I.B.; Spruss, A.; Wagnerberger, S.; Königsrainer, A.; Bischoff, S.C.; Bergheim, I. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD). Dig. Dis. Sci. 2012, 57, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Paik, Y.H.; Seki, E. Toll-like receptor signaling and liver fibrosis. Gastroenterol. Res. Pract. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Gastaldelli, A.; Vanni, E.; Gambino, R.; Cassader, M.; Baldi, S.; Ponti, V.; Pagano, G.; Ferrannini, E.; Rizzetto, M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: Sites and mechanisms. Diabetologia 2005, 48, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Sabate, J.M.; Jouet, P.; Harnois, F.; Mechler, C.; Msika, S.; Grossin, M.; Coffin, B. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: A contributor to severe hepatic steatosis. Obes. Surg. 2008, 18, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, A.; Mottershead, M.; Syn, W.K.; Jones, R.; Smith, S.; Nwokolo, C.U. Ciprofloxacin suppresses bacterial overgrowth, increases fasting insulin but does not correct low acylated ghrelin concentration in non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2005, 22, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Shanab, A.A.; Scully, P.; Crosbie, O.; Buckley, M.; O’Mahony, L.; Shanahan, F.; Gazareen, S.; Murphy, E.; Quigley, E.M. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: Association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig. Dis. Sci. 2011, 56, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Wigg, A.J.; Roberts-Thomson, I.C.; Dymock, R.B.; McCarthy, P.J.; Grose, R.H.; Cummins, A.G. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 2001, 48, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Riordan, S.M.; Duncombe, V.M.; Thomas, M.C.; Nagree, A.; Bolin, T.D.; McIver, C.J.; Williams, R. Small intestinal bacterial overgrowth, intestinal permeability, and non-alcoholic steatohepatitis. Gut 2002, 50, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Mues, M.; Koutrolos, M.; Rasbi, Z.A.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Erdman, S.E.; Rao, V.P.; Olipitz, W.; Taylor, C.L.; Jackson, E.A.; Levkovich, T.; Lee, C.W.; Horwitz, B.H.; Fox, J.G.; Ge, Z.; et al. Unifying roles for regulatory T cells and inflammation in cancer. Int. J. Cancer 2010, 126, 1651–1665. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Bedogni, G.; Baviera, G.; Giorgio, V.; Porro, E.; Paris, C.; Giammaria, P.; Reali, L.; Anania, F.; Nobili, V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2014, 39, 1276–1285. [Google Scholar] [PubMed]
- Mykhal’chyshyn, H.P.; Bodnar, P.M.; Kobyliak, N.M. Effect of probiotics on proinflammatory cytokines level in patients with type 2 diabetes and nonalcoholic fatty liver disease. Likars’ka Sprava 2013, 2, 56–62. [Google Scholar] [PubMed]
- Solga, S.F.; Buckley, G.; Clark, J.M.; Horska, A.; Diehl, A.M. The effect of a probiotic on hepatic steatosis. J. Clin. Gastroenterol. 2008, 42, 1117–1119. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (n = 49) | Study Group (n = 26) | Control Group (n = 23) | p Value |
|---|---|---|---|---|
| Steatosis | 0.321 ¶ | |||
| 5%–33% | 12 (24.5%) | 4 (15.4%) | 8 (34.78%) | |
| 34%–66% | 27 (55.1%) | 17 (65.4%) | 10 (43.5%) | |
| >66% | 10 (20.4%) | 5 (19.2%) | 5 (21.7%) | |
| Lobular Inflammation | 0.08 ¶ | |||
| <2 foci ×200 | 31 (63.3%) | 17 (65.4%) | 14 (60.9%) | |
| 2–4 foci ×200 | 14 (28.6%) | 5 (21.7%) | 9 (34.6%) | |
| >4 foci ×200 | 4 (8.2%) | 4 (17.4%) | 0 (0.0%) | |
| Ballooning | 0.166 ¶ | |||
| Few balloon cells | 24 (49.0%) | 16 (61.5%) | 8 (34.8%) | |
| Prominent balloon cells | 25 (51.0%) | 10 (38.5%) | 15 (65.2%) | |
| Fibrosis Stage | 0.502 ¶ | |||
| F0 | 22 (44.9%) | 12 (46.2%) | 10 (43.5%) | |
| F1 | 16 (32.7%) | 7 (26.9%) | 9 (39.1%) | |
| F2 | 3 (6.1%) | 3 (11.5%) | 0 (0.0%) | |
| F3 | 5 (10.2%) | 3 (11.5%) | 2 (8.7%) | |
| F4 | 3 (6.1%) | 1 (3.8%) | 2 (8.7%) | |
| NAS Score | 0.9095 ¶ | |||
| 3 | 4 (8%) | 2 (7.7%) | 2 (8.7%) | |
| 4 | 21 (42%) | 12 (46.2%) | 9 (39.1%) | |
| ≥5 | 24 (48%) | 12 (46.2%) | 12 (52.2%) |
| Variable | Total (n = 50) | Study Group (n = 27) | Control Group (n = 23) | p Value |
|---|---|---|---|---|
| SteatosisGrades on MRI ** | ||||
| MRI PDFF (median, range) | 9 (2.9; 27.4) | 14.9 (2.9; 27.4) | 6.4 (3.9; 23.4) | 0.040 † |
| Grades 0–1 steatosis (n/%) | 35 (70.0%) | 16 (59.2%) | 19 (82.6%) | 0.073 * |
| Grades 2–3 steatosis (n/%) | 15 (30.0%) | 11 (40.7%) | 4 (17.4%) | |
| Fibrosis on Elastography | ||||
| Shear stiffness (kPa) (median, range) | 3.23 (2.31; 8.74) | 3.41 (2.46; 7.59) | 3.05(2.31; 8.74) | 0.416 † |
| Normal liver tissue (n/%) | 16 (34.0%) | 7 (29.2%) | 9 (39.1%) | 0.471 * |
| Any grade of fibrosis (n/%) | 31 (66.0%) | 17 (70.8%) | 14 (60.9%) | |
| NAFLD Fibrosis Score | 0.552 ¶ | |||
| Absence of significant fibrosis (n/%) | 17 (43.0%) | 9 (33.3%) | 8 (34.8%) | |
| Indeterminate (n/%) | 25 (50.0%) | 15 (55.6%) | 10 (43.5%) | |
| Presence of significant fibrosis (n/%) | 8 (16.0%) | 3 (11.1%) | 5 (21.7%) |
| Variable | Total (n = 50) | Study Group (n = 27) | Control Group (n = 23) | p Value |
|---|---|---|---|---|
| Metabolic Characteristics | ||||
| Obesity (n/%) | 49 (98.0%) | 27 (100.0%) | 22 (95.3%) | 0.460 ¶ |
| High body fat percentage (n/%) | 40 (87.0%) | 23 (85.0%) | 17 (88.5%) | 1.000 ¶ |
| Hypertension (n/%) | 38 (76.0%) | 19 (70.4%) | 19 (82.6%) | 0.313 * |
| Hypercholesterolemia (n/%) | 38 (76.0%) | 21 (77.8%) | 17 (73.9%) | 0.750 * |
| Low HDL-c (n/%) | 20 (40.0%) | 11 (40.7%) | 9 (39.1%) | 0.980 * |
| Hypertriglyceridemia (n/%) | 31 (62.0%) | 19 (70.4%) | 12 (52.2%) | 0.186 * |
| Insulin resistance | 7 (14%) | 7 (25.0%) | 0 (0%) | 0.176 § |
| Glucose intolerance (n/%) | 14 (28.0%) | 6 (21.4%) | 8 (36.4%) | |
| Type 2 diabetes | 19 (38.0%) | 9 (32.1%) | 10 (45.5%) | |
| Metabolic Syndrome (n/%) | 49 (98.0%) | 26 (96.3%) | 23 (100.0%) | 1.000 ¶ |
| Sedentarism | 35 (70.0%) | 16 (59.3%) | 16 (82.6%) | 0.073 * |
| Hepatic Biochemistry | ||||
| AST (× ref. value) (median, range) | 0.9 (0.4;3.8) | 0.9 (0.4;3.8) | 0.9 (0.5;3.7) | 0.869 † |
| ALT (× ref. value) (median, range) | 0.9 (0.3;5.5) | 0.9 (0.3;5.5) | 0.9 (0.4;3.2) | 0.899 † |
| ALP (× ref. value) (median, range) | 0.7 (0.3;7.4) | 0.7 (0.3;7.4) | 0.7 (0.5;3.1) | 0.442 † |
| GGT (× ref. value) (median, range) | 1.7 (0.3;21.7) | 1.6 (0.5;17.1) | 1.8 (0.3;21.7) | 0.719 † |
| Albumin (g/dL) (mean ± SD) | 4.4 ± 0.3 | 4.4 ± 0.3 | 4.3 ± 0.4 | 0.429 # |
| Total bilirubin (mg/dL) (median, range) | 0.6 (0.2;2.4) | 0.6 (0.2;2.4) | 0.6 (0.3;2.3) | 0.5000 † |
| Platelets (/mm3) (mean ± SD) | 239,560 ± 75,955 | 242,555 ± 71,910 | 236,043 ± 81,941 | 0.766 # |
| Intestinal Parameters | Total (n = 50) | Study Group (n = 27) | Control Group (n = 23) | p Value |
|---|---|---|---|---|
| SIBO (n/%) | 2 (4.0%) | 1 (3.7%) | 1 (4.3%) | 1.00 ¶ |
| LPS (EU/mL) (median, range) | 0.67 (0.28;1.66) | 0.69 (0.34;1.43) | 0.63 (0.28;1.66) | 0.365 † |
| Intestinal Permeability Test | ||||
| % lactulose excretion (median, range) | 0.222 (0.010;1.140) | 0.270 (0.010;0.590) | 0.175 (0.010;1.140) | 0.780 † |
| Altered excretion of lactulose | 23 (51.1%) | 15 (55.6%) | 8 (44.4%) | 0.465 * |
| Normal excretion of lactulose | 22 (48.9%) | 12 (44.4%) | 10 (55.6%) | |
| % mannitol excretion (mean ± SD) | 17.65±6.61 | 16.61 ± 5.53 | 18.70 ± 7.69 | 0.294 # |
| Altered excretion of mannitol | 2 (4.4%) | 1 (3.7%) | 1 (5.6%) | 1.00 ¶ |
| Normal excretion of mannitol | 43 (95.6%) | 26 (96.3%) | 17 (94.4%) | |
| Lactulose/mannitol (median, range) | 0.014 (0.001;0.146) | 0.016 (0.001;0.146) | 0.011 (0.001;0.116) | 0.677 † |
| Altered ratio lactulose/mannitol | 23 (51.1%) | 15 (55.6%) | 8 (44.4%) | 0.465 * |
| Normal ratio lactulose/mannitol | 22 (48.9%) | 12 (44.4%) | 10 (55.6%) |
| Variable | Study Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Before | After | p Value | Before | After | p Value | |
| Metabolic Variables | ||||||
| Body weight (kg) | 85.2 ± 14.6 | 83.9 ± 14.0 | 0.006 # | 84.5 ± 20.4 | 84.5 ± 20.2 | 0.837 † |
| BMI (kg/m2) | 32.5 ± 4.0 | 32.1 ± 3.8 | 0.005 # | 32.5 ± 4.0 | 32.3 ± 5.8 | 0.924 # |
| WC (cm) | 107.8 ± 10.8 | 105.9 ± 11.2 | 0.001 # | 104.0 ± 13.55 | 104.9 ±13.8 | 0.600 # |
| Body fat (%) * | 37.3 (27.2; 44.1) | 36.6 (11.8; 45.0) | 0.987 † | 32.6 ± 5.85 | 31.5 (22.1; 45.8) | 0.576 # |
| BEE (kcal) * | 1645 (1232; 2599) | 1545 (1207; 2508) | 0.256 † | 1560 (11,061; 3191) | 1609 (1345; 2980) | 0.573 † |
| Ferritin (ng/dL) | 129 (23.3; 685.0) | 131 (14.2; 530.0) | 0.903 † | 134 (16.2; 377.5) | 151 (11.1; 943) | 0.920 † |
| Glucose (mg/dL) | 99.0 (80; 293) | 101.0 (83; 322) | 0.207 † | 109 (85; 280) | 127 (84; 326) | 0.123 † |
| Cholesterol (mg/dL) | 205.4 ± 34.6 | 208.9 ± 38.4 | 0.605 # | 190.9 ± 42.5 | 195.5 ± 30.4 | 0.458 # |
| LDL-c (mg/dL) | 125.2 ± 28.0 | 125.8 ± 35.5 | 0.928 # | 109.3 ± 32.7 | 104.2 ± 24.8 | 0.365 # |
| HDL-c (mg/dL) | 44.9 ± 10.3 | 44.3 ± 8.9 | 0.685 # | 46.7 ± 11.7 | 46.2 ± 10.5 | 0.822 # |
| VLDL-c (mg/dL) | 35 (20; 66) | 35 (16; 122) | 0.485 † | 29 (13.9; 130) | 37 (13.3; 133) | 0.006 † |
| Triglycerides (mg/dL) | 176 (100; 328) | 173 (81; 572) | 0.461 † | 147 (69–610) | 183 (66.4; 663) | 0.006 † |
| Uric acid (mg/dL) | 5.5 ± 1.4 | 4.9±1.2 | 0.006 # | 5.7 ± 1.9 | 5.2 ± 1.6 | 0.167 † |
| Liver Biochemistry and Platelet Count | ||||||
| ALT (× ref. value) | 0.9 (0.3; 5.5) | 0.8 (0.2; 4.5) | 0.568 † | 0.9 (0.4; 3.1) | 1.0 (0.4; 4.4) | 0.738 † |
| AST (× ref. value) | 0.9 (0.4; 3.8) | 0.9 (0.3; 4.1) | 0.422 † | 0.9 (0.5; 3.7) | 0.9 (0.3; 4.3) | 0.584 † |
| ALP (× ref. value) | 0.7 (0.3; 7.4) | 0.7 (0.3; 1.2) | 0.939 † | 0.7 (0.5; 3.1) | 0.8 (0.5; 2.5) | 0.196 † |
| GGT (× ref. value) | 1.6 (0.5; 17.1) | 1.7 (0.6; 9.4) | 0.990 † | 1.8 (0.3; 21.7) | 1.5 (0.5; 12.1) | 0.858 † |
| Albumin (mg/dL) | 4.4 ± 0.3 | 4.4 ± 0.4 | 0.194 # | 4.3 ± 0.4 | 4.2 ± 0.4 | 0.173 # |
| Total bilirubin (mg/dL) | 0.6 (0.2; 2.4) | 0.5 (0.3; 2.2) | 0.137 † | 0.6 (0.3; 2.3) | 0.7 (0.3; 2.3) | 0.179 † |
| Platelets (/mm3) | 242,556 ± 71,911 | 239,519 ± 85,325 | 0.518 † | 236,044 ± 242,556 | 248,391 ± 78,480 | 0.078 # |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferolla, S.M.; Couto, C.A.; Costa-Silva, L.; Armiliato, G.N.A.; Pereira, C.A.S.; Martins, F.S.; Ferrari, M.D.L.A.; Vilela, E.G.; Torres, H.O.G.; Cunha, A.S.; et al. Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients 2016, 8, 397. https://doi.org/10.3390/nu8070397
Ferolla SM, Couto CA, Costa-Silva L, Armiliato GNA, Pereira CAS, Martins FS, Ferrari MDLA, Vilela EG, Torres HOG, Cunha AS, et al. Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients. 2016; 8(7):397. https://doi.org/10.3390/nu8070397
Chicago/Turabian StyleFerolla, Silvia M., Cláudia A. Couto, Luciana Costa-Silva, Geyza N. A. Armiliato, Cristiano A. S. Pereira, Flaviano S. Martins, Maria De Lourdes A. Ferrari, Eduardo G. Vilela, Henrique O. G. Torres, Aloísio S. Cunha, and et al. 2016. "Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis" Nutrients 8, no. 7: 397. https://doi.org/10.3390/nu8070397
APA StyleFerolla, S. M., Couto, C. A., Costa-Silva, L., Armiliato, G. N. A., Pereira, C. A. S., Martins, F. S., Ferrari, M. D. L. A., Vilela, E. G., Torres, H. O. G., Cunha, A. S., & Ferrari, T. C. A. (2016). Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients, 8(7), 397. https://doi.org/10.3390/nu8070397