Effect of Immune-Enhancing Enteral Nutrition Enriched with or without Beta-Glucan on Immunomodulation in Critically Ill Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Randomization and Intervention
2.3. Anthropometric Parameters and Blood Collection
2.4. Serum Lipid Profiles and Glucose
2.5. Serum Nutritional Status
2.6. Serum Liver and Renal Function
2.7. Leukocyte Count and Serum High-Sensitivity C-Reactive Protein
2.8. Peripheral Blood Mononuclear Cells
2.9. Cytokine Assay in Serum and PBMC Supernatants
2.10. NK Cell Activity
2.11. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Effects on NK Cell Activity and Serum Prealbumin Following Seven Days of Tube Feeding of the Control and the IMHP Groups with and without β-Glucan
3.3. Effects on Serum CRP and Cytokines and PBMC Cytokine Production Following Seven Days of Tube Feeding in the Control and the IMHP Groups with and without β-Glucan
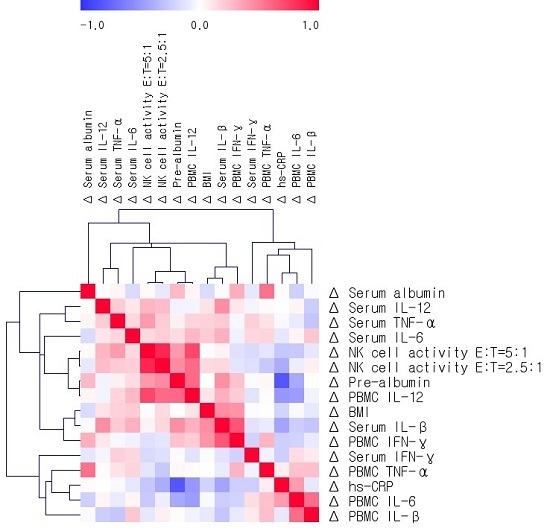
3.4. Relationships among Changes in BMI, Serum Albumin, Prealbumin, Cytokines, PBMC Cytokine Production, and NK Cell Activity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BEE | basal energy expenditure |
| BMI | body mass index |
| BUN | blood urea nitrogen |
| E | effector cell |
| γ-GTP | gamma-glutamyl transpeptidase |
| GOT | glutamic oxalacetic transaminase |
| GPT | glutamic pyruvate transaminase |
| HDL | High-density lipoprotein |
| hs-CRP | high-sensitivity C-reactive protein |
| ICU | intensive care unit |
| IFN | interferon |
| IL | interleukin |
| IMHP | high-protein enteral nutrition with immune-modulating nutrients |
| LDL | Low-density lipoprotein |
| NK | natural killer |
| PBMC | peripheral blood mononuclear cell |
| T | target cell |
| TNF | tumor necrosis factor |
References
- Kreymann, K.G.; Berger, M.M.; Deutz, N.E.; Hiesmayr, M.; Jolliet, P.; Kazandjiev, G.; Nitenberg, G.; van den Berghe, G.; Wernerman, J.; DGEM (German Society for Nutritional Medicine); et al. ESPEN guidelines on enteral nutrition: Intensive care. Clin. Nutr. 2006, 25, 210–223. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Martindale, R.G.; Vanek, V.W.; McCarthy, M.; Roberts, P.; Taylor, B.; Ochoa, J.B.; Napolitano, L.; Cresci, G.; A.S.P.E.N. Board of Directors; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N). JPEN J. Parenter. Enteral Nutr. 2009, 33, 277–316. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, R.A.; Wischmeyer, P.E. Clinical review: Optimizing enteral nutrition for critically ill patients—A simple data-driven formula. Crit. Care 2011, 15, 234. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 Fatty acids, inflammation, and immunity—Relevance to postsurgical and critically ill patients. Lipids 2004, 39, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, J.; Kong, F.; Lin, J.; Gao, Y. Induction of immunomodulating cytokines by a new polysaccharide-peptide complex from culture mycelia of Lentinus edodes. Immunopharmacology 1998, 40, 187–198. [Google Scholar] [CrossRef]
- Ren, L.; Perera, C.; Hemar, Y. Antitumor activity of mushroom polysaccharides: A review. Food Funct. 2012, 3, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vashishta, A.; Saraswat-Ohri, S.; Vetvickova, J. Immunological effects of yeast- and mushroom-derived beta-glucans. J. Med Food. 2008, 11, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Stanilka, J.M.; Rowe, C.A.; Esteves, E.A.; Nieves, C., Jr.; Spaiser, S.J.; Christman, M.C.; Langkamp-Henken, B.; Percival, S.S. Consuming Lentinula edodes (Shiitake) Mushrooms Daily Improves Human Immunity: A Randomized Dietary Intervention in Healthy Young Adults. J. Am. Coll. Nutr. 2015, 34, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Ziaei, J.E.; Esfahani, A.; Jafarabadi, M.A.; Movassaghpourakbari, A.; Farrin, N. Effect of beta glucan on white blood cell counts and serum levels of IL-4 and IL-12 in women with breast cancer undergoing chemotherapy: A randomized double-blind placebo-controlled clinical trial. Asian Pac. J. Cancer Prev. 2014, 15, 5733–5739. [Google Scholar] [CrossRef] [PubMed]
- Kirmaz, C.; Bayrak, P.; Yilmaz, O.; Yuksel, H. Effects of glucan treatment on the Th1/Th2 balance in patients with allergic rhinitis: A double-blind placebo-controlled study. Eur. Cytokine Netw. 2005, 16, 128–134. [Google Scholar] [PubMed]
- Bergendiova, K.; Tibenska, E.; Majtan, J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 2011, 111, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Richter, J.; Svozil, V.; Rajnohová Dobiášová, L.; Král, V. Placebo-driven clinical trials of yeast-derived β-(1–3) glucan in children with chronic respiratory problems. Ann. Transl. Med. 2013, 1, 26. [Google Scholar] [PubMed]
- Richter, J.; Svozil, V.; Král, V.; Rajnohová Dobiášová, L.; Stiborová, I.; Vetvicka, V. Clinical trials of yeast-derived β-(1,3) glucan in children: Effects on innate immunity. Ann. Transl. Med. 2014, 2, 15. [Google Scholar] [PubMed]
- Mantovani, M.S.; Bellini, M.F.; Angeli, J.P.; Oliveira, R.J.; Silva, A.F.; Ribeiro, L.R. beta-Glucans in promoting health: Prevention against mutation and cancer. Mutat. Res. 2008, 658, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Davis, J.M.; Carmichael, M.D. Immune modulating effects of β-glucan. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Akramiene, D.; Kondrotas, A.; Didziapetriene, J.; Kevelaitis, E. Effects of beta-glucans on the immune system. Medicina (Kaunas) 2007, 43, 597–606. [Google Scholar] [PubMed]
- Zeković, D.B.; Kwiatkowski, S.; Vrvić, M.M.; Jakovljević, D.; Moran, C.A. Natural and modified (1→3)-beta-D-glucans in health promotion and disease alleviation. Natural and modified (1→3)-beta-D-glucans in health promotion and disease alleviation. Crit. Rev. Biotechnol. 2005, 25, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Carlet, J.M.; Masur, H.; Gerlach, H.; Calandra, T.; Cohen, J.; Gea-Banacloche, J.; Keh, D.; Marshall, J.C.; Parker, M.M.; et al. Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit. Care Med. 2004, 32, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 2008, 36, 296–327. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: http://www.clinicaltrials.gov (accessed on 4 October 2015).
- Donaldson-Andersen, J.; Fitzsimmons, L. Metabolic requirements of the critically ill, mechanically ventilated trauma patient: Measured versus predicted energy expenditure. Nutr. Clin. Pract. 1998, 13, 25–31. [Google Scholar] [CrossRef]
- Moretta, A.; Bottino, C.; Mingari, M.C.; Biassoni, R.; Moretta, L. What is a natural killer cell? Nat. Immunol. 2002, 3, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Meyer, F.; Matthies, B.; Pross, M.; Koenig, W.; Lippert, H. Immunomodulation by perioperative administration of n-3 fatty acids. Br. J. Nutr. 2002, 87, S89–S94. [Google Scholar] [CrossRef] [PubMed]
- Forel, J.M.; Chiche, L.; Thomas, G.; Mancini, J.; Farnarier, C.; Cognet, C.; Guervilly, C.; Daumas, A.; Vély, F.; Xéridat, F.; et al. Phenotype and functions of natural killer cells in critically-ill septic patients. PLoS ONE 2012, 7, e50446. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, A.G.; Nielsen, J.S.; Tønnesen, E.; Krog, J. Expression of NK cell and monocyte receptors in critically ill patients--potential biomarkers of sepsis. Scand. J. Immunol. 2015, 81, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Pelizon, A.C.; Kaneno, R.; Soares, A.M.; Meira, D.A.; Sartori, A. Immunomodulatory activities associated with beta-glucan derived from Saccharomyces cerevisiae. Physiol. Res. 2005, 54, 557–564. [Google Scholar] [PubMed]
- Yatawara, L.; Wickramasinghe, S.; Nagataki, M.; Takamoto, M.; Nomura, H.; Ikeue, Y.; Watanabe, Y.; Agatsuma, T. Aureobasidium-derived soluble branched (1,3–1,6) beta-glucan (Sophy beta-glucan) enhances natural killer activity in Leishmania amazonensis-infected mice. Korean J. Parasitol. 2009, 47, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fonseca-Guimaraes, F.; Adib-Conquy, M.; Cavaillon, J.M. Natural killer (NK) cells in antibacterial innate immunity: Angels or devils? Mol. Med. 2012, 18, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Narni-Mancinelli, E.; Jaeger, B.N.; Bernat, C.; Fenis, A.; Kung, S.; De Gassart, A.; Mahmood, S.; Gut, M.; Heath, S.C.; Estellé, J.; et al. Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science 2012, 335, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.P.; Shih, C.C.; Chu, C.M.; Huang, C.Y.; Hua, C.C.; Liu, Y.C.; Chuang, D.Y. Effect of interleukin-17 on in vitro cytokine production in healthy controls and patients with severe sepsis. J. Formos. Med. Assoc. 2015, 114, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Mukaro, V.R.; Costabile, M.; Murphy, K.J.; Hii, C.S.; Howe, P.R.; Ferrante, A. Leukocyte numbers and function in subjects eating n-3 enriched foods: Selective depression of natural killer cell levels. Arthritis Res. Ther. 2008, 10, R57. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Jensen, G.L.; Koletzko, B.V.; Singer, P.; Wanten, G.J. Lipid emulsions in parenteral nutrition of intensive care patients: Current thinking and future directions. Intensive Care Med. 2010, 36, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Ott, J.; Hiesgen, C.; Mayer, K. Lipids in critical care medicine. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Barros, K.V.; Cassulino, A.P.; Schalch, L.; Della Valle Munhoz, E.; Manetta, J.A.; Calder, P.C.; Flor Silveira, V.L. Pharmaconutrition: Acute fatty acid modulation of circulating cytokines in elderly patients in the ICU. JPEN J. Parenter. Enter. Nutr. 2014, 38, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Giger, U.; Büchler, M.; Farhadi, J.; Berger, D.; Hüsler, J.; Schneider, H.; Krähenbühl, S.; Krähenbühl, L. Preoperative immunonutrition suppresses perioperative inflammatory response in patients with major abdominal surgery-a randomized controlled pilot study. Ann. Surg. Oncol. 2007, 14, 2798–2806. [Google Scholar] [CrossRef] [PubMed]


| Nutrient | Control | IMHP with β-Glucan | IMHP |
|---|---|---|---|
| Calories (kcal) | 200 | 200 | 200 |
| Protein (g) | 10.0 | 12.0 | 12.0 |
| Total fat (g) | 6.7 | 6.7 | 6.7 |
| Total carbohydrate (g) | 28.5 | 23.0 | 23.0 |
| Vitamin A (μgRE) | 150.00 | 150.00 | 150.00 |
| VitaminB1 (mg) | 0.24 | 0.24 | 0.24 |
| VitaminB2 (mg) | 0.30 | 0.30 | 0.30 |
| VitaminB6 (mg) | 0.30 | 0.30 | 0.30 |
| VitaminB12 (μg) | 0.48 | 0.48 | 0.48 |
| Vitamin C (mg) | 20.00 | 40.00 | 40.00 |
| VitaminD3 (μg) | 1.00 | 1.00 | 1.00 |
| Vitamin E (mgα-TE) | 2.00 | 4.80 | 4.80 |
| VitaminK1 (μg) | 9.75 | 15.00 | 15.00 |
| Folic acid (μg) | 80.00 | 80.00 | 80.00 |
| Niacin (mg) | 3.20 | 3.20 | 3.20 |
| Biotin (μg) | 6.00 | 6.00 | 6.00 |
| Pantothenic acid (mg) | 1.00 | 1.00 | 1.00 |
| Calcium (mg) | 140.00 | 150.00 | 150.00 |
| Phosphorus (mg) | 140.00 | 140.00 | 140.00 |
| Magnesium (mg) | 58.00 | 44.20 | 44.20 |
| Zinc (mg) | 2.00 | 4.00 | 4.00 |
| Iron (mg) | 2.00 | 2.00 | 2.00 |
| Sodium (mg) | 155.00 | 141.35 | 141.35 |
| Chloride (mg) | 170.00 | 121.20 | 121.20 |
| Potassium (mg) | 260.00 | 240.39 | 240.39 |
| Manganese (mg) | 0.46 | 1.60 | 1.60 |
| Iodine (μg) | 19.50 | 30.00 | 30.00 |
| Copper (mg) | 0.10 | 0.32 | 0.32 |
| Selenium (μg) | 0.00 | 22.00 | 22.00 |
| Chromium (μg) | 0.00 | 5.00 | 5.00 |
| Molybdenum (μg) | 0.00 | 2.50 | 2.50 |
| Taurine (mg) | 22.00 | 22.00 | 22.00 |
| L-Carnitine (mg) | 22.00 | 22.00 | 22.00 |
| Choline (mg) | 73.00 | 73.00 | 73.00 |
| β-glucan (mg) | 0.00 | 50.00 | 0.00 |
| Variables | Total (n = 22) | pa | pb | |||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 7) | IMHP with β-Glucan (n = 8) | IMHP (n = 7) | ||||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |||
| Age (year) | 53.7 ± 6.18 | 64.1 ± 6.03 | 72.6 ± 3.23 | 0.141 | ||||
| Male/Female n, (%) | 4 (57.1)/3 (42.9) | 5 (62.5)/3 (37.5) | 6 (85.7)/1 (14.3) | 0.488 | ||||
| BMI (kg/m2) | 24.1 ± 1.31 | 23.4 ± 1.38 | 22.1 ± 1.20 | 21.9 ± 1.37 | 20.8 ± 1.50 | 20.2 ± 1.39 | 0.213 | 0.114 |
| APACHE II score | 16.7 ± 3.07 | 16.9 ± 3.39 | 18.5 ± 2.69 | 0.862 | ||||
| TCI (kcal/d), mean | ||||||||
| Day 1 | 736.3 ± 176.0 | 738.1 ± 211.5 | 790.3 ± 129.1 | 0.688 | ||||
| Day 3 | 1253.5 ± 214.3 | 1191.0 ± 69.8 | 1059.3 ± 151.1 | 0.546 | ||||
| Day 7 | 1507.7 ± 157.2 | 1065.9 ± 153.3 | 1102.0 ± 156.7 | 0.170 | ||||
| Glucose (mg/dL)∮ | 136.1 ± 8.40 | 143.1 ± 5.86 | 179.0 ± 29.3 | 173.8 ± 29.1 | 306.9 ± 117.4 | 154.6 ± 17.4 | 0.052 | 0.959 |
| Triglyceride (mg/dL)∮ | 104.0 ± 19.6 | 79.3 ± 8.83 | 116.3 ± 16.3 | 109.0 ± 12.1 | 79.0 ± 13.6 | 84.4 ± 11.2 | 0.335 | 0.145 |
| Total-cholesterol (mg/dL)∮ | 105.7 ± 10.9 | 118.4 ± 13.2 | 108.0 ± 13.2 | 132.0 ± 8.85 | 112.0 ± 13.9 | 145.0 ± 7.75 * | 0.998 | 0.293 |
| HDL-cholesterol (mg/dL)∮ | 27.9 ± 6.34 | 31.6 ± 4.08 | 25.9 ± 4.67 | 24.9 ± 3.54 | 34.6 ± 4.19 | 38.6 ± 3.88 | 0.645 | 0.057 |
| LDL-cholesterol (mg/dL)∮ | 57.1 ± 7.76 | 71.0 ± 12.7 | 58.9 ± 11.7 | 85.3 ± 7.69 | 61.6 ± 12.6 | 89.4 ± 6.71 * | 0.924 | 0.526 |
| Leukocyte counts (Χ103/μL)∮ | 6.99 ± 0.48 | 12.2 ± 2.26 * | 12.3 ± 2.21 | 12.7 ± 2.24 | 12.9 ± 1.83 | 10.8 ± 1.28 | 0.089 | 0.839 |
| Serum albumin (mg/dL)∮ | 2.71 ± 0.20 | 2.94 ± 0.21 | 2.83 ± 0.10 | 2.94 ± 0.18 | 2.69 ± 0.23 | 2.93 ± 0.19 * | 0.722 | 0.979 |
| Variables | Total (n = 22) | pa | pb | pc | pd | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 7) | IMHP with β-Glucan (n = 8) | IMHP (n = 7) | ||||||||||||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |||||||||||
| NK cell activity 5:1 (%)∮ | 13.6 ± 3.69 | 12.7 ± 5.62 | 11.0 ± 2.40 | 31.4 ± 6.36 * | 10.7 ± 7.41 | 18.2 ± 4.63 | 0.266 | 0.155 | ||||||||
| Change | −0.86 ± 4.17 b | 20.4 ± 5.93 a | 7.47 ± 3.59 a,b | 0.019 | 0.037 | |||||||||||
| NK cell activity 2.5:1 (%)∮ | 14.1 ± 2.14 | 11.8 ± 3.60 | 9.88 ± 5.14 | 29.3 ± 4.76 * | 7.56 ± 5.37 | 18.4 ± 6.11 | 0.841 | 0.059 | ||||||||
| Change | −2.34 ± 3.08 b | 19.4 ± 6.67 a | 10.8 ± 6.18 a,b | 0.034 | 0.055 | |||||||||||
| Prealbumin (mg/dL)∮ | 11.9 ± 2.15 | 10.9 ± 1.86 b | 10.4 ± 0.84 | 13.3 ± 0.80 b,* | 9.00 ± 1.21 | 19.3 ± 1.87 a,* | 0.589 | 0.017 | ||||||||
| Change | −1.00 ± 1.05 c | 2.88 ± 0.83 b | 10.3 ± 2.12 a | 0.002 | 0.001 | |||||||||||
| hs-CRP (mg/dL)∮ | 84.7 ± 20.0 | 99.7 ± 18.9 a | 130.3 ± 33.0 | 65.7 ± 13.9 a,* | 164.1 ± 23.8 | 19.8 ± 9.87 b,* | 0.093 | 0.006 | ||||||||
| Change | 15.0 ± 11.9 a | −64.6 ± 24.5 b | −144.3 ± 28.5 b | 0.002 | 0.006 | |||||||||||
| Serum | ||||||||||||||||
| IL-12 (pg/mL) | 9.54 ± 8.84 | 7.67 ± 4.58 | 3.21 ± 3.21 | 10.6 ± 9.55 | 3.90 ± 3.90 | 0.00 ± 0.00 | 0.696 | 0.193 | ||||||||
| IFN- γ (pg/mL) | 0.03 ± 0.03 | 0.03 ± 0.03 | 0.24 ± 0.24 | 0.28 ± 0.28 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.613 | 0.613 | ||||||||
| TNF-α (pg/mL)∮ | 6.23 ± 1.87 | 3.78 ± 0.46 | 6.55 ± 1.39 | 6.06 ± 1.79 | 7.80 ± 0.81 | 6.67 ± 1.79 | 0.889 | 0.634 | ||||||||
| IL-6 (pg/mL)∮ | 66.8 ± 23.2 | 49.5 ± 23.6 | 39.5 ± 9.94 | 23.5 ± 6.07 | 72.3 ± 51.6 | 13.2 ± 3.02 | 0.456 | 0.090 | ||||||||
| IL-1β (pg/mL)∮ | 7.27 ± 5.65 | 4.31 ± 3.47 | 0.82 ± 0.08 | 0.75 ± 0.07 | 0.92 ± 0.17 | 1.05 ± 0.34 | 0.217 | 0.321 | ||||||||
| Nonstimulated PBMCs | ||||||||||||||||
| IL-12 (pg/mL) | 24.1 ± 6.06 | 12.2 ± 5.47 * | 15.3 ± 4.73 | 20.2 ± 4.55 | 18.8 ± 2.19 | 20.7 ± 3.16 | 0.519 | 0.305 | ||||||||
| Change | −11.9 ± 3.38 b | 4.83 ± 2.61 a | 1.89 ± 1.68 a | 0.004 | 0.003 | |||||||||||
| IFN- γ (pg/mL) | 1.00 ± 0.31 | 0.67 ± 0.19 | 0.54 ± 0.17 | 0.75 ± 0.23 | 0.78 ± 0.18 | 0.76 ± 0.20 | 0.520 | 0.999 | ||||||||
| TNF-α (pg/mL)∮ | 9.66 ± 4.98 | 25.4 ± 16.9 | 3.17 ± 2.06 | 3.95 ± 0.96 | 5.71 ± 2.27 | 3.92 ± 0.99 | 0.651 | 0.578 | ||||||||
| IL-6 (pg/mL)∮ | 26.9 ± 5.19 | 119.5 ± 70.5 a | 12.1 ± 3.07 | 21.1 ± 9.65 a,b | 24.2 ± 8.06 | 12.6 ± 2.25 b | 0.054 | 0.046 | ||||||||
| IL-1β (pg/mL)∮ | 4.08 ± 1.49 | 9.32 ± 5.54 | 1.93 ± 0.64 | 2.52 ± 1.15 | 2.35 ± 0.58 | 1.87 ± 0.60 | 0.297 | 0.312 | ||||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.G.; Kim, Y.S.; Lee, Y.J.; Ahn, H.Y.; Kim, M.; Kim, M.; Cho, M.J.; Cho, Y.; Lee, J.H. Effect of Immune-Enhancing Enteral Nutrition Enriched with or without Beta-Glucan on Immunomodulation in Critically Ill Patients. Nutrients 2016, 8, 336. https://doi.org/10.3390/nu8060336
Lee JG, Kim YS, Lee YJ, Ahn HY, Kim M, Kim M, Cho MJ, Cho Y, Lee JH. Effect of Immune-Enhancing Enteral Nutrition Enriched with or without Beta-Glucan on Immunomodulation in Critically Ill Patients. Nutrients. 2016; 8(6):336. https://doi.org/10.3390/nu8060336
Chicago/Turabian StyleLee, Jae Gil, Young Sam Kim, Young Ju Lee, Hyeon Yeong Ahn, Minjoo Kim, Minkyung Kim, Min Jung Cho, Younsoo Cho, and Jong Ho Lee. 2016. "Effect of Immune-Enhancing Enteral Nutrition Enriched with or without Beta-Glucan on Immunomodulation in Critically Ill Patients" Nutrients 8, no. 6: 336. https://doi.org/10.3390/nu8060336
APA StyleLee, J. G., Kim, Y. S., Lee, Y. J., Ahn, H. Y., Kim, M., Kim, M., Cho, M. J., Cho, Y., & Lee, J. H. (2016). Effect of Immune-Enhancing Enteral Nutrition Enriched with or without Beta-Glucan on Immunomodulation in Critically Ill Patients. Nutrients, 8(6), 336. https://doi.org/10.3390/nu8060336