Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting
Abstract
:Simple Summary
Abstract
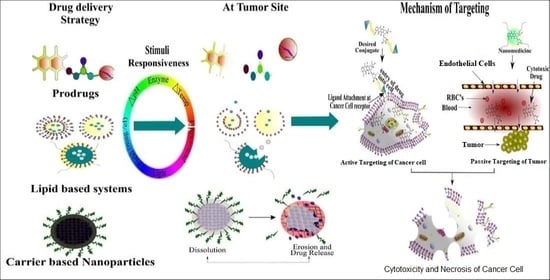
1. Introduction
2. Stimuli-Responsive Drug Delivery Systems
2.1. Physical Stimuli-Responsive Drug Delivery Systems
2.1.1. Thermoresponsive Drug Delivery Systems
2.1.2. Magnetic/Electric Field-Responsive Drug Delivery Systems
- Constituted particles at the nano-size range to allow perfusion at the capillary level;
- They should have adequate magnetic responsiveness;
- They should possess the ability to carry a wide variety of active therapeutic agents;
- They can be designed to function as controlled or targeted drug delivery systems;
- They have high biocompatibility and biodegradability and minimal antigenicity and toxicity.
2.1.3. Ultrasound-Responsive Drug Delivery Systems (URDDS)
2.1.4. Light-Responsive Drug Delivery Systems (LRDDS)
2.1.5. Stimuli-Responsive Lipids
Temperature-Responsive Lipids
Electric/Magnetic Field-Responsive Lipids
Sound-Responsive Lipids
Light-Responsive Lipids
pH-Responsive Lipids
Enzyme-Responsive Lipids
2.2. Chemical Stimuli-Responsive Drug Delivery Systems
2.2.1. pH-Responsive Drug Delivery Systems
2.2.2. Enzymes-Responsive Drug Delivery Systems
2.2.3. Stimuli-Responsive Prodrugs
Temperature-Responsive Prodrugs
Magnetic/Electric Field-Responsive Prodrugs
Ultrasound-Responsive Prodrugs
Light-Responsive Prodrugs
pH-Responsive Prodrugs
2.2.4. Stimuli-Responsive Carriers/Polymers
- Temperature-responsive carriers/polymers;
- Magnetic/electric field-responsive carriers/polymers;
- Ultrasound-responsive carriers/polymers;
- Light-responsive carriers/polymers;
- pH-responsive carriers/polymers;
- Enzyme-responsive carriers/polymers.
Temperature-Responsive Carriers/Polymers
Magnetic/Electric Field-Responsive Carriers/Polymers
Ultrasound-Responsive Carriers/Polymers
Light-Responsive Carriers/Polymers
pH-Responsive Carriers/Polymers
Enzyme-Responsive Carriers/Polymers
3. Molecular Dynamics (MD) Simulations Associated with Stimuli-Based Tumor Targeting
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Leao, D.; Craig, P.; Godoy, L.; Leite, C.; Policeni, B. Response Assessment in Neuro-Oncology Criteria for Gliomas: Practical Approach Using Conventional and Advanced Techniques. Am. J. Neuroradiol. 2020, 41, 10–20. [Google Scholar] [CrossRef]
- Medina, M.A.; Oza, G.; Sharma, A.; Arriaga, L.; Hernández Hernández, J.M.; Rotello, V.M.; Ramirez, J.T. Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. Int. J. Environ. Res. Public Health 2020, 17, 2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorat, N.D.; Bauer, J. Functional smart hybrid nanostructures based nanotheranostic approach for advanced cancer treatment. Appl. Surf. Sci. 2020, 146809. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wu, H.; Huang, J.; Qian, W.; Martinson, D.E.; Ji, B.; Li, Y.; Wang, Y.A.; Yang, L.; Mao, H. Probing and Enhancing Ligand-Mediated Active Targeting of Tumors Using Sub-5 nm Ultrafine Iron Oxide Nanoparticles. Theranostics 2020, 10, 2479. [Google Scholar] [CrossRef]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef]
- Sun, H.; Zhong, Z. 100th Anniversary of Macromolecular Science Viewpoint: Biological Stimuli-Sensitive Polymer Prodrugs and Nanoparticles for Tumor-Specific Drug Delivery. ACS Macro Lett. 2020, 9, 1292–1302. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-Responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921. [Google Scholar] [CrossRef] [Green Version]
- Paris, J.L.; Baeza, A.; Vallet-Regí, M. Overcoming the stability, toxicity, and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics. Exp. Opin. Drug Deliv. 2019, 16, 1095–1112. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Ahmad, S.A.; Hoda, M.N.; Rishishwar, S.; Rishishwar, P.; Nayak, A.K. Stimuli-Responsive carbon nanotubes for targeted drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Application; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Ghamkhari, A.; Mahmoodzadeh, F.; Ghorbani, M.; Hamishehkar, H. A novel gold nanorods coated by stimuli-responsive ABC triblock copolymer for chemotherapy of solid tumors. Eur. Polym. J. 2019, 115, 313–324. [Google Scholar] [CrossRef]
- Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Hoang Thi, T.T. Recent progress and advances of multi-stimuli-responsive dendrimers in drug delivery for cancer treatment. Pharmaceutics 2019, 11, 591. [Google Scholar] [CrossRef] [Green Version]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials. 2019, 9, 638. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yang, W.-W.; Xu, D.-G. Stimuli-Responsive nanoscale drug delivery systems for cancer therapy. J. Drug Target. 2019, 27, 423–433. [Google Scholar] [CrossRef]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-Responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef] [Green Version]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmi, R.; Scherz, A.; Van Der Boom, M.E.; Kraatz, H.-B. Stimuli responsive materials: New avenues toward smart organic devices. J. Mater. Chem. 2005, 15, 4480–4487. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, X.; Tham, H.P.; Phua, S.Z.F.; Cheng, W.; Zeng, W.; Shi, H.; Mei, L.; Zhao, Y. NIR-Light-Activated Combination Therapy with a Precise Ratio of Photosensitizer and Prodrug Using a Host-Guest Strategy. Angew. Chem. 2019, 131, 7723–7728. [Google Scholar] [CrossRef]
- Samanta, D.; Hosseini-Nassab, N.; Zare, R.N. Electroresponsive nanoparticles for drug delivery on demand. Nanoscale 2016, 8, 9310–9317. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.; Mia, M.; Rahman, M.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.S.; Ghazali, H.S.; Ghazali, Z.S.; Khunjush, B.; Akbari, M. Stimuli-Responsive Hydrogels for Local Post-Surgical Drug Delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Yu, B.; Gao, L.; Cong, H.; Song, N.; Lu, C. Stimuli responsive nanoparticles for controlled anti-cancer drug release. Cur. Med. Chem. 2018, 25, 1837–1866. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, K.T.; Al-Jamal, W.T.; Wang, J.T.; Rubio, N.; Buddle, J.; Gathercole, D.; Zloh, M.; Kostarelos, K. Cationic poly-L-lysine dendrimer complexes doxorubicin and delays tumor growth in vitro and in vivo. ACS Nano 2013, 7, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Antoniraj, M.G.; Kumar, C.S.; Kandasamy, R. Synthesis and characterization of poly (N-isopropylacrylamide)-g-carboxymethyl chitosan copolymer-based doxorubicin-loaded polymeric nanoparticles for thermoresponsive drug release. Colloid Polym. Sci. 2016, 294, 527–535. [Google Scholar] [CrossRef]
- Cheng, Y.; Hao, J.; Lee, L.A.; Biewer, M.C.; Wang, Q.; Stefan, M.C. Thermally controlled release of anticancer drug from self-assembled γ-substituted amphiphilic poly (ε-caprolactone) micellar nanoparticles. Biomacromolecules 2012, 13, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.D.; Cheon, S.H.; Song, S.-C. Controlled release of doxorubicin from thermosensitive poly (organophosphazene) hydrogels. Int. J. Pharm. 2006, 319, 29–36. [Google Scholar] [CrossRef]
- Peller, M.; Willerding, L.; Limmer, S.; Hossann, M.; Dietrich, O.; Ingrisch, M.; Sroka, R.; Lindner, L.H. Surrogate MRI markers for hyperthermia-induced release of doxorubicin from thermosensitive liposomes in tumors. J. Control. Release 2016, 237, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ren, F.; Ding, B.; Sun, N.; Liu, X.; Ding, X.; Gao, S. A thermo-sensitive PLGA-PEG-PLGA hydrogel for sustained release of docetaxel. J. Drug Target. 2011, 19, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, M.; Li, R.; Ding, Y.; Qian, X.; Yu, L.; Jiang, X. The antitumor effect of novel docetaxel-loaded thermosensitive micelles. Eur. J. Pharm. Biopharm. 2008, 69, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.H.; Ramasamy, T.; Kim, D.-W.; Cho, H.J.; Kim, Y.-I.; Cho, K.H.; Yong, C.S.; Kim, J.O.; Choi, H.-G. Docetaxel-Loaded thermosensitive liquid suppository: Optimization of rheological properties. Arch. Pharm. Res. 2013, 36, 1480–1486. [Google Scholar] [CrossRef]
- Carter, K.A.; Shao, S.; Hoopes, M.I.; Luo, D.; Ahsan, B.; Grigoryants, V.M.; Song, W.; Huang, H.; Zhang, G.; Pandey, R.K. Porphyrin-phospholipid liposomes permeabilized by near-infrared light. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Joniec, A.; Sek, S.; Krysinski, P. Magnetoliposomes as potential carriers of doxorubicin to tumours. Chem. A Eur. J. 2016, 22, 17715–17724. [Google Scholar] [CrossRef]
- Sherlock, S.P.; Tabakman, S.M.; Xie, L.; Dai, H. Photothermally enhanced drug delivery by ultrasmall multifunctional FeCo/graphitic shell nanocrystals. ACS Nano 2011, 5, 1505–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brulé, S.; Levy, M.; Wilhelm, C.; Letourneur, D.; Gazeau, F.; Ménager, C.; Le Visage, C. Doxorubicin release triggered by alginate embedded magnetic nanoheaters: A combined therapy. Adv. Mater. 2011, 23, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shi, M.; Liu, P.; Ko, A.; Zhong, W.; Liao, W.; Xing, M.M. Reducible polyamidoamine-magnetic iron oxide self-assembled nanoparticles for doxorubicin delivery. Biomaterials 2014, 35, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Tarasi, R.; Khoobi, M.; Niknejad, H.; Ramazani, A.; Ma’mani, L.; Bahadorikhalili, S.; Shafiee, A. Β-cyclodextrin functionalized poly (5-amidoisophthalicacid) grafted Fe3O4 magnetic nanoparticles: A novel biocompatible nanocomposite for targeted docetaxel delivery. J. Mag. Magn. Mater. 2016, 417, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Panda, J.; Satapathy, B.S.; Majumder, S.; Sarkar, R.; Mukherjee, B.; Tudu, B. Engineered polymeric iron oxide nanoparticles as potential drug carrier for targeted delivery of docetaxel to breast cancer cells. J. Magn. Magn. Mat. 2019, 485I, 165–173. [Google Scholar] [CrossRef]
- Wang, S.; Dormidontova, E.E. Nanoparticle design optimization for enhanced targeting: Monte Carlo simulations. Biomacromolecules 2010, 11, 1785–1795. [Google Scholar] [CrossRef] [Green Version]
- Murdan, S. Electro-Responsive drug delivery from hydrogels. J. Control. Release 2003, 92, 1–17. [Google Scholar] [CrossRef]
- Han, M.; Huang-Fu, M.-Y.; Guo, W.-W.; Guo, N.-N.; Chen, J.; Liu, H.-N.; Xie, Z.-Q.; Lin, M.-T.; Wei, Q.-C.; Gao, J.-Q. MMP-2-sensitive HA end-conjugated poly (amidoamine) dendrimers via click reaction to enhance drug penetration into solid tumor. ACS Appl. Mater. Interfaces 2017, 9, 42459–42470. [Google Scholar] [CrossRef]
- Baghbani, F.; Moztarzadeh, F. Bypassing multidrug resistant ovarian cancer using ultrasound responsive doxorubicin/curcumin co-deliver alginate nanodroplets. Colloid Surf. B Biointerfaces 2017, 153, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kawakami, S.; Kono, Y.; Un, K.; Higuchi, Y.; Maruyama, K.; Yamashita, F.; Hashida, M. Enhancement of the anti-tumor effect of DNA vaccination using an ultrasound-responsive mannose-modified gene carrier in combination with doxorubicin-encapsulated PEGylated liposomes. Int. J. Pharm. 2014, 475, 401–407. [Google Scholar] [CrossRef]
- Lentacker, I.; Geers, B.; Demeester, J.; De Smedt, S.C.; Sanders, N.N. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: Cytotoxicity and mechanisms involved. Mol. Ther. 2010, 18, 101–108. [Google Scholar] [CrossRef]
- Wang, Z.; He, Q.; Zhao, W.; Luo, J.; Gao, W. Tumor-Homing, pH-and ultrasound-responsive polypeptide-doxorubicin nanoconjugates overcome doxorubicin resistance in cancer therapy. J. Control. Release 2017, 264, 66–75. [Google Scholar] [CrossRef]
- Hu, J.-J.; Liu, L.-H.; Li, Z.-Y.; Zhuo, R.-X.; Zhang, X.-Z. MMP-Responsive theranostic nanoplatform based on mesoporous silica nanoparticles for tumor imaging and targeted drug delivery. J. Mater. Chem. B 2016, 4, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Khatun, Z.; Nurunnabi, M.; Nafiujjaman, M.; Reeck, G.R.; Khan, H.A.; Cho, K.J.; Lee, Y.-K. A hyaluronic acid nanogel for photo–chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale 2015, 7, 10680–10689. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Trewyn, B.G.; Lin, V.S.Y. Light- and pH-responsive release of doxorubicin from a mesoporous silica-based nanocarrier. Chem. A Eur. J. 2011, 17, 3338–3342. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Carter, K.A.; Razi, A.; Geng, J.; Shao, S.; Giraldo, D.; Sunar, U.; Ortega, J.; Lovell, J.F. Doxorubicin encapsulated in stealth liposomes conferred with light-triggered drug release. Biomaterials 2016, 75, 193–202. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Zhang, G.; Li, C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano 2010, 4, 1033–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Cui, Z.; Yu, P.; Guo, C.; Feng, B.; Jiang, T.; Wang, S.; Yin, Q.; Zhong, D.; Yang, X. pH-and NIR light-responsive micelles with hyperthermia-triggered tumor penetration and cytoplasm drug release to reverse doxorubicin resistance in breast cancer. Adv. Funct. Mater. 2015, 25, 2489–2500. [Google Scholar] [CrossRef]
- Hao, Y.; Dong, M.; Zhang, T.; Peng, J.; Jia, Y.; Cao, Y.; Qian, Z. Novel approach of using near-Infrared Responsive PEGylated Gold Nanorod Coated Poly (L-lactide) microneedles to enhance the antitumor efficiency of Docetaxel-Loaded MPEG-PDLLA Micelles for Treating an A431 tumor. ACS Appl. Mater. Interface 2017, 9, 15317–15327. [Google Scholar] [CrossRef]
- Du, B.; Han, S.; Zhao, F.; Lim, K.H.; Xi, H.; Su, X.; Yao, H.; Zhou, J. A smart upconversion-based light-triggered polymer for synergetic chemo-photodynamic therapy and dual-modal MR/UCL imaging. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2071–2080. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nair, A.; Rejinold, N.S.; Maya, S.; Nair, S. Doxorubicin-Loaded pH-responsive chitin nanogels for drug delivery to cancer cells. Carbohydr. Polym. 2012, 87, 2352–2356. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.-Q.; Zhang, X. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Unsoy, G.; Khodadust, R.; Yalcin, S.; Mutlu, P.; Gunduz, U. Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur. J. Pharm. Sci. 2014, 62, 243–250. [Google Scholar] [CrossRef]
- Dadsetan, M.; Taylor, K.E.; Yong, C.; Bajzer, Ž.; Lu, L.; Yaszemski, M.J. Controlled release of doxorubicin from pH-responsive microgels. Acta Biomater. 2013, 9, 5438–5446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourjavadi, A.; Hosseini, S.H.; Alizadeh, M.; Bennett, C. Magnetic pH-responsive nanocarrier with long spacer length and high colloidal stability for controlled delivery of doxorubicin. Colloid Surf. B Biointerfaces 2014, 116, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Li, Z.; Qiao, M.; Long, M.; Wang, M.; Zhang, X.; Tian, C.; Chen, D. Self-Assembled pH-responsive hyaluronic acid–g-poly (l-histidine) copolymer micelles for targeted intracellular delivery of doxorubicin. Acta Biomater. 2014, 10, 2024–2035. [Google Scholar] [CrossRef]
- Tian, Y.; Glogowska, A.; Zhong, W.; Klonisch, T.; Xing, M. Polymeric mesoporous silica nanoparticles as a pH-responsive switch to control doxorubicin intracellular delivery. J. Mater. Chem. B 2013, 1, 5264–5272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, G.; Alves, C.S.; Tomás, H.; Xiong, Z.; Shen, M.; Rodrigues, J.O.; Shi, X. Multifunctional dendrimer-entrapped gold nanoparticles conjugated with doxorubicin for pH-responsive drug delivery and targeted computed tomography imaging. Langmuir 2018, 34, 12428–12435. [Google Scholar] [CrossRef]
- Lee, J.M.; Park, H.; Oh, K.T.; Lee, E.S. pH-Responsive hyaluronated liposomes for docetaxel delivery. Int. J. Pharm. 2018, 547, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Ramasamy, T.; Choi, J.Y.; Nguyen, H.T.; Pham, T.T.; Jeong, J.-H.; Ku, S.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Tumor-targeting, pH-sensitive nanoparticles for docetaxel delivery to drug-resistant cancer cells. Int. J. Nanomed. 2015, 10, 5249. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhu, W.; Zhang, H.; Han, J.; Zhang, L.; Lin, Q.; Ai, F. Carboxymethyl chitosan/phospholipid bilayer-capped mesoporous carbon nanoparticles with pH-responsive and prolonged release properties for oral delivery of the antitumor drug, Docetaxel. Int. J. Pharm. 2017, 532, 384–392. [Google Scholar] [CrossRef]
- Hami, Z.; Amini, M.; Ghazi-Khansari, M.; Rezayat, S.M.; Gilani, K. Synthesis and in vitro evaluation of a pH-sensitive PLA–PEG–folate based polymeric micelle for controlled delivery of docetaxel. Colloid Surf. B Biointerfaces 2014, 116, 309–317. [Google Scholar] [CrossRef]
- Kalafatovic, D.; Nobis, M.; Son, J.; Anderson, K.I.; Ulijn, R.V. MMP-9 triggered self-assembly of doxorubicin nanofiber depots halts tumor growth. Biomaterials 2016, 98, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.Y.; Park, K.; Kim, S.Y.; Byun, Y. MMPs-Specific PEGylated peptide–DOX conjugate micelles that can contain free doxorubicin. Eur. J. Pharm. Biopharm. 2007, 67, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Nazli, C.; Demirer, G.S.; Yar, Y.; Acar, H.Y.; Kizilel, S. Targeted delivery of doxorubicin into tumor cells via MMP-sensitive PEG hydrogel-coated magnetic iron oxide nanoparticles (MIONPs). Colloid Surf. B Biointerfaces 2014, 122, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, A.-N.; Liu, Y.-J.; Zhang, W.-J.; Pang, N.; Cheng, S.-X.; Qi, X.-R. Amphiphilic dendrimer engineered nanocarrier systems for co-delivery of siRNA and paclitaxel to matrix metalloproteinase-rich tumors for synergistic therapy. NPG Asia Mater. 2018, 10, 238–254. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Li, P.-J.; Wang, Y.; Chang, L.; Wan, D.; Wang, H. Active targeted drug delivery of MMP-2 sensitive polymeric nanoparticles. Chem. Commun. 2018, 54, 11092–11095. [Google Scholar] [CrossRef]
- Zheng, J.; Wan, Y.; Elhissi, A.; Zhang, Z.; Sun, X. Targeted paclitaxel delivery to tumors using cleavable PEG-conjugated solid lipid nanoparticles. Pharm. Res. 2014, 31, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Perche, F.; Wang, T.; Torchilin, V.P. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials 2014, 35, 4213–4222. [Google Scholar] [CrossRef] [Green Version]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Tagami, T.; Foltz, W.D.; Ernsting, M.J.; Lee, C.M.; Tannock, I.F.; May, J.P.; Li, S.-D. MRI monitoring of intratumoral drug delivery and prediction of the therapeutic effect with a multifunctional thermosensitive liposome. Biomaterials 2011, 32, 6570–6578. [Google Scholar] [CrossRef]
- Gulzar, A.; Gai, S.; Yang, P.; Li, C.; Ansari, M.B.; Lin, J. Stimuli responsive drug delivery application of polymer and silica in biomedicine. J. Mater. Chem. B 2015, 3, 8599–8622. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-J.; Chaung, E.-Y.; Wey, S.-P.; Lin, K.-J.; Cheng, F.; Lin, C.-C.; Liu, H.-L.; Tseng, H.-W.; Liu, C.-P.; Wei, M.-C. Hyperthermia-mediated local drug delivery by a bubble-generating liposomal system for tumor-specific chemotherapy. ACS Nano 2014, 8, 5105–5115. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. control. Rel. 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Rapoport, N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci. 2007, 32, 962–990. [Google Scholar] [CrossRef]
- Sponchioni, M.; Palmiero, U.C.; Moscatelli, D. Thermo-Responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahle, F.F.; Gulfam, M.; Lowe, T.L. Design strategies for physical-stimuli-responsive programmable nanotherapeutics. Drug Discov. Today 2018, 23, 992–1006. [Google Scholar] [CrossRef]
- Bonini, M.; Berti, D.; Baglioni, P. Nanostructures for magnetically triggered release of drugs and biomolecules. Curr. Opin. Colloid Interface Sci. 2013, 18, 459–467. [Google Scholar] [CrossRef]
- De Cuyper, M.; Joniau, M. Magnetoliposomes. Formation and structural characterization. Eur. Biophys. J. 1988, 15, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Senyei, A.; Widder, K.; Czerlinski, G. Magnetic guidance of drug-carrying microspheres. J. Appl. Phys. 1978, 49, 3578–3583. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Wang, J.; Gao, N.; Ren, J.; Zhao, A.; Qu, X. Electrically pulsatile responsive drug delivery platform for treatment of Alzheimer’s disease. Nano Res. 2015, 8, 2400–2414. [Google Scholar] [CrossRef]
- D’Emanuele, A.; Staniforth, J.N. An electrically modulated drug delivery device: I. Pharm. Res. 1991, 8, 913–918. [Google Scholar] [CrossRef]
- Hosseini-Nassab, N.; Samanta, D.; Abdolazimi, Y.; Annes, J.P.; Zare, R.N. Electrically controlled release of insulin using polypyrrole nanoparticles. Nanoscale 2017, 9, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, C.P.; Brady, C.; Cowley, J.F.; McGlinchey, S.M.; McGoldrick, N.; Kinnear, D.J.; Andrews, G.P.; Jones, D.S. Triggered drug delivery from biomaterials. Exp. Opin. Drug Deliv. 2010, 7, 605–616. [Google Scholar] [CrossRef]
- Kim, J.A.; Lee, W.G. Role of weakly polarized nanoparticles in electroporation. Nanoscale 2011, 3, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Riviere, J.E.; Heit, M.C. Electrically-Assisted transdermal drug delivery. Pharm. Res. 1997, 14, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Inamdar, S.Z.; Das, K.K.; Akamanchi, K.G.; Patil, A.V.; Inamadar, A.C.; Reddy, K.R.; Raghu, A.V.; Kulkarni, R.V. Tailor-Made electrically-responsive poly (acrylamide)-graft-pullulan copolymer based transdermal drug delivery systems: Synthesis, characterization, in-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 56, 101525. [Google Scholar] [CrossRef]
- Schroeder, A.; Kost, J.; Barenholz, Y. Ultrasound, liposomes, and drug delivery: Principles for using ultrasound to control the release of drugs from liposomes. Chem. Phys. Lipids 2009, 162, 1–16. [Google Scholar] [CrossRef]
- Di, J.; Kim, J.; Hu, Q.; Jiang, X.; Gu, Z. Spatiotemporal drug delivery using laser-generated-focused ultrasound system. J. Control. Release 2015, 220, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Sirsi, S.R.; Borden, M.A. State-of-the-Art materials for ultrasound-triggered drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Gu, L.; Ren, W.; Liu, Y. Stimuli-Responsive polymers for anti-cancer drug delivery. Mater. Sci. Eng. C 2014, 45, 600–608. [Google Scholar] [CrossRef]
- Xia, H.; Zhao, Y.; Tong, R. Ultrasound-Mediated polymeric micelle drug delivery, in: Therapeutic Ultrasound, Country. Ther. Ultrasound 2016. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Du, L.-N.; Lu, C.-T.; Jin, Y.-G.; Ge, S.-P. Potential and problems in ultrasound-responsive drug delivery systems. Int. J. Nanomed. 2013, 8, 1621. [Google Scholar] [CrossRef] [Green Version]
- Zardad, A.-Z.; Choonara, Y.E.; Toit, L.C.D.; Kumar, P.; Mabrouk, M.; Kondiah, P.P.D.; Pillay, V. A review of thermo-and ultrasound-responsive polymeric systems for delivery of chemotherapeutic agents. Polymers 2016, 8, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, L.; Li, C.; Wang, Y.; Gong, Y.; Su, F.; Li, S. Novel thermosensitive polymer-modified liposomes as nano-carrier of hydrophobic antitumor drugs. J. Pharm. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, W.; Gu, Z. Stimuli-Responsive nanomaterials for therapeutic protein delivery. J. Control. Release 2014, 194, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Q.; Shen, J.; Zhang, Z.; Yu, H.; Li, Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv. Drug Deliv. Rev. 2013, 65, 1699–1715. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-Sensitive intelligent drug delivery systems. Photochem. Photobiol. 2009, 85, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, H.; Yao, Q.; Ge, H.; Fan, J.; Sun, W.; Wang, J.; Peng, X. Hypoxia-Activated NIR photosensitizer anchoring in the mitochondria for photodynamic therapy. Chem. Sci. 2019, 10, 10586–10594. [Google Scholar] [CrossRef] [Green Version]
- Duwa, R.; Emami, F.; Lee, S.; Jeong, J.-H.; Yook, S. Polymeric and lipid-based drug delivery systems for treatment of glioblastoma multiforme. J. Ind. Eng. Chem. 2019, 79, 261–273. [Google Scholar] [CrossRef]
- LaManna, C.M.; Lusic, H.; Grinstaff, M.W. Stimuli responsive charge-switchable lipids: Capture and release of nucleic acids. Chem. Phys. Lipids 2016, 196, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Kurisawa, M.; Yokoyama, M.; Okano, T. Gene expression control by temperature with thermo-responsive polymeric gene carriers. J. Control. Release 2000, 69, 127–137. [Google Scholar] [CrossRef]
- de Smet, M.; Langereis, S.; van den Bosch, S.; Grüll, H. Temperature-Sensitive liposomes for doxorubicin delivery under MRI guidance. J. Control. Release 2010, 143, 120–127. [Google Scholar] [CrossRef]
- Rehman, M.; Ihsan, A.; Madni, A.; Bajwa, S.Z.; Shi, D.; Webster, T.J.; Khan, W.S. Solid lipid nanoparticles for thermoresponsive targeting: Evidence from spectrophotometry, electrochemical, and cytotoxicity studies. Int. J. Nanomed. 2017, 12, 8325. [Google Scholar] [CrossRef] [Green Version]
- AAllam, A.; Potter, S.J.; Bud’ko, S.L.; Shi, D.; Mohamed, D.F.; Habib, F.S.; Pauletti, G.M. Lipid-Coated superparamagnetic nanoparticles for thermoresponsive cancer treatment. Int. J. Pharm. 2018, 54, 297–304. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Magin, R.L.; Cysyk, R.L.; Zaharko, D.S. Treatment of solid L1210 murine tumors with local hyperthermia and temperature-sensitive liposomes containing methotrexate. Cancer Res. 1980, 40, 1388–1395. [Google Scholar] [PubMed]
- Yoshida, M.; Watanabe, Y.; Sato, M.; Maehara, T.; Aono, H.; Naohara, T.; Hirazawa, H.; Horiuchi, A.; Yukumi, S.; Sato, K. Feasibility of chemohyperthermia with docetaxel-embedded magnetoliposomes as minimally invasive local treatment for cancer. Int. J. Cancer 2010, 126, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Zhaowu, Z.; Xiaoli, W.; Yangde, Z.; Xingyan, L.; Weihua, Z.; Nianfeng, L. Preparation and characterization of Tegafur magnetic thermosensitive liposomes. Pharm. Dev. Technol. 2009, 14, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Eleršič, K.; Pavlič, J.I.; Iglič, A.; Vesel, A.; Mozetič, M. Electric-Field controlled liposome formation with embedded superparamagnetic iron oxide nanoparticles. Chem. Phys. Lipids 2012, 165, 120–124. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, J.-C. Temperature and electric field-triggerable liposomes incorporating poly (hydroxyethyl acrylate-co-hexadecyl acrylate-co-carboxyethyl acrylate). J. Ind. Eng. Chem. 2018, 62, 383–391. [Google Scholar] [CrossRef]
- Nobuto, H.; Sugita, T.; Kubo, T.; Shimose, S.; Yasunaga, Y.; Murakami, T.; Ochi, M. Evaluation of systemic chemotherapy with magnetic liposomal doxorubicin and a dipole external electromagnet. Int. J. Cancer 2004, 109, 627–635. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Javadi, M.; Belnap, D.M.; Barrow, J.R.; Pitt, W.G. Ultrasound sensitive eLiposomes containing doxorubicin for drug targeting therapy. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 67–76. [Google Scholar] [CrossRef]
- Lin, W.; Ma, X.; Zhou, C.; Yang, H.; Yang, Y.; Xie, X.; Yang, C.; Han, C. Development and characteristics of novel sonosensitive liposomes for vincristine bitartrate. Drug Deliv. 2019, 26, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Gosangari, S.L.; Watkin, K.L. Enhanced release of anticancer agents from nanoliposomes in response to diagnostic ultrasound energy levels. Pharm. Dev. Technol. 2012, 17, 383–388. [Google Scholar] [CrossRef]
- You, J.; Zhang, P.; Hu, F.; Du, Y.; Yuan, H.; Zhu, J.; Wang, Z.; Zhou, J.; Li, C. Near-Infrared light-sensitive liposomes for the enhanced photothermal tumor treatment by the combination with chemotherapy. Pharm. Res. 2014, 31, 554–565. [Google Scholar] [CrossRef]
- Yavlovich, A.; Singh, A.; Blumenthal, R.; Puri, A. A novel class of photo-triggerable liposomes containing DPPC: DC8, 9PC as vehicles for delivery of doxorubcin to cells. Biochim. Biophys. Acta Biomembr. 2011, 1808, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, W.; Di, H.; Luo, L.; Zhu, C.; Yang, J.; Yin, X.; Yin, H.; Gao, J.; Du, Y. A photosensitive liposome with NIR light triggered doxorubicin release as a combined photodynamic-chemo therapy system. J. Control. Release 2018, 277, 114–125. [Google Scholar] [CrossRef]
- Li, Q.; Tang, Q.; Zhang, P.; Wang, Z.; Zhao, T.; Zhou, J.; Li, H.; Ding, Q.; Li, W.; Hu, F. Human epidermal growth factor receptor-2 antibodies enhance the specificity and anticancer activity of light-sensitive doxorubicin-labeled liposomes. Biomaterials 2015, 57, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sudimack, J.J.; Guo, W.; Tjarks, W.; Lee, R.J. A novel pH-sensitive liposome formulation containing oleyl alcohol. Biochim. Biophys. Acta Biomembr. 2002, 1564, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Reddy, J.A.; Dean, D.; Kennedy, M.D.; Low, P.S. Optimization of folate-conjugated liposomal vectors for folate receptor-mediated gene therapy. J. Pharm. Sci. 1999, 88, 1112–1118. [Google Scholar] [CrossRef]
- Moku, G.; Gulla, S.K.; Nimmu, N.V.; Khalid, S.; Chaudhuri, A. Delivering anti-cancer drugs with endosomal pH-sensitive anti-cancer liposomes. Biomater. Sci. 2016, 4, 627–638. [Google Scholar] [CrossRef]
- Chang, M.; Lu, S.; Zhang, F.; Zuo, T.; Guan, Y.; Wei, T.; Shao, W.; Lin, G. RGD-Modified pH-sensitive liposomes for docetaxel tumor targeting. Colloid Surf. B Biointerfaces 2015, 129, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.; Larsen, T.B.; Jølck, R.I.; Eliasen, R.; Holm, R.; Gjetting, T.; Andresen, T.L. Investigation of enzyme-sensitive lipid nanoparticles for delivery of siRNA to blood-brain barrier and glioma cells. Int. J. Nanomed. 2015, 10, 5995. [Google Scholar] [CrossRef] [Green Version]
- Pourhassan, H.; Clergeaud, G.; Hansen, A.E.; Østrem, R.G.; Fliedner, F.P.; Melander, F.; Nielsen, O.L.; O’Sullivan, C.K.; Kjær, A.; Andresen, T.L. Revisiting the use of sPLA2-sensitive liposomes in cancer therapy. J. Control. Release 2017, 261, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Amit, K.; Chalasani, K.; Jain, A.; Chourasia, M.; Jain, A.; Jain, N. Enzyme triggered pH sensitive liposomes for insulin delivery. J. Drug Deliv. Sci. Technol. 2007, 17, 399–405. [Google Scholar] [CrossRef]
- Taylor, K.M.; Morris, R.M. Thermal analysis of phase transition behaviour in liposomes. Thermochim. Acta 1995, 248, 289–301. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Lindner, L.H.; Eichhorn, M.E.; Eibl, H.; Teichert, N.; Schmitt-Sody, M.; Issels, R.D.; Dellian, M. Novel temperature-sensitive liposomes with prolonged circulation time. Clin. Cancer Res. 2004, 10, 2168–2178. [Google Scholar] [CrossRef] [Green Version]
- Needham, D.; Anyarambhatla, G.; Kong, G.; Dewhirst, M.W. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000, 60, 1197–1201. [Google Scholar]
- Lu, Y.-J.; Chuang, E.-Y.; Cheng, Y.-H.; Anilkumar, T.; Chen, H.-A.; Chen, J.-P. Thermosensitive magnetic liposomes for alternating magnetic field-inducible drug delivery in dual targeted brain tumor chemotherapy. Chem. Eng. J. 2019, 373, 720–733. [Google Scholar] [CrossRef]
- Yousefpour, A.; Amjad-Iranagh, S.; Goharpey, F.; Modarress, H. Effect of drug amlodipine on the charged lipid bilayer cell membranes DMPS and DMPS + DMPC: A molecular dynamics simulation study. Eur. Biophys. J. 2018, 47, 939–950. [Google Scholar] [CrossRef]
- Arsov, Z.; González-Ramírez, E.J.; Goñi, F.M.; Tristram-Nagle, S.; Nagle, J.F. Phase behavior of palmitoyl and egg sphingomyelin. Chem. Phys. Lipids 2018, 213, 102–110. [Google Scholar] [CrossRef]
- Kalyanram, P.; Ma, H.; Marshall, S.; Goudreau, C.; Cartaya, A.; Zimmermann, T.; Stadler, I.; Nangia, S.; Gupta, A. Interaction of amphiphilic coumarin with DPPC/DPPS lipid bilayer: Effects of concentration and alkyl tail length. Phys. Chem. Chem. Phys. 2020, 22, 15197–15207. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, D.; Li, J.; Wang, Y.; Guo, J.-X.; Chen, Z.-P.; Cai, B.-C.; Yang, T. Influence of lipid composition on the phase transition temperature of liposomes composed of both DPPC and HSPC. Drug Dev. Ind. Pharm. 2013, 39, 197–204. [Google Scholar] [CrossRef]
- Morini, M.A.; Sierra, M.B.; Pedroni, V.I.; Alarcon, L.M.; Appignanesi, G.A.; Disalvo, E.A. Influence of temperature, anions and size distribution on the zeta potential of DMPC, DPPC and DMPE lipid vesicles. Colloids Surf. B Biointerfaces 2015, 131, 54–58. [Google Scholar] [CrossRef]
- Chen, W.; Duša, F.; Witos, J.; Ruokonen, S.-K.; Wiedmer, S.K. Determination of the main phase transition temperature of phospholipids by nanoplasmonic sensing. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korani, M.; Nikoofal-Sahlabadi, S.; Nikpoor, A.R.; Ghaffari, S.; Attar, H.; Mashreghi, M.; Jaafari, M.R. The effect of phase transition temperature on therapeutic efficacy of liposomal bortezomib. Anti Cancer Agents Med. Chem. 2020, 20, 700–708. [Google Scholar] [CrossRef]
- Hong, M.-S.; Lim, S.-J.; Oh, Y.-K.; Kim, C.-K. pH-Sensitive, serum-stable and long-circulating liposomes as a new drug delivery system. J. Pharm. Pharmacol. 2002, 54, 51–58. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Golzio, M.; Rols, M.-P. Electric field-responsive nanoparticles and electric fields: Physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug Deliv. Rev. 2019, 138, 56–67. [Google Scholar] [CrossRef]
- Lim, J.K.; Zhou, H.; Tilton, R.D. Liposome rupture and contents release over coplanar microelectrode arrays. J. Colloid Interfaces Sci. 2009, 332, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.P.T.H.J.; Chung, T.W.; Liu, Y.Y.H.D.Z. Liposomes incorporated with cholesterol for drug release triggered by magnetic field. J. Med. Biol. Eng. 2007, 27, 29–34. [Google Scholar]
- Riva, E.R.; Sinibaldi, E.; Grillone, A.F.; del Turco, S.; Mondini, A.; Li, T.; Takeoka, S.; Mattoli, V. Enhanced In Vitro Magnetic Cell Targeting of Doxorubicin-Loaded Magnetic Liposomes for Localized Cancer Therapy. Nanomaterials 2020, 10, 2104. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv. Drug Deliv. Rev. 2008, 60, 1193–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dromi, S.; Frenkel, V.; Luk, A.; Traughber, B.; Angstadt, M.; Bur, M.; Poff, J.; Xie, J.; Libutti, S.K.; Li, K.C. Pulsed-High intensity focused ultrasound and low temperature–sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin. Cancer Res. 2007, 13, 2722–2727. [Google Scholar] [CrossRef] [Green Version]
- de Matos, M.B.; Deckers, R.; van Elburg, B.; Lajoinie, G.; de Miranda, B.S.; Versluis, M.; Schiffelers, R.; Kok, R.J. Ultrasound-sensitive liposomes for triggered macromolecular drug delivery: Formulation and in vitro characterization. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Guo, D.; Wang, X.; Tan, M.; Liu, M.; Ran, H.; Wang, Z. Effect of sound sensitive nano-liposomes combined with low intensity focused ultrasound on proliferation of HepG2 cells and its CT imaging in vitro. J. Third Mil. Med. Univ. 2018, 40, 2180–2189. [Google Scholar]
- Sun, L.; Xu, Y.; Zhang, X.; Gao, Y.; Chen, J.; Zhou, A.; Lu, Q.; Wang, Z.; Shao, K.; Wu, H. Mesenchymal Stem Cells Functionalized Sonodynamic Treatment for Improving Therapeutic Efficacy and Compliance of Orthotopic Oral Cancer. Adv. Mater. 2020, 32, 2005295. [Google Scholar] [CrossRef]
- Yavlovich, A.; Smith, B.; Gupta, K.; Blumenthal, R.; Puri, A. Light-Sensitive lipid-based nanoparticles for drug delivery: Design principles and future considerations for biological applications. Mol. Membr. Biol. 2010, 27, 364–381. [Google Scholar] [CrossRef] [Green Version]
- Pramanik, S.K.; Pal, U.; Choudhary, P.; Singh, H.; Reiter, R.J.; Ethirajan, A.; Swarnakar, S.; Das, A. Stimuli-Responsive Nanocapsules for the Spatiotemporal Release of Melatonin: Protection against Gastric Inflammation. ACS Appl. Biol. Mater. 2019, 2, 5218–5226. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef]
- Simões, S.; Moreira, J.N.; Fonseca, C.; Düzgüneş, N.; de Lima, M.C.P. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 2004, 56, 947–965. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Guo, W.; Stephenson, S.M.; Lee, R.J. Efficient intracellular drug and gene delivery using folate receptor-targeted pH-sensitive liposomes composed of cationic/anionic lipid combinations. J. Control. Release 2002, 80, 309–319. [Google Scholar] [CrossRef]
- Liu, C.; Ewert, K.K.; Yao, W.; Wang, N.; Li, Y.; Safinya, C.R.; Qiao, W. A multifunctional lipid incorporating active targeting and dual-control release capabilities for precision drug delivery. ACS Appl. Mater. Interfaces 2019, 12, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Andresen, T.L.; Thompson, D.H.; Kaasgaard, T. Enzyme-Triggered nanomedicine: Drug release strategies in cancer therapy (Invited Review). Mol. Membr. Biol. 2010, 27, 353–363. [Google Scholar] [CrossRef]
- de la Rica, R.; Aili, D.; Stevens, M.M. Enzyme-Responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef]
- Meers, P. Enzyme-Activated targeting of liposomes. Adv. Drug Deliv. Rev. 2001, 53, 265–272. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, S.-M.; Chu, J.-H.; Liu, X.-Z.; Zhang, L.; He, S.-Y.; Yang, S.-M.; Ju, R.-J.; Li, X.-T. Tumor Microenvironmental Responsive Liposomes Simultaneously Encapsulating Biological and Chemotherapeutic Drugs for Enhancing Antitumor Efficacy of NSCLC. Int. J. Nanomed. 2020, 15, 6451. [Google Scholar] [CrossRef] [PubMed]
- VanderMolen, K.M.; McCulloch, W.; Pearce, C.J.; Oberlies, N.H. Romidepsin (Istodax, NSC 630176, FR901228, FK228, depsipeptide): A natural product recently approved for cutaneous T-cell lymphoma. J. Antibiot. 2011, 64, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. 2011 FDA drug approvals. Nat. Rev. Drug Discov. 2012, 11, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Dotan, E.; Aggarwal, C.; Smith, M.R. Impact of rituximab (Rituxan) on the treatment of B-cell non-Hodgkin’s lymphoma. Pharm. Ther. 2010, 35, 148. [Google Scholar]
- U.S. Food and Drug Administration. FDA Approves Gemtuzumab Ozogamicin for CD33-Positive AML; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-gemtuzumab-ozogamicin-cd33-positive-aml (accessed on 1 February 2021).
- Blick, S.K.; Scott, L.J. Cetuximab. Drugs 2007, 67, 2585–2607. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil-the first FDA-approved nano-drug: From an idea to a product. In Handbook of Harnessing Biomaterials in Nanomedicine; Pan Stanford Publishing: Singapore, 2012; pp. 335–398. [Google Scholar]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artific. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [Green Version]
- Gardikis, K.; Tsimplouli, C.; Dimas, K.; Micha-Screttas, M.; Demetzos, C. New chimeric advanced Drug Delivery nano Systems (chi-aDDnSs) as doxorubicin carriers. Int. J. Pharm. 2010, 402, 231–237. [Google Scholar] [CrossRef]
- Burade, V.; Bhowmick, S.; Maiti, K.; Zalawadia, R.; Ruan, H.; Thennati, R. Lipodox®(generic doxorubicin hydrochloride liposome injection): In vivo efficacy and bioequivalence versus Caelyx®(doxorubicin hydrochloride liposome injection) in human mammary carcinoma (MX-1) xenograft and syngeneic fibrosarcoma (WEHI 164) mouse models. BMC Cancer 2017, 17, 405. [Google Scholar] [CrossRef] [Green Version]
- Chou, H.-H.; Wang, K.-L.; Chen, C.-A.; Wei, L.-H.; Lai, C.-H.; Hsieh, C.-Y.; Yang, Y.-C.; Twu, N.-F.; Chang, T.-C.; Yen, M.-S. Pegylated liposomal doxorubicin (Lipo-Dox®) for platinum-resistant or refractory epithelial ovarian carcinoma: A Taiwanese gynecologic oncology group study with long-term follow-up. Gynecol. Oncol. 2006, 101, 423–428. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Li, N.; Qiu, Z.; Zhou, S.; Li, C.; Zhao, J.; Song, H.; Chen, X. Pharmacokinetics and biodistribution study of paclitaxel liposome in Sprague-Dawley rats and Beagle dogs by liquid chromatography-tandem mass spectrometry. Drug Res. 2013, 63, 603–606. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Huang, X.-E.; Gao, L.-L. A clinical study on the premedication of paclitaxel liposome in the treatment of solid tumors. Biomed. Pharmacother. 2009, 63, 603–607. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Fassas, A.; Anagnostopoulos, A. The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia. Leuk. Lymphoma 2005, 46, 795–802. [Google Scholar] [CrossRef]
- Zhang, H. Onivyde for the therapy of multiple solid tumors. OncoTargets Ther. 2016, 9, 3001. [Google Scholar] [CrossRef] [Green Version]
- FDA. Approves Onivyde Combo Regimen for Advanced Pancreatic Cancer. Oncol. Times 2015. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.N.; Merchant, M.S.; Cole, D.E.; Jayaprakash, N.; Bernstein, D.; Delbrook, C.; Richards, K.; Widemann, B.C.; Wayne, A.S. Vincristine sulfate liposomes injection (VSLI, Marqibo®): Results from a phase I study in children, adolescents, and young adults with refractory solid tumors or leukemias. Pediatr. Blood Cancer 2016, 63, 997–1005. [Google Scholar] [CrossRef]
- Phuphanich, S.; Maria, B.; Braeckman, R.; Chamberlain, M. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt®) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J. Neuro Oncol. 2007, 81, 201–208. [Google Scholar] [CrossRef]
- Ginat, D.T. HiDAC (High-Dose Ara-C; Cytarabine; Cytosine Arabinoside; Cytosar-U; Depocyt). In Neuroimaging Pharmacopoeia; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Sartor, O. Eligard: Leuprolide acetate in a novel sustained-release delivery system. Urology 2003, 61, 25–31. [Google Scholar] [CrossRef]
- Sartor, O. Eligard® 6: A new form of treatment for prostate cancer. Eur. Urol. Suppl. 2006, 5, 905–910. [Google Scholar] [CrossRef]
- Groisberg, R.; Hong, D.S.; Behrang, A.; Hess, K.; Janku, F.; Piha-Paul, S.; Naing, A.; Fu, S.; Benjamin, R.; Patel, S. Characteristics and outcomes of patients with advanced sarcoma enrolled in early phase immunotherapy trials. J. Immunother. Cancer 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Kojima, C. Design of stimuli-responsive dendrimers. Expert Opin. Drug Deliv. 2010, 7, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, L.; Liu, Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241. [Google Scholar] [CrossRef]
- Bawa, P.; Pillay, V.; Choonara, Y.E.; Toit, L.C.D. Stimuli-Responsive polymers and their applications in drug delivery. Biomed. Mater. 2009, 4, 022001. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- Kitano, H.; Akatsuka, Y.; Ise, N. pH-Responsive liposomes which contain amphiphiles prepared by using lipophilic radical initiator. Macromolecules 1991, 24, 42–46. [Google Scholar] [CrossRef]
- Dai, S.; Ravi, P.; Tam, K.C. pH-Responsive polymers: Synthesis, properties and applications. Soft Matter 2008, 4, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Kim, J.H.; Koo, H.; Bae, S.M.; Shin, H.; Kim, M.S.; Lee, B.-H.; Park, R.-W.; Kim, I.-S.; Choi, K. Tumor-Targeting peptide conjugated pH-responsive micelles as a potential drug carrier for cancer therapy. Bioconjug. Chem. 2010, 21, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ren, X.; Wang, W.; Ke, L.; Ning, E.; Du, L.; Bradshaw, J. A 5-fluorouracil-loaded pH-responsive dendrimer nanocarrier for tumor targeting. Int. J. Pharm. 2011, 420, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Hira, S.K.; Mitra, K.; Srivastava, P.; Singh, S.; Vishwakarma, S.; Singh, R.; Ray, B.; Manna, P.P. Doxorubicin loaded pH responsive biodegradable ABA-type Amphiphilic PEG-b-aliphatic Polyketal-b-PEG block copolymer for therapy against aggressive murine lymphoma. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102128. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.-J. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Guo, Z. Endogenous stimuli-responsive nanocarriers for drug delivery. Chem. Lett. 2016, 45, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Ulijn, R.V. Enzyme-Responsive materials: A new class of smart biomaterials. J. Mater. Chem. 2006, 16, 2217–2225. [Google Scholar] [CrossRef]
- Yao, Q.; Kou, L.; Tu, Y.; Zhu, L. MMP-Responsive “smart” drug delivery and tumor targeting. Trends Pharmacol. Sci. 2018, 39, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef]
- Taresco, V.; Alexander, C.; Singh, N.; Pearce, A.K. Stimuli-Responsive Prodrug Chemistries for Drug Delivery. Adv. Ther. 2018, 1, 1800030. [Google Scholar] [CrossRef] [Green Version]
- Xie, A.; Hanif, S.; Ouyang, J.; Tang, Z.; Kong, N.; Kim, N.Y.; Qi, B.; Patel, D.; Shi, B.; Tao, W. Stimuli-Responsive prodrug-based cancer nanomedicine. EBioMedicine 2020, 56. [Google Scholar] [CrossRef]
- Talelli, M.; Morita, K.; Rijcken, C.; Aben, R.; Lammers, T.; Scheeren, H.; van Nostrum, C.; Storm, G.; Hennink, W. Synthesis and characterization of biodegradable and thermosensitive polymeric micelles with covalently bound doxorubicin-glucuronide prodrug via click chemistry. Bioconjug. Chem. 2011, 22, 2519–2530. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Chen, Y.; Wang, Y.; Li, H.; Han, H.; Chen, T.; Jin, Q.; Ji, J. pH-and NIR Light-Responsive Polymeric Prodrug Micelles for Hyperthermia-Assisted Site-Specific Chemotherapy to Reverse Drug Resistance in Cancer Treatment. Small 2016, 12, 2731–2740. [Google Scholar] [CrossRef]
- Zhang, Y.; Teh, C.; Li, M.; Ang, C.Y.; Tan, S.Y.; Qu, Q.; Korzh, V.; Zhao, Y. Acid-Responsive polymeric doxorubicin prodrug nanoparticles encapsulating a near-infrared dye for combined photothermal-chemotherapy. Chem. Mater. 2016, 28, 7039–7050. [Google Scholar] [CrossRef]
- Han, Y.; Li, J.; Zan, M.; Luo, S.; Ge, Z.; Liu, S. Redox-Responsive core cross-linked micelles based on cypate and cisplatin prodrugs-conjugated block copolymers for synergistic photothermal-chemotherapy of cancer. Polym. Chem. 2014, 5, 3707–3718. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.; Chen, H.; Lim, W.Q.; Phua, F.S.Z.; An, G.; Yang, P.; Zhao, Y. Reduction-Sensitive fluorescence enhanced polymeric prodrug nanoparticles for combinational photothermal-chemotherapy. Biomaterials 2018, 163, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Frullano, L.; Tejerina, B.; Meade, T.J. Synthesis and Characterization of a Doxorubicin-Gd (III) Contrast Agent Conjugate: A New Approach toward Prodrug-Procontrast Complexes. Inorg. Chem. 2006, 45, 8489–8491. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Gil, J.; Cobaleda-Siles, M.; Zabaleta, A.; Salassa, L.; Calvo, J.; Mareque-Rivas, J.C. An iron oxide nanocarrier loaded with a Pt (IV) prodrug and immunostimulatory dsRNA for combining complementary cancer killing effects. Adv. Healthc. Mater. 2015, 4, 1034–1042. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Z.; Hao, Y.; Zhu, C.; Jiao, Y.; Chen, H.; Wang, Y.-M.; Yan, J.; Guo, Z.; Wang, X. Glutathione boosting the cytotoxicity of a magnetic platinum (IV) nano-prodrug in tumor cells. Chem. Sci. 2016, 7, 2864–2869. [Google Scholar] [CrossRef] [Green Version]
- Lopez, R.F.; Bentley, M.V.L.; Delgado-Charro, M.B.; Guy, R.H. Optimization of aminolevulinic acid delivery by iontophoresis. J. Control. Release 2003, 88, 65–70. [Google Scholar] [CrossRef]
- Luo, W.; Wen, G.; Yang, L.; Tang, J.; Wang, J.; Wang, J.; Zhang, S.; Zhang, L.; Ma, F.; Xiao, L. Dual-Targeted and pH-sensitive doxorubicin prodrug-microbubble complex with ultrasound for tumor treatment. Theranostics 2017, 7, 452. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yu, B.; Li, C.; Xu, M.; Cao, Z.; Xie, X.; Wang, W.; Liu, J. Ultrasound triggered phase-change nanodroplets for doxorubicin prodrug delivery and ultrasound diagnosis: An in vitro study. Colloids Surf. B Biointerfaces 2019, 174, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Bourre, L.; Giuntini, F.; Eggleston, I.M.; Wilson, M.; MacRobert, A.J. 5-Aminolaevulinic acid peptide prodrugs enhance photosensitization for photodynamic therapy. Mol. Cancer Ther. 2008, 7, 1720–1729. [Google Scholar] [CrossRef] [Green Version]
- Hossion, A.M.; Bio, M.; Nkepang, G.; Awuah, S.G.; You, Y. Visible light controlled release of anticancer drug through double activation of prodrug. ACS Med. Chem. Lett. 2013, 4, 124–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.H.; Qiu, W.X.; Li, B.; Zhang, C.; Sun, L.F.; Wan, S.S.; Rong, L.; Zhang, X.Z. A Red Light Activatable Multifunctional Prodrug for Image-Guided Photodynamic Therapy and Cascaded Chemotherapy. Adv. Funct. Mater. 2016, 26, 6257–6269. [Google Scholar] [CrossRef]
- Thapa, P.; Li, M.; Karki, R.; Bio, M.; Rajaputra, P.; Nkepang, G.; Woo, S.; You, Y. Folate-PEG conjugates of a far-red light-activatable paclitaxel prodrug to improve selectivity toward folate receptor-positive cancer cells. ACS Omega 2017, 2, 6349–6360. [Google Scholar] [CrossRef]
- Yang, B.; Wang, K.; Zhang, D.; Sun, B.; Ji, B.; Wei, L.; Li, Z.; Wang, M.; Zhang, X.; Zhang, H. Light-Activatable dual-source ROS-responsive prodrug nanoplatform for synergistic chemo-photodynamic therapy. Biomater. Sci. 2018, 6, 2965–2975. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Chen, Y.; Jin, Q.; Ji, J. A biomimic pH-sensitive polymeric prodrug based on polycarbonate for intracellular drug delivery. Polym. Chem. 2014, 5, 854–861. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, J.; Liu, G.; Wang, Y. A pH-Sensitive Phospholipid Polymeric Prodrug Based on Branched Polyethylenimine for Intracellular Drug Delivery. Macromol. Chem. Phys. 2016, 217, 2049–2055. [Google Scholar] [CrossRef]
- He, J.; Wang, W.; Zhou, H.; She, P.; Zhang, P.; Cao, Y.; Zhang, X. A novel pH-sensitive polymeric prodrug was prepared by SPAAC click chemistry for intracellular delivery of doxorubicin and evaluation of its anti-cancer activity in vitro. J. Drug Deliv. Sci. Technol. 2019, 53, 101130. [Google Scholar] [CrossRef]
- Du, C.; Deng, D.; Shan, L.; Wan, S.; Cao, J.; Tian, J.; Achilefu, S.; Gu, Y. A pH-sensitive doxorubicin prodrug based on folate-conjugated BSA for tumor-targeted drug delivery. Biomaterials 2013, 34, 3087–3097. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, R.; Bhuvaneshwar, G.; Sharma, C.P. Hemocompatible curcumin-dextran micelles as pH sensitive pro-drugs for enhanced therapeutic efficacy in cancer cells. Carbohydr. Polym. 2016, 137, 497–507. [Google Scholar] [CrossRef]
- Knox, R.J.; Connors, T.A. Prodrugs in cancer chemotherapy. Pathol. Oncol. Res. 1997, 3, 309. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; di Ilio, C.; Federici, L. Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Gao, C.; Wu, J. Covalent Layer-by-Layer Functionalization of Multiwalled Carbon Nanotubes by Click Chemistry. Langmuir 2009, 25, 5814–5824. [Google Scholar] [CrossRef]
- Zhang, Y.; Ang, C.Y.; Li, M.; Tan, S.Y.; Qu, Q.; Zhao, Y. Polymeric prodrug grafted hollow mesoporous silica nanoparticles encapsulating near-infrared absorbing dye for potent combined photothermal-chemotherapy. ACS Appl. Mater. Interface 2016, 8, 6869–6879. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Ding, Y.; Qian, J.; Zhang, R.; Dong, C.M. Dual drug-paired polyprodrug nanotheranostics reverse multidrug resistant cancers via mild photothermal-cocktail chemotherapy. J. Mater. Chem. B 2019, 7, 5306–5319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lin, H.; Huang, J.; Li, A.; Sun, C.; Richmond, J.; Gao, J. A gadolinium-complex-based theranostic prodrug for in vivo tumour-targeted magnetic resonance imaging and therapy. Chem. Commun. 2019, 55, 4546–4549. [Google Scholar] [CrossRef] [PubMed]
- Aoi, A.; Watanabe, Y.; Mori, S.; Takahashi, M.; Vassaux, G.; Kodama, T. Herpes simplex virus thymidine kinase-mediated suicide gene therapy using nano/microbubbles and ultrasound. Ultrasound Med. Biol. 2008, 34, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Bezagu, M.; Clarhaut, J.; Renoux, B.; Monti, F.; Tanter, M.; Tabeling, P.; Cossy, J.; Couture, O.; Papot, S.; Arseniyadis, S. In situ targeted activation of an anticancer agent using ultrasound-triggered release of composite droplets. Eur. J. Med. Chem. 2017, 142, 2–7. [Google Scholar] [CrossRef]
- Juzeniene, A.; Peng, Q.; Moan, J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem. Photobiol. Sci. 2007, 6, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of long-circulating zwitterionic cross-linked micelles for active-targeted drug delivery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Cheng, R.; Zhong, Z. A6 Peptide-Tagged Core-Disulfide-Cross-Linked Micelles for Targeted Delivery of Proteasome Inhibitor Carfilzomib to Multiple Myeloma In Vivo. Biomacromolecules 2020, 21, 2049–2059. [Google Scholar] [CrossRef]
- Mari, C.; Pierroz, V.; Leonidova, A.; Ferrari, S.; Gasser, G. Towards Selective Light-Activated RuII-Based Prodrug Candidates. Eur. J. Inorg. Chem. 2015, 2015, 3879–3891. [Google Scholar] [CrossRef]
- Bio, M.; Rajaputra, P.; Lim, I.; Thapa, P.; Tienabeso, B.; Hurst, R.E.; You, Y. Efficient activation of a visible light-activatable CA4 prodrug through intermolecular photo-unclick chemistry in mitochondria. Chem. Commun. 2017, 53, 1884–1887. [Google Scholar] [CrossRef]
- Phua, S.Z.F.; Xue, C.; Lim, W.Q.; Yang, G.; Chen, H.; Zhang, Y.; Wijaya, C.F.; Luo, Z.; Zhao, Y. Light-Responsive prodrug-based supramolecular nanosystems for site-specific combination therapy of cancer. Chem. Mater. 2019, 31, 3349–3358. [Google Scholar] [CrossRef]
- Zhang, Q.; Kuang, G.; He, S.; Lu, H.; Cheng, Y.; Zhou, D.; Huang, Y. Photoactivatable Prodrug-Backboned Polymeric Nanoparticles for Efficient Light-Controlled Gene Delivery and Synergistic Treatment of Platinum-Resistant Ovarian Cancer. Nano Lett. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Jin, Q.; Jia, F.; Wang, H.; Ji, J. Biomimic pH/reduction dual-sensitive reversibly cross-linked hyaluronic acid prodrug micelles for targeted intracellular drug delivery. Polymer 2015, 76, 237–244. [Google Scholar] [CrossRef]
- Yu, L.; Tan, S.; Li, Z.; Zheng, Z.; Zhou, L.; Su, Y.; Wang, X. Mixed polycarbonate prodrug nanoparticles with reduction/pH dual-responsive and charge conversional properties. React. Funct. Polym. 2017, 120, 74–82. [Google Scholar] [CrossRef]
- Xing, T.; Yan, L. pH-Responsive amphiphilic block copolymer prodrug conjugated near infrared fluorescence probe. RSC Adv. 2014, 4, 28186–28194. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Li, M.; Chen, X.; Xiao, C.; Zhuang, X.; Huang, Y.; Chen, X. One-step “click chemistry”-synthesized cross-linked prodrug nanogel for highly selective intracellular drug delivery and upregulated antitumor efficacy. ACS Appl. Mater. Interface 2016, 8, 10673–10682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, C.; Wang, W.; Liu, J.; Liu, Q.; Huang, F.; Chu, L.; Gao, H.; Li, C.; Kong, D.; et al. Co-Delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci. Rep. 2016, 6, 21225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Fan, X.; Li, L. pH-Sensitive polymeric micelles formed by doxorubicin conjugated prodrugs for co-delivery of doxorubicin and paclitaxel. Carbohydr. Polym. 2016, 137, 19–29. [Google Scholar] [CrossRef]
- Li, S.-Y.; Liu, L.-H.; Jia, H.-Z.; Qiu, W.-X.; Rong, L.; Cheng, H.; Zhang, X.-Z. A pH-responsive prodrug for real-time drug release monitoring and targeted cancer therapy. Chem. Commun. 2014, 50, 11852–11855. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, Y.; Liu, X.; Zhou, J.; Wang, X.; Feng, H.; Liu, H. Synergistic Combination Chemotherapy of Lung Cancer: Cisplatin and Doxorubicin Conjugated Prodrug Loaded, Glutathione and pH Sensitive Nanocarriers. Drug Des. Dev. Ther. 2020, 14, 5205–5215. [Google Scholar] [CrossRef]
- Hoffman, A.S. Stimuli-Responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef]
- Liu, S.; Tong, Y.; Yang, Y.-Y. Incorporation and in vitro release of doxorubicin in thermally sensitive micelles made from poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide)-b-poly (D, L-lactide-co-glycolide) with varying compositions. Biomaterials 2005, 26, 5064–5074. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; Jiang, G.; Liu, X.; Li, Z.; Gao, G.; Liu, F. Thermosensitive poly (N-isopropylacrylamide) hydrophobic associated hydrogels: Optical, swelling/deswelling, and mechanical properties. J. Mater. Sci. 2013, 48, 774–784. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Chennazhi, K.; Nair, S.; Tamura, H.; Jayakumar, R. Biodegradable and thermo-sensitive chitosan-g-poly (N-vinylcaprolactam) nanoparticles as a 5-fluorouracil carrier. Carbohydr. Polym. 2011, 83, 776–786. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, D.; Dinda, H.; Chakraborty, I.; Shashank, L.; Bhattacharyya, R.; Sarma, J.D.; Shunmugam, R. Super paramagnetic norbornene copolymer functionalized with biotin and doxorubicin: A potential unique site-Specific theranostic agent. Macromolecules 2016, 49, 2411–2418. [Google Scholar] [CrossRef]
- Zha, Y.; Thaker, H.D.; Maddikeri, R.R.; Gido, S.P.; Tuominen, M.T.; Tew, G.N. Nanostructured block-random copolymers with tunable magnetic properties. J. Am. Chem. Soc. 2012, 134, 14534–14541. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Shin, H.S.; Lee, Y.M.; Jeong, C.N. Properties of electroresponsive poly (vinyl alcohol)/poly (acrylic acid) IPN hydrogels under an electric stimulus. J. Appl. Polym. Sci. 1999, 73, 1675–1683. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Oh, I.-K.; Kee, C.-D.; Kim, S.-J. Bacterial cellulose actuator with electrically driven bending deformation in hydrated condition. Sens. Actuat. B Chem. 2010, 146, 307–313. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.I.; Shin, S.R.; Kim, S.I. Electrical behavior of chitosan and poly (hydroxyethyl methacrylate) hydrogel in the contact system. J. Appl. Polym. Sci. 2004, 92, 915–919. [Google Scholar] [CrossRef]
- Hirano, T.; Komatsu, M.; Uenohara, H.; Takahashi, A.; Takayama, K.; Yoshimoto, T. A novel method of drug delivery for fibrinolysis with Ho: YAG laser-induced liquid jet. Lasers Med. Sci. 2002, 17, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.Y.; Kennedy, A.M.; Shea, J.E.; Scaife, C.L.; Nam, K.-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J. Control. Release 2009, 138, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, N. Combined cancer therapy by micellar-encapsulated drug and ultrasound. Int. J. Pharm. 2004, 277, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Kelch, S.; Lendlein, A. Polymers move in response to light. Adv. Mater. 2006, 18, 1471–1475. [Google Scholar] [CrossRef]
- Nakayama, Y.; Matsuda, T. Preparation and characteristics of photocrosslinkable hydrophilic polymer having cinnamate moiety. J. Polym. Sci. A Polym. Chem. 1992, 30, 2451–2457. [Google Scholar] [CrossRef]
- Nakayama, Y.; Matsuda, T. Surface fixation of poly (ethylene glycol) via photodimerization of cinnamate group. J. Polym. Sci. A Polym Chem. 1993, 31, 3299–3305. [Google Scholar] [CrossRef]
- Walker, J.M.; Zaleski, J.M. Non-Enzymatic remodeling of fibrin biopolymers via photothermally triggered radical-generating nanoparticles. Chem. Mater. 2014, 26, 5120–5130. [Google Scholar] [CrossRef]
- Khan, S.; Akhtar, N.; Minhas, M.U.; Badshah, S.F. pH/Thermo-Dual Responsive Tunable In Situ Cross-Linkable Depot Injectable Hydrogels Based on Poly (N-Isopropylacrylamide)/Carboxymethyl Chitosan with Potential of Controlled Localized and Systemic Drug Delivery. AAPS PharmSciTech 2019, 20, 119. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chang, C.-H.; Wu, Y.-S.; Hsu, Y.-M.; Chiou, S.-F.; Chen, Y.-J. Development of pH-responsive chitosan/heparin nanoparticles for stomach-specific anti-Helicobacter pylori therapy. Biomaterials 2009, 30, 3332–3342. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Ma, L.; Chen, J. Thermo-and pH-sensitive comb-type grafted poly (N, N-diethylacrylamide-co-acrylic acid) hydrogels with rapid response behaviors. Eur. Polym. J. 2009, 45, 2060–2067. [Google Scholar] [CrossRef]
- Ferguson, E.L.; Duncan, R. Dextrin-Phospholipase A2: Synthesis and evaluation as a bioresponsive anticancer conjugate. Biomacromolecules 2009, 10, 1358–1364. [Google Scholar] [CrossRef]
- Vicent, M.J.; Greco, F.; Nicholson, R.I.; Paul, A.; Griffiths, P.C.; Duncan, R. Polymer therapeutics designed for a combination therapy of hormone-dependent cancer. Angew. Chem. 2005, 117, 4129–4134. [Google Scholar] [CrossRef]
- De la Rica, R.; Fratila, R.M.; Szarpak, A.; Huskens, J.; Velders, A.H. Multivalent nanoparticle networks as ultrasensitive enzyme sensors. Angew. Chem. 2011, 123, 5822–5825. [Google Scholar] [CrossRef]
- De laRica, R.; Fratila, R.M.; Szarpak, A.; Huskens, J.; Velders, A.H. Multivalent nanoparticle networks as ultrasensitive enzyme sensors. Angew. Chem. Int. Ed. 2011, 50, 5704–5707. [Google Scholar] [CrossRef]
- Wei, H.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Thermo-Sensitive polymeric micelles based on poly (N-isopropylacrylamide) as drug carriers. Prog. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- Ruel-Gariepy, E.; Leroux, J.-C. In situ-forming hydrogel—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Shin, H.H.; Choi, H.W.; Lim, J.H.; Kim, J.W.; Chung, B.G. Near-Infrared Light-Triggered Thermo-responsive Poly (N-Isopropylacrylamide)-Pyrrole Nanocomposites for Chemo-photothermal Cancer Therapy. Nanosc. Res. Lett. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Manouras, T.; Vamvakaki, M. Field responsive materials: Photo-, electro-, magnetic-and ultrasound-sensitive polymers. Polym. Chem. 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Filipcsei, G.; Csetneki, I.; Szilágyi, A.; Zrínyi, M. Magnetic field-responsive smart polymer composites. In Oligomers—Polymer Composites Molecular Imprinting; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Zrinyi, M. Intelligent polymer gels controlled by magnetic fields. Colloid Polym. Sci. 2000, 278, 98–103. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Li, X.; Kang, A.; Sun, L.; Sun, M.; Yang, F.; Xu, C. Magnetic And pH Dual-Responsive Nanoparticles For Synergistic Drug-Resistant Breast Cancer Chemo/Photodynamic Therapy. Int. J. Nanomed. 2019, 14, 7665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evjen, T.J.; Hagtvet, E.; Nilssen, E.A.; Brandl, M.; Fossheim, S.L. Sonosensitive dioleoylphosphatidylethanolamine-containing liposomes with prolonged blood circulation time of doxorubicin. Eur. J. Pharm. Sci. 2011, 43, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Wu, J.; Niu, S.; Sun, T.; Li, F.; Bai, Y.; Jin, L.; Lin, L.; Shi, Q.; Zhu, L.-M. Biodegradable, pH-Sensitive Hollow Mesoporous Organosilica Nanoparticle (HMON) with Controlled Release of Pirfenidone and Ultrasound-Target-Microbubble-Destruction (UTMD) for Pancreatic Cancer Treatment. Theranostics 2019, 9, 6002. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, C.; Huang, Y.; Zhang, F.; Lin, G. Study of the pH-sensitive mechanism of tumor-targeting liposomes. Colloids Surf. B Biointerfaces 2017, 151, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Bae, Y.H. Cancer nanomedicines targeting tumor extracellular pH. Colloids Surf. B Biointerfaces 2012, 99, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Poon, Z.; Chang, D.; Zhao, X.; Hammond, P.T. Layer-by-Layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano 2011, 5, 4284–4292. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Jiang, Y.; Zhang, J.; Klok, H.-A.; Zhong, Z. pH-Sensitive coiled-coil peptide-cross-linked hyaluronic acid nanogels: Synthesis and targeted intracellular protein delivery to CD44 positive cancer cells. Biomacromolecules 2018, 19, 555–562. [Google Scholar] [CrossRef]
- Lin, W.; Ma, G.; Yuan, Z.; Qian, H.; Xu, L.; Sidransky, E.; Chen, S. Development of Zwitterionic Polypeptide Nanoformulation with High Doxorubicin Loading Content for Targeted Drug Delivery. Langmuir 2018, 35, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Z.; Niu, J.; Ma, H.-J.; Dad, H.A.; Shao, H.-T.; Yuan, T.-J.; Peng, L.-H. Transdermal siRNA delivery by pH-switchable micelles with targeting effect suppress skin melanoma progression. J. Control. Release 2020, 322, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Barve, A.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Enzyme-Responsive polymeric micelles of cabazitaxel for prostate cancer targeted therapy. Acta Biomater. 2020, 113, 501–511. [Google Scholar] [CrossRef]
- Haddish-Berhane, N.; Rickus, J.L.; Haghighi, K. The role of multiscale computational approaches for rational design of conventional and nanoparticle oral drug delivery systems. Int. J. Nanomed. 2007, 2, 315. [Google Scholar]
- Engelmann, U.M.; Shasha, C.; Teeman, E.; Slabu, I.; Krishnan, K.M. Predicting size-dependent heating efficiency of magnetic nanoparticles from experiment and stochastic Néel-Brown Langevin simulation. J. Magn. Magn. Mater. 2019, 471, 450–456. [Google Scholar] [CrossRef]
- Molla, M.R.; Rangadurai, P.; Pavan, G.M.; Thayumanavan, S. Experimental and theoretical investigations in stimuli responsive dendrimer-based assemblies. Nanoscale 2015, 7, 3817–3837. [Google Scholar] [CrossRef]
- Pavan, G.M.; Albertazzi, L.; Danani, A. Ability to adapt: Different generations of PAMAM dendrimers show different behaviors in binding siRNA. J. Phys. Chem. B 2010, 114, 2667–2675. [Google Scholar] [CrossRef]
- Tian, W.-D.; Ma, Y.-Q. Molecular dynamics simulations of a charged dendrimer in multivalent salt solution. J. Phys. Chem. B 2009, 113, 13161–13170. [Google Scholar] [CrossRef]
- Nguyen, H.-N.; Wey, S.-P.; Juang, J.-H.; Sonaje, K.; Ho, Y.-C.; Chuang, E.-Y.; Hsu, C.-W.; Yen, T.-C.; Lin, K.-J.; Sung, H.-W. The glucose-lowering potential of exendin-4 orally delivered via a pH-sensitive nanoparticle vehicle and effects on subsequent insulin secretion in vivo. Biomaterials 2011, 32, 2673–2682. [Google Scholar] [CrossRef]
- Sekar, V.; Rajendran, K.; Vallinayagam, S.; Deepak, V.; Mahadevan, S. Synthesis and characterization of chitosan ascorbate nanoparticles for therapeutic inhibition for cervical cancer and their in silico modeling. J. Ind. Eng. Chem. 2018, 62, 239–249. [Google Scholar] [CrossRef]
- Yang, K.; Ma, Y.-Q. Computer simulation of the translocation of nanoparticles with different shapes across a lipid bilayer. Nat. Nanotechnol. 2010, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.A.; Bevan, M.A. Computational design of nanoparticle drug delivery systems for selective targeting. Nanoscale 2015, 7, 15332–15340. [Google Scholar] [CrossRef] [Green Version]
- Khoshoei, A.; Ghasemy, E.; Poustchi, F.; Shahbazi, M.-A.; Maleki, R. Engineering the pH-sensitivity of the graphene and carbon nanotube based nanomedicines in smart cancer therapy by grafting trimetyl Chitosan. Pharm. Res. 2020, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]







| S. No | Stimulus | Drug | Drug Delivery System | Reference |
|---|---|---|---|---|
| 1. | Temperature | Doxorubicin | Liposomes, Micellar nanoparticles, Hydrogels, Polymeric nanoparticles, Dendrimers | [25,26,27,28,29] |
| Docetaxel | Micelles, Hydrogel, Liquid Suppository, Liposomes | [30,31,32,33] | ||
| 2. | Magnetic Field | Doxorubicin | Magneto-liposomes, FeCo/Graphite shell Nanocrystals, Alginate embedded Magnetic Nanoheaters, Magnetic iron oxide nanoparticles | [34,35,36,37] |
| Docetaxel | Docetaxel grafted magnetic nanoparticles, Nanocomposite, Polymeric iron oxide nanoparticles | [33,38,39] | ||
| 3. | Electric Field | Antisense oligonucleotides | Liposome nanoparticles, Hydrogels | [40,41] |
| 4. | Ultrasound | Doxorubicin | Polypeptide doxorubicin nanoconjugates, Alginate nanodroplets, PEGylated Liposomes, Microbubbles | [42,43,44,45] |
| Docetaxel | Nanobubbles, Lipid microbubbles, | [46,47] | ||
| 5. | Light | Doxorubicin | Gold nanospheres, Stealth Liposomes, Micelles, Mesoporous silica nanocarriers, Nanogels, | [48,49,50,51,52] |
| Docetaxel | PEGylated Gold Nanorod Coated Poly(l-lactide) Microneedles, Nanocomposites, | [53,54] | ||
| 6. | pH | Doxorubicin | Nanogels, Liposomes, Magnetic chitosan nanoparticles, Microgels, Micelles, Mesoporous silica nanoparticles, Magnetic nanoparticles, Dendrimers | [55,56,57,58,59,60,61,62] |
| Docetaxel | Liposomes, Lipid polymer hybrid nanoparticles, Mesoporous carbon nanoparticles, Micelles | [63,64,65,66] | ||
| 7. | Enzymes | Doxorubicin | Magnetic iron-oxide nanoparticles, Polymer-peptide-drug conjugates, Nanofibers, Dendrimers | [42,67,68,69] |
| Paclitaxel | Polymeric nanoparticles, Solid lipid nanoparticles, Dendrimers, Micelles | [70,71,72,73] |
| S. No | Stimulus | Drug | Lipids | Drug Delivery System | Targeting | Major Findings | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Temperature | Doxorubicin | DPPC:HSPC:Chol:DPPE-PEG | Liposomes | Passive | Hyperthermia assisted rapid drug release and enhanced in vitro cytotoxicity. | [110] |
| 5-fluorouracil | Lauric acid (LA):oleic acid (OA):linoleic acid (LIA) | SLN | Passive | Mild hyperthermia (39 °C) based quick drug release in diffusion-controlled manner thus showed 2–3 times higher cytotoxicity against cancer cells. | [111] | ||
| Camptothecin | DPPC:DPPG | Lipid-coated nanoparticles | Passive | The formulation showed thermoresponsive controlled drug release, greater cytotoxicity and synergistic activity against cancer cells. | [112] | ||
| Methotrexate | DPPC:DSPC | Liposomes | Passive | Delayed tumor growth and 4–6 fold improved cytotoxicity than free methotrexate through developed Liposomes | [113] | ||
| 2. | Magnetic field | Docetaxel | DPLC:DOPE:TMAG/FeFe2O4 | Magnetoliposomes | Dual active and passive | These magnetoliposomes showed the dual hyperthermia and magnetic field-assisted enhanced release and cytotoxicity of anticancer agent. | [114] |
| Tegafur | DPPC:MPPC:DSPE-PEG2000 | Liposomes | Passive | The result of these magnetic field sensitive liposomes of tegafur showed greater stability and specificity towards tumor cells. | [115] | ||
| Doxorubicin | DPPC:Chol:DSPE-PEG2000-Folate | Lipid-coated nanoparticles | Dual active and passive | Enhanced tumor-specific cytotoxicity, cell uptake and synergistic effects of biological and magnetic field-assisted targeting by magnetic field responsive liposomes relative to non-magnetic liposomes. | [116] | ||
| 3. | Electric field | Iron oxide nanoparticles | POPC:Chol-γ-Fe2O3 | Nanoparticles | Passive | Efficient and novel method to manufacture SPIONs for effective targeting the tumor cells by applying an external stimuli. | [117] |
| Calcein | EPC-P(HEA-HDA-CEA) | Liposomes | Passive | Tumor-specific targeting and triggered drug release from electric field-responsive liposomes for dermal and transdermal drug delivery. | [118] | ||
| Doxorubicin | EPC:CHOL/Fe3O4 | Liposomes | Passive | 3–4 fold higher DOX concentration through DOX loaded liposomes at the tumor. Moreover, decrease tumor growth and suppressed lung metastasis through target specific localization of DOX was achieved. | [119] | ||
| 4. | Ultrasound | Doxorubicin | DPPC, Chol, DSPE-PEG2000-amine, α-tocopherol, & (PFC5) | Nanoliposomes | Passive | Perfluoropentane and DPPC based DOX loaded liposomes showed controlled and target-specific release upon insonation with low-intensity ultrasound. | [120] |
| Vincristine | HSPC:DSPE-PEG2000/DSPE-PEG2000-Mal/HMME | Emulsion liposomes (eLiposomes) | Passive | Site-specific delivery of vincristine triggered by ultrasound. In addition, the ultrasound also caused ROS-based tumor cell death through triggered release of drugs. | [121] | ||
| Doxorubicin | DOPE:DSPC:DSPE-PEG:Chol | Liposomes | Passive | These ultrasound-sensitive liposomes showed long blood circulation of drug for higher tumor uptake. | [122] | ||
| 5. | Light | Doxorubicin | DPPC:HSPC:Chol:DSPE-PEG2000/OMP-HauNS | Liposomes | Dual active and passive | These liposomes showed a promising delivery of chemotherapeutics as light-triggered targeted drug release. | [123] |
| DPPC:DC8,9PC:DSPE-PEG2000 | Lipid nanoparticles | Passive | Spatial and temporal release of therapeutic agents at tumor site and preferable taken up by permeable tumor vasculature was achieved and tumor-specific targeting was achieved by developed liposomes. | [124] | |||
| ICG-ODA,S100, PLsPC,Chol,DSPE-PEG2000 & DSPE-PEG2000-NH-DSC | Nanoparticles | Dual active and passive | These light-responsive nanoparticles structured with s light-responsive lipids showed tumor-specific DOX release and tumor growth inhibition compared to chemotherapy alone. | [125] | |||
| DPPC,HSPC,Chol,DSPE-PEG2000-NH-maleimide, OMP-HAuNS, HER2ab | Liposomes | Dual active and passive | Controlled and photo thermal release of DOX through NIR-responsive liposomes showed greater cytotoxicity than DOX nanoparticles alone. Moreover, DOX in conjunction with NIR laser showed significant antitumor efficacy. | [126] | |||
| 6. | pH | Cytosine-h-D-arabinofuranoside | PC:CHEMS:T-80:OAlc Or DOPE:CHEMS | Liposomes | Dual active and passive | These liposomes showed excellent stability at pH 7.4 and rapid destabilization upon acidic environment of cancer cells thus showed greater and targeted drug release. | [127] |
| DNA plasmid | DOPE:C-DOPE/FA-PEG-DOPG | Liposomes | Dual active and passive | pH-dependent release of endosome entrapped DNA into the cytoplasm and tumor-specific targeting was achieved with limited cytotoxic effects on normal cells. | [128] | ||
| Curcumin & Paclitaxel | DOPC:DOPE:Cholesterol: DSPE-PEG2000 | Lipid-coated nanoparticles | Passive | The acidic pH-based tumor-specific targeting and tumor inhibition was achieved. | [129] | ||
| Docetaxel | PE/CHOL/CHEMS/RGD-CHEMS | Liposomes | Dual active and passive | Enhanced tumor-specific delivery compared to conventional pH-sensitive liposomes was achieved. Moreover, the higher uptake of drug by cancer cells was also achieved. | [130] | ||
| 7. | Enzyme | siRNA | DSPE-PEG2000/DSPE-PEG2000 angiopep/DOTAP/POPC/DODAP/DOPE-Rhb | Lipid nanoparticles | Dual active and passive | These lipid nanoparticles of siRNA showed safe, stable and effective delivery for treating the central nervous system disorders. | [131] |
| Oxaliplatin | DPPC:DPPG:DSPC:DSPG: DSPE-PEG2000:HSPC:Chol | Nanoparticles | Passive | These liposomes showed improved anti-tumor efficiency towards the enzymes directed by lysolipids and serum protein. | [132] | ||
| Insulin | PC:DPPE:Chol:OA/PA/SA | Liposomes | Passive | These enzyme-triggered pH-sensitive liposomes showed improved tumor specificity, rapid release at cancer cells and ability of a drug to reach the systemic circulation in a controlled manner. | [133] |
| S. No | Lipids | Abbreviation | Transition Temperature Tc (°C) | References |
|---|---|---|---|---|
| 1. | Dipalmitoyl phosphatidylcholine | DPPC | 41 °C | [135] |
| 2. | Dipalmitoyl phosphatidylglycerol | DPPG | 41 °C | [135] |
| 3. | Dimyristoyl phosphatidylserine | DMPS | 38 °C | [139] |
| 4. | Egg spingomyline | ESM | 40 °C | [140] |
| 5. | Dipalmitoyl phosphatidylserine | DPPS | 51 °C | [141] |
| 6. | Hydrogenated soybean phosphatidylcholine | HSPC | 52 °C | [142] |
| 7. | Dimyristoyl phosphatidylethanolamine | DMPE | 50 °C | [143] |
| 8. | Dimyristoyl phosphatidylcholine | DSPC | 55 °C | [144] |
| 9. | Dimyristoyl phosphatidylglycerol | DSPG | 55 °C | [145] |
| 10. | Dipalmitoyl phosphatidylethanolamine | DPPE | 60 °C | [146] |
| Product Name | Drug Delivery System | Drug | Indication | Clinical Status | Reference |
|---|---|---|---|---|---|
| Istodax | Prodrug | Romidepsin | Cutaneous T-cell Lymphoma | FDA (US) approved in 2009 | [166] |
| Zytiga | Prodrug | Abiraterone acetate | Metastatic castration-resistant prostate cancer | FDA (US) approved in 2011 | [167] |
| Rituxan | Prodrug | Rituximab | β-cell non-Hodgkin’s lymphoma and Refractory low-grade lymphoma | FDA approved | [168] |
| Mylotag | Prodrug | Gemtuzumab ozogamicin | Acute myeloid leukemia | US and EU approved in 2018 | [169] |
| Erbitux | Prodrug | Cetuximab | Colorectal cancer | FDA approved in 2009 | [170] |
| Doxil | Liposomes | Doxorubicin | Ovarian and breast cancer, Kaposi’s sarcoma | FDA approved in 1995, EMA approved in 1996 | [171] |
| Myocet | Liposomes | Doxorubicin | Metastatic breast cancer | FDA approved in 2000 | [172,173] |
| Lipodox | Liposomes | Doxorubicin | Kaposi’s sarcoma, breast and ovarian cancer | FDA approved in 2013 | [174,175] |
| Lipusu | Liposomes | Paclitaxel | Solid tumor and ovarian cancer | FDA approved in 2006 | [176,177] |
| DaunoXome | Liposomes | Daunorubicin | Hematological malignancy and Kaposi’s sarcoma | FDA approved in 1996 | [178,179] |
| Onivyde | Liposomes | Irinotecan | Metastatic pancreatic cancer and multiple solid tumor | FDA approved in 2015 | [180,181] |
| Marqibo | Liposomes | Vincristine sulfate | Acute lymphoblastic leukemia | FDA approved in 2012 | [182,183] |
| DepoCyt | Liposomes | Cytarabine | Neoplastic meningitis, lymphoma and solid tumor | FDA approved in 2007 | [184,185] |
| Eligard | Liposomes | Leuprolide acetate | Prostate cancer | FDA approved in 2002 | [186,187] |
| Mepact | Liposomes | Mifamurtide | Non metastatic osteosarcoma, | EMA approved in 2009 | [188,189] |
| Cellular Compartment/Tissue | pH |
|---|---|
| Early endosome | 6.0–6.5 |
| Late endosome | 5.0–6.0 |
| Lysosome | 4.5–5.0 |
| Golgi | 6.4 |
| Stomach | 1.0–3.0 |
| Duodenum | 4.8–8.2 |
| Colon | 7.0–7.5 |
| Blood | 7.35–7.45 |
| Tumor | 6.5–7.2 |
| S. No | Stimulus | Drug | Prodrug | Targeting | Major Findings | Reference |
|---|---|---|---|---|---|---|
| 1. | Temperature | Doxorubicin | mPEG5000-b-PMAmLac2-r-AzEMA)-DOX-propGA3 | Dual active and passive | Improved in vitro and in vivo cytotoxicity of cancer cells was achieved through hyperthermia in the presence of beta glucuronidase. | [206] |
| P-cypate/P-DOX | Passive | The micelles showed enhanced in vitro drug release at acidic pH. Micelles also displayed both in vitro and in vivo cytotoxicity at cancer cells in the presence of light source and the light source caused hyperthermia. So collectively micelles showed triple stimuli responsiveness. | [52] | |||
| P(MAOEPC)-b-P(MEMA)-Hz-DOX + IR-780 (PDOX/IR-780) | Passive | Enhanced pH and photothermal in vitro drug release, cytotoxicity and drug internalization at tumor site and cancer cells was achieved by using pH-responsive polymeric prodrug micelles and NIR light source. | [207] | |||
| PNHNH2-b-POEGMA-FA-DOX + IR-825 (PDOX/IR-825) | Dual active and Passive | Enhanced in vitro and in vivo cytotoxicity of cancer cells was achieved at acidic pH in the presence of light source. | [208] | |||
| Cisplatin | P(Pt-Cyp-MEO2MA-co-MASI)-b-PHPMA | Passive | Enhanced photothermal responsiveness, drug efflux and in vitro cytotoxicity was achieved. | [209] | ||
| Camptothecin | Nap-CPT-Ad + HA-CD + IR-825 | Passive | Enhanced in vitro and in vivo cytotoxicity of cancer cells was achieved through light and temperature chemotherapeutics. | [210] | ||
| 2. | Magnetic Field | Doxorubicin | DOX-Gd(III) | Passive | Enhanced magnetic field and pH-responsive in vitro drug release was achieved. | [211] |
| Cisplatin | poly(I:C)-Pt(IV)-IONPs | Passive | Enhanced in vitro cytotoxicity using various cancer cell lines and immune cell-facilitated anti-neoplastic effects were achieved. | [212] | ||
| HSPt–PEG-SPIONs | Passive | Enhanced in vitro and in vivo cytotoxicity of cancer cells and theranostic application of Pt(IV) prodrug-loaded superparamagnetic iron nanoparticles (nanocomposites) was established. | [213] | |||
| 3. | Electric field | 5-aminolaevulinic acid (ALA) | ALA + Iontophoresis | Passive (diffusion) | Enhanced efflux of ALA across the skin and in dermis through iontophoresis was achieved. | [214] |
| 4. | Ultrasound | Doxorubicin | Heparin-FA-PEG-cRGD-DOX-MB | Dual active and Passive | Enhanced in vitro and in vivo cytotoxicity of cancer cells through combination of ultrasound and pH responsiveness was established. | [215] |
| per-fluoro-pentane/C9F17-Pasp(DET)/cis-aconityl-DOX/PGA-g-mPEGn-prodrug nanodroplets | Dual active and Passive | Enhanced in vitro cytotoxicity of cancer cells and theranostic application was achieved. | [216] | |||
| 5. | Light | 5-aminolaevulinic acid (ALA), | Ac-LPheALAOMe | Dual active and Passive | Enhanced stability, hydrophilicity photosensitization and cancer cells uptake were achieved. | [217] |
| Coumarin | D-L-dps/hυ | Passive | Enhanced photosensitization, quick in vitro drug release and singlet oxygen-cleavable onco-cytotoxicity in the presence of specific light intensity was achieved. | [218] | ||
| Gemcitabine | GEM-L-mTPP/658 nm Light source | Passive Targeting | Enhanced photosensitization, singlet oxygen-cleavable cross liker based in vitro cytotoxicity, cascaded drug release at tumor site and combination of photodynamic therapy and chemotherapy was achieved in the presence of suitable light source. | [219] | ||
| Paclitaxel | FA-PEG2000,5000-Pc-L-PTX | Dual active and Passive Targeting | Achievement of amphiphilic prodrug with enhanced light-mediated oncoreceptors overexpression, improved cytotoxicity associated with suitable chain length of ligands and enhanced cellular uptake was established in the presence of photosensitizer and singlet oxygen-cleavable cross linkers. | [220] | ||
| Cabazitaxel | Ppa⸞CTX-S/Se-OA⸞DSPE-PEG2000 NPs | Passive | Enhanced in vitro cytotoxicity, cellular uptake and intracellular release of anticancer agent was achieved in the presence of light source (of suitable wavelength). | [221] | ||
| 6. | pH | Doxorubicin | PMAC-graft-(ADPC-co-Mal-DOX | Passive | Enhanced drug internalization, cellular uptake and in vitro cytotoxicity at cancer pH was observed in the presence of esterases (dual pH and enzyme responsiveness). | [222] |
| ADPC-PEI-Mal-DOX | Passive | Improved in vitro drug release, cytotoxicity, internalization and cellular uptake was achieved at acidic pH. | [223] | |||
| mPEG-b-PLA-g-DOX | Passive | Enhanced in vitro release and cytotoxicity of doxorubicin at acidic pH and cancer cells were established. | [224] | |||
| FA-BSA-CA-DOX | Dual active and Passive | Enhanced tumor selectivity, efficacy, in vitro cytotoxicity and release of doxorubicin at the tumor site and cancer pH was achieved. | [225] | |||
| Curcumin | Cu-Dex micelles | Passive | Quick in vitro release and enhanced cytotoxicity at tumor site and cancer pH was established. | [226] | ||
| 7. | Enzyme (s) | Chemotherapeutic agent (s) | Antibody directed enzyme prodrug therapy, Gene directed enzyme prodrug therapy and Glutathione transferases based prodrug therapy | Dual active and Passive | N/A * | [227,228] |
| S. No. | Stimulus | Drug | Polymer | Drug Delivery System | Targeting | Major Findings | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Temperature | Doxorubicin | Poly (N-isopropylacrylamide-co-N, N-dimethylacrylamide)-b-poly (D, L-Lactide-co-glycolide) | Micelles | Passive | Thermosensitive doxorubicin-loaded micelles showed greater cytotoxicity at the temperature above the lower critical solution temperature. | [252] |
| Poly (N-isopropylacrylamide) | Hydrogel | Passive | Thermoresponsive Poly (N-isopropylacrylamide) hydrogels have good optical transparencies, mechanical properties and better swelling and deswelling properties. | [253] | |||
| 5-fluorouracil | Poly (N-vinyl caprolactam) based chitosan | Polymeric nanoparticles | Passive | Thermosensitive Poly (N-vinyl caprolactam) based chitosan 5-FU nanoparticles showed increased apoptosis to tumor cells. | [254] | ||
| 2. | Magnetic field | Biotin and Doxorubicin | Poly (ethylene glycol) | Magnetic nanoparticles | Dual active and Passive | Super paramagnetic norbornene copolymers biotin and doxorubicin are potential theranostic agents for tumor diagnosis. | [255] |
| Oxanorbornene | (Poly 1-Poly 7,With C16 homo block and Co-r-Fe) | Magnetic nanoparticles | Passive | The dipolar interactions of the cobalt nanoparticles with the phase-separated domains were responsible for the phenomenon of room temperature ferromagnetic particles of the block co-polymers. | [256] | ||
| 3. | Electric Field | N/A * | Poly (vinyl alcohol) (PVA)/Poly(acrylic acid) (PAA) | Hydrogels | Passive | Poly (vinyl alcohol) (PVA)/Poly(acrylic acid) (PAA) IPN hydrogels showed bending behavior upon exposure of electric field. | [257] |
| Bacterial Cellulose | Actuator | Passive | Bacterial cellulose actuator as an electro-active bio-polymer for implantable devices in wet environments. | [258] | |||
| chitosan and poly (hydroxyethyl methacrylate) | Hydrogels | Passive | Upon the exposure of electric field, the degree of bending deformation increases. | [259] | |||
| 4. | Ultrasound | N/A * | Gelatin | laser therapy | Passive | Fibrinolysis promoted by injecting fibrinolytics deeply into thrombus by means of Ho:YAG laser-induced liquid jet. | [260] |
| Paclitaxel | poly (ethylene oxide)-co-poly(L-lactide) (PEG-PLLA) or poly (ethylene oxide-co-polycaprolactone (PEG-PCL) | Nanoemulsion/Microbubbles | Active | Upon exposure of ultrasound, the drug-loaded nanoemulsion is converted into microbubbles (in-situ). | [261] | ||
| Doxorubicin | Poly (ethylene oxide)-co-poly(prolylene oxide)-co-poly(ethylene oxide), poly(prolylene oxide) (PPO) | Micelles | Passive | Enhanced cell viability, sensitization of multi-drug resistant cells, efflux, and cancer cell killing in presence of ultrasound stimuli due to pluronic triblock copolymer. | [262] | ||
| 5. | Light | N/A * | Cinnamic acid as cinnamon derivative | Passive | Enables the photo-induced shape changes in response to light. | [263] | |
| Cinnamylidene acetic acid as cinnamon derivative | Passive | The exposure of light on the cinnamon derivative results in higher yield of cross-linked polymers and less swelling behavior. | [264,265] | ||||
| Fibrin | PEG7500 | Gold nanoparticles | Dual Active and Passive | Gold nanoparticles have significant effect on disease state in deep vein thrombosis via local and catheter-delivered approaches. | [266] | ||
| 6. | pH | 5-fluorouracil | Poly (N-isopropylacrylamide)/carboxymethyl chitosan | Injectable hydrogels | Passive | In situ cross-linked depot injectable hydrogels are dual pH and thermoresponsive having the potential of onco-intracellular drug delivery through parenteral delivery. | [267] |
| Heparin | Chitosan | Polymeric nanoparticles | Passive | PH-sensitive chitosan/heparin nanoparticles can infiltrate the cell-to-cell junction and also adhere locally with the H-pylori at the infection site in the stomach. | [268] | ||
| Poly (N,N-diethylacrylamide-co-acrylic acid) | Hydrogel | Passive | Thermo and pH dual-responsive grafted hydrogels showed better thermo-reversible behavior as compared to normal hydrogels. | [269] | |||
| 7. | Enzyme | N/A * | Dextran | Polymeric nanoparticles | Active | Dextrin-phospholipase A2 showed reduced toxicity and α-amylase triggered activity. | [270] |
| Doxorubicin | N-(2-hydroxypropyl) methacrylamide (HPMA) | Polymeric nanoparticles | Passive | Polymer conjugates have combine effect of endocrine therapy and chemotherapy. | [271] | ||
| N/A * | Perthiolated β-cyclodextrin | Gold nanoparticles | Active | Sensitivity of nanoparticles biosensors enhanced by assembling through supramolecular multi-valent interactions. | [272,273] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahim, M.A.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z.; et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers 2021, 13, 670. https://doi.org/10.3390/cancers13040670
Rahim MA, Jan N, Khan S, Shah H, Madni A, Khan A, Jabar A, Khan S, Elhissi A, Hussain Z, et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers. 2021; 13(4):670. https://doi.org/10.3390/cancers13040670
Chicago/Turabian StyleRahim, Muhammad Abdur, Nasrullah Jan, Safiullah Khan, Hassan Shah, Asadullah Madni, Arshad Khan, Abdul Jabar, Shahzeb Khan, Abdelbary Elhissi, Zahid Hussain, and et al. 2021. "Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting" Cancers 13, no. 4: 670. https://doi.org/10.3390/cancers13040670
APA StyleRahim, M. A., Jan, N., Khan, S., Shah, H., Madni, A., Khan, A., Jabar, A., Khan, S., Elhissi, A., Hussain, Z., Aziz, H. C., Sohail, M., Khan, M., & Thu, H. E. (2021). Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers, 13(4), 670. https://doi.org/10.3390/cancers13040670