Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer
Abstract
:1. Introduction
2. Methods
3. History and Origins
4. Chemical and Pharmacological Characteristics
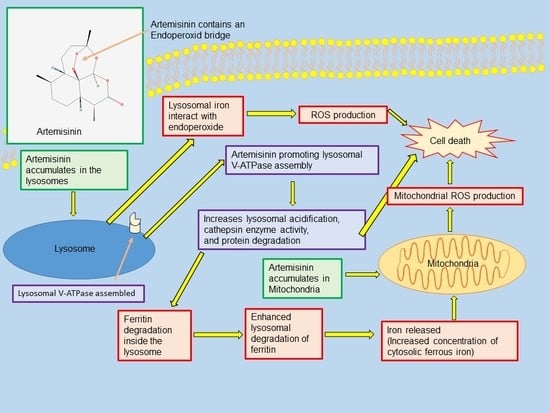
5. Mode of Action
6. Artemisinin as a Treatment for Cancer
6.1. Artemisinin and Its Derivatives Used as a Synergistic Agent
6.2. Artemisinin and Its Derivatives Used to Sensitize Cancer Cells
7. Discussion and Conclusions
Author Contributions
Conflicts of Interest
Funding
References
- Guo, Z. Artemisinin anti-malarial drugs in China. Acta Pharmacol. Sin. B 2016, 6, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. 2016, 55, 10210–10226. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Qinghaosu (artemisinin): Chemistry and pharmacology. Acta Pharmacol. Sin. 2012, 33, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Terkuile, F.; White, N.J.; Holloway, P.; Pasvol, G.; Krishna, S. Plasmodium falciparum: In Vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp. Parasitol. 1993, 76, 85–95. [Google Scholar] [CrossRef]
- Chen, P.Q.; Li, G.Q.; Guo, X.B.; He, K.R.; Fu, Y.X.; Fu, L.C.; Song, Y.Z. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chin. Med. J. 1994, 107, 709–711. [Google Scholar] [PubMed]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012, 247597. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.C.; Singh, N.P.; Sasaki, T. Development of artemisinin compounds for cancer treatment. Investog. New Drugs 2013, 31, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Bergamaschi, G.; Dezza, L.; Arosio, P. Manipulations of cellular iron metabolism for modulating normal and malignant cell proliferation: Achievements and prospects. Blood 1990, 75, 1903–1919. [Google Scholar] [PubMed]
- Waknine-Grinberg, J.H.; Hunt, N.; Bentura-Marciano, A.; McQuillan, J.; Chan, H.W.; Chan, W.C.; Barenholz, Y.; Haynes, R.K.; Golenser, J. Artemisone effective against cerebral malaria. Malar J. 2010, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Nobelprize.org. The Nobel Prize in Physiology or Medicine 2015. Nobel Media AB 2014. Available online: http://www.nobelprize.org/nobel_prizes/medicine/laureates/2015/ (accessed on 24 June 2017).
- Ashton, M.; Nguyen, D.S.; Nguyen, V.H.; Toufigh, G.; Trinh, N.H.; Huong, D.X.; Nguyen, T.N.; Le Dinh, C. Artemisinin kinetics and dynamics during oral and rectal treatment of uncomplicated malaria. Clin. Pharmacol. Ther. 1998, 63, 482–493. [Google Scholar] [CrossRef]
- Ashton, M.; Hai, T.N.; Nguyen, D.S.; Huong, D.X.; Nguyen, V.H.; Nguyen, T.N.; Le Dinh, C. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab. Dispos. 1998, 26, 25–27. [Google Scholar] [PubMed]
- Lai, H.; Singh, N.P. Oral artemisinin prevents and delays the development of 7,12-dimethylbenz[a]antrane (DMBA) induced breast cancer in rats. Cancer Lett. 2006, 231, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, H.; Kunda, P.; Chen, X.; Liu, Q.-L.; Liu, T. Artesunate exerts specific cytotoxicity in retinoblastoma cells via CD71. Oncol. Rep. 2013, 30, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.R.; Olliaro, P.L. Safety of artemisinin and its derivatives. A review of published and unpublished clinical trials. Med. Trop. (Mars) 1998, 58, 50–53. [Google Scholar] [PubMed]
- Ju, R.-J.; Cheng, L.; Peng, X.-M.; Wang, T.; Li, C.-Q.; Song, X.-L.; Liu, S.; Chao, J.-P.; Li, X.-T. Octreotide-modified liposomes containing daunorubicin and dihydroartemisinin for treatment of invasive breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- König, M.; von Hagens, C.; Hoth, S.; Baumann, I.; Walter-Sack, I.; Edler, L.; Sertel, S. Investigation of ototoxicity of artesunate as add-on therapy in patients with metastatic or locally advanced breast cancer: New audiological results from a prospective, open, uncontrolled, monocentric phase I study. Cancer Chemother. Pharmacol. 2016, 77, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guiguemde, A.W.; Bentura-Marciano, A.; Clark, J.; Haynes, R.K.; Chan, W.-C.; Wong, H-N.; Hunt, N.H.; Guy, R.K.; Golenser, J. Synthesis of artemiside and its effects in combination with conventional drugs against severe murine Malaria. Antimicrob. Agents Chemother. 2012, 56, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Price, R.; van Vugt, M.; Phalpun, L.; Luxemburger, C.; Simpson, J.; McGready, R.; Terkuile, F.; Kham, A.; Chongsuphajaisiddhi, T.; White, N.J.; et al. Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. Am. J. Trop. Med. Hyg. 1999, 60, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.L.; Haynes, R.K.; Meunier, B.; Yuthavong, Y. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 2001, 17, 122–126. [Google Scholar] [CrossRef]
- Meshnick, S.R.; Thomas, A.; Ranz, A.; Xu, C.M.; Pan, H.Z. Artemisinin (qinghaosu): The role of intracellular hemin in its mechanism of antimalarial action. Mol. Biochem. Parasitol. 1991, 49, 181–189. [Google Scholar] [CrossRef]
- Zhang, S.; Gerhard, G.S. Heme activates artemisinin more efficiently than hemin, inorganic iron, or hemoglobin. Bioorg. Med. Chem. 2008, 16, 7853–7861. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Singh, N.P. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett. 1995, 91, 41–46. [Google Scholar] [CrossRef]
- Reizenstein, P. Iron, free radicals and cancer. Med. Oncol. Tumor Pharmacother. 1991, 8, 229–233. [Google Scholar] [PubMed]
- Lui, G.Y.L.; Kovacevic, Z.; Richardson, V.; Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Targeting cancer cells by binding iron: Dissecting cellular signaling pathways. Oncotarget 2015, 6, 18748–18779. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012, 21, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, Y.; Kohgo, Y.; Nishisato, T.; Kondo, H.; Kato, J.; Urushizaki, Y.; Urushizaki, I. Transferrin receptors in human cancerous tissues. Tohoku J. Exp. Med. 1987, 153, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Du, J.H.; Zhang, H.D.; Ma, Z.J.; Ji, K.M. Artesunate induces oncosis-like cell death in vitro and has antitumor activity against pancreatic cancer xenografts in vivo. Cancer Chemother. Pharmacol. 2010, 65, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Mercer, A.E.; Copple, I.M.; Maggs, J.L.; O’Neill, P.M.; Park, B.K. The role of heme and the mitochondrion in the chemical and molecular mechanisms of mammalian cell death induced by the artemisinin antimalarials. J. Biol. Chem. 2011, 283, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.D.; Tan, S.H.; Ng, S.; Shi, Y.; Zhou, J.; Tank, K.S.; Wong, W.S.; Shen, H.M. Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin. J. Biol. Chem. 2014, 48, 33425–33441. [Google Scholar] [CrossRef] [PubMed]
- Hamacher-Brady, A.; Stein, H.A.; Turschner, S.; Toegel, I.; Mora, R.; Jennewein, N.; Efferth, T.; Eils, R.; Brady, N.R. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J. Biol. Chem. 2011, 286, 6587–6601. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, J.A.; Sundar, S.N.; Cheung, M.; Tin, A.S.; Modiano, J.; Firestone, G.L. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J. Biol. Chem. 2009, 284, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Gallis, B.; Takatani-Nakase, T.; Oh, S.; Lacoste, E.; Singh, N.P.; Goodlett, D.R.; Tanaka, S.; Futaki, S.; Lai, H.; et al. Transferrin receptor-dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009, 274, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Mercer, A.E.; Maggs, J.L.; Sun, X.M.; Cohen, G.M.; Chadwick, J.; O’Neill, P.M.; Park, B.K. Evidence for the involvement of carbon-centered radicals in the induction of apoptotic cell death by artemisinin compounds. J. Biol. Chem. 2007, 282, 9372–9382. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Giaisi, M.; Merling, A.; Krammer, P.H.; Li-Weber, M. Artesunate induces ROS-mediated apoptosis in doxorubicinresistant T leukemia cells. PLoS ONE 2007, 2, e693. [Google Scholar] [CrossRef] [PubMed]
- Hooft van Huijsduijnen, R.; Guy, K.R.; Chibale, K.; Haynes, R.K.; Peitz, I.; Kelter, G.; Philipps, M.A.; Vennerstrom, J.L.; Yuthavong, Y.; Wells, T.N.C. Anticancer properties of distinct antimalarial drug classes. PLoS ONE 2013, 8, e82962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilaoui, M.; Mouse, H.A.; Jaafari, A.; Zyad, A. Differential effect of artemisinin against cancer cell lines. Nat. Prod. Bioprospect. 2014, 4, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Luo, H.S.; Li, M.; Tan, S. Artesunate inhibits the growth and induces apoptosis of human gastric cancer cells by downregulating COX-2. OncoTargets Ther. 2015, 8, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zuo, L.F.; Zuo, J. Artesunate induces apoptosis and inhibits growth of Eca109 and Ec9706 human esophageal cancer cell lines in vitro and in vivo. Mol. Med. Rep. 2015, 12, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Weifeng, T.; Feng, S.; Xiangji, L.; Changging, S.; Zhiquian, Q.; Huazhong, Z.; Peining, Y.; Yong, Y.; Mengchao, W.; Xiaoqing, J.; et al. Artemisinin inhibits in vitro and in vivo invasion and metastasis of human hepatocellular carcinoma cells. Phytomedicine 2011, 18, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Woerdenbag, H.J.; Moskal, T.A.; Pras, N.; Malingré, T.M.; el-Feraly, F.S.; Kampinga, H.H.; Konings, A.W. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J. Nat. Prod. 1993, 56, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, C.; Cooper, A.J.; Barbu, E.; Birch, B.R.; Lwaleed, B.A. Artemisinin as potential anticancer agents: Uptake detection in erythrocytes using Fourier transform infrared spectroscopy and cytotoxicity against bladder cancer cells. J. Clin. Pathol. 2016, 69, 962–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Guo, X.; Yue, W.; Wang, J.; Yang, J.; Chen, J. Artemether suppresses cell proliferation and induces apoptosis in diffuse large B cell lymphoma cells. Exp. Ther. Med. 2017, 14, 4083–4090. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, T.; Zhiqun, S.; Peng, L.; Mengqing, G.; Hermine, O.; Rousseaux, S.; Khochbin, S.; Mi, J.Q.; Wang, J. Induction of autophagy and autophagy-dependent apoptosis in diffuse large B-cell lymphoma by a new antimalarial artemisinin derivative, SM1044. Cancer Med. 2017, 7, 380–396. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.; Gallis, B.; Solazzi, J.W.; Kim, B.J.; Gulati, R.; Vakar-Lopez, F.; Goodlett, D.R.; Vessella, R.L.; Sasaki, T. Effect of artemisinin derivatives on apoptosis and cell cycle in prostate cancer cells. Anticancer Drugs 2010, 21, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Fei, A.M.; Nie, R.M.; Wang, J.; Li, Y.; Wang, Z.Y.; Mi, J.Q. A new artemisinin derivative SM1044 induces apoptosis of Kasumi-1 cells and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2011, 19, 607–611. [Google Scholar] [PubMed]
- Rutterman, G.R.; Erich, S.A.; Mol, J.A.; Spee, B.; Grinwis, G.C.; Fleckenstein, L.; London, C.A.; Efferth, T. Safety and efficacy field study of artesunate for dogs with non-resectable tumours. Anticancer Res. 2013, 33, 1819–1827. [Google Scholar]
- Singh, N.P.; Verma, K.B. Case report of a laryngeal squamous cell carcinoma treated with artesunate. Arch. Oncol. 2002, 10, 279–280. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Yu, S.Q.; Miao, L.Y.; Huang, X.Y.; Zhang, X.P.; Zhu, Y.P.; Xia, X.H.; Li, D.Q. Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: A randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 2008, 6, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.H.; Adoubi, I.; Jansen, N.; JC, K.C.; Cnodder, T.D.E.; Tschulakow, A.; Efferth, T. First study of oral Artenimol-R in advanced cervical cancer: Clinical benefit, tolerability and tumor markers. Anticancer Res. 2011, 31, 4417–4422. [Google Scholar] [PubMed]
- Singh, N.P.; Lai, H.C. Synergistic cytotoxicity of artemisinin and sodium butyrate on human cancer cells. Anticancer Res. 2005, 25, 4325–4331. [Google Scholar] [PubMed]
- Wang, S.J.; Gao, Y.; Chen, H.; Kong, R.; Jiang, H.C.; Pan, S.H.; Xue, D.B.; Bai, X.W.; Su, B. Dihydroartemisinin inactivates NF-κB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett. 2010, 293, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.J.; Zhang, J.L.; Li, A.; Wang, Z.; Lou, X.E. Dihydroartemisinin improves the efficiency of chemotherapeutics in lung carcinomas in vivo and inhibits murine Lewis lung carcinoma cell line growth in vitro. Cancer Chemother. Pharmacol. 2010, 66, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Schwarzer, A.; Gläser, D.; Mugge, L.O.; Uhlig, L.O.; Heyn, S.; Kragl, B.; Mohren, M.; Hoffmann, F.A.; Lange, T.; et al. Lenalidomide in combination with bendamustine and prednisolone in relapsed/refractory multiple myeloma: Results of a phase 2 clinical trial (OSHO-#077). J. Cancer Res. Clin. Oncol. 2017, 143, 2545–2553. [Google Scholar] [PubMed]
- Liu, W.M.; Gravett, A.M.; Dalgleish, A.G. The antimalarial agent artesunate possesses anticancer properties that can be enhanced by combination strategies. Int. J. Cancer 2011, 128, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, C.; Chen, P.; Cheng, B.; Cheng, Y. RACKI induces chemotherapy resistance in esophageal carcinoma by upregulating the PI3K/AKT pathway and Bcl-2 expression. OncoTargets Ther. 2018, 11, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Reungpatthanaphong, P.; Mankhetkorn, S. Modulation of multidrug resistance by artemisinin, artesunate and dihydroartemisinin in K562/adr and GLC4/adr resistant cell lines. Biol. Pharm. Bull. 2002, 25, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Soomro, S.; Langenberg, T.; Mahringer, A.; Badireenath Konkimalla, V.; Hordewel, C.; Holenya, P.; Brand, A.; Cetin, C.; Fricker, G.; Dewerchin, M.; et al. Design of novel artemisinin-like derivatives with cytotoxic and anti-angiogenic properties. J. Cell Mol. Med. 2011, 15, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hou, D.; Liu, Q.; Wu, T.; Guo, H.; Zhang, X.; Zou, Y.; Liu, Z.; Liu, J.; Wei, J.; et al. Artesunate sensitizes ovarian cancer cells to cisplatin by downregulating RAD51. Cancer Biol. Ther. 2015, 16, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, D.; Zhang, R.; Wang, H. Experimental therapy of hepatoma with artemisinin and its derivatives: In Vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin. Cancer Res. 2008, 14, 5519–5530. [Google Scholar] [CrossRef] [PubMed]
- Kobasa, T.; Talundzic, E.; Sug-Aram, R.; Boondat, P.; Goldman, I.F.; Lucchi, N.W.; Dharmarak, P.; Sintasath, D.; Fukuda, M.; Whistler, T.; et al. The emergence and spread of kelch 13 mutations associated with artemisinin resistance in Plasmodium falciparum parasites in twelve Thai provinces from 2007–2016. Antimicrob. Agents Chemother. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hemming-Schroeder, E.; Umukoro, E.; Lo, E.; Fung, B.; Tomás-Domingo, P.; Zhou, G.; Zhong, D.; Dixit, A.; Atieli, H.; Githeko, A.; et al. Impacts of antimalarial drugs on Plasmodium falciparum drug resistance markers, Western Kenya, 2003–2015. Am. J. Trop. Med. Hyg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.; Fichtner, I.; Killian, P.H.; Kronski, E.; Pfeffer, U.; Efferth, T. Development of resistance towards artesunate in MDA-MB-231 human breast cancer cells. PLoS ONE 2011, 6, e20550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anfosso, L.; Efferth, T.; Albini, A.; Pfeffer, U. Microarray expression profiles of angiogenesis-related genes predict tumor cell response to artemisinins. Pharmacogenomics 2006, 6, 269–278. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstat-Korzenny, E.; Ascencio-Aragón, J.A.; Niezen-Lugo, S.; Vázquez-López, R. Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer. Med. Sci. 2018, 6, 19. https://doi.org/10.3390/medsci6010019
Konstat-Korzenny E, Ascencio-Aragón JA, Niezen-Lugo S, Vázquez-López R. Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer. Medical Sciences. 2018; 6(1):19. https://doi.org/10.3390/medsci6010019
Chicago/Turabian StyleKonstat-Korzenny, Enrique, Jorge Alberto Ascencio-Aragón, Sebastian Niezen-Lugo, and Rosalino Vázquez-López. 2018. "Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer" Medical Sciences 6, no. 1: 19. https://doi.org/10.3390/medsci6010019