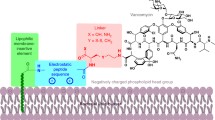
Abstract
The discovery of new antimicrobial and anticancer drugs, and overcoming the problem of resistance to current anti-infective and anticancer drug therapies require innovation in the pharmaceutical and scientific research community. A further challenge of drug design is to make the therapeutic agent specific, long lasting, of minimal toxicity, and affordable. Microbial and cancer cell surfaces present molecular features that can differentially prefocus drugs within the human host. This property can localize drugs near cell-surface targets, thereby reducing opportunities for adverse effects, or the emergence of drug resistance caused by intracellular drug and target modification and by the induction of drug efflux pumps. The solubility demands on cell-surface targeting drugs should also be less stringent than for those drugs requiring transmembrane transport or internalization in order to reach intracellular targets.
Cationic peptides have provided an increasingly important research focus in this regard. Although the cationic antimicrobial peptides are distributed widely in nature and provide localized primary defenses against microbial attack, the susceptibility of L-peptides to proteolysis and the known properties of successful antimicrobials have led to a focus on circularized peptides, D,L-peptides, and peptides containing unusual amino acids. New on the scene as lead antifungal agents are D-octapeptides and their derivatives that were developed from a combinatorial library produced through solid-phase peptide synthesis protocols. These peptides contain an amidated C-terminal tri-arginine motif, which confers membrane impermeability and focuses the peptides near the fungal cell surface. To date, the octapeptides and their derivatives also require some aromaticity, preferably the indole ring of tryptophan. In some cases, a single 4-methoxy-2,3,6-trimethylbenzenesulfonyl moiety remaining on the peptide after incomplete cleavage of the peptide from the solid phase produces a peptide with activity, whereas the parent shows little or no activity in the screen. Recent research advances that support the polycationic cell surface approach include the RGD (Arg-Gly-Asp) tripeptide and its mimetics, as well as aminoglycoside arginine drugs (e.g. neomycin coupled to small arginine polymers) and prodrugs. In the case of polycationic peptides, D-peptides could be used for intravenous injection and direct-surface drug applications, but mimetics will probably be needed for oral delivery.


Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement
References
Jones D. Overcoming inhibition. Nat Rev Drug Discov 2004; 3: 551
Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med 2002; 53: 615–27
Fauci AS. Infectious diseases: considerations for the 21st Century. Clin Infect Dis 2001; 32: 675–85
Projan SJ. Why is big pharma getting out of antibacterial drug discovery? Curr Opin Microbiol 2003; 6: 427–30
Levy SB. The antibiotic paradox. New York: Plenum Press, 1992: 7
Bush K. Why it is important to continue antibacterial drug discovery. ASM News 2004; 70: 282–7
Overbye KM, Barrett JF. Antibiotics: where did we go wrong? Drug Discov Today 2005; 10: 45–52
Shlaes DM, Projan SJ, Edwards JEJ. Antibiotic discovery: state of the state. ASM News 2004; 70: 275–81
Bush K, Macielag M, Weidner-Wells M. Taking inventory: antibacterial agents currently at or beyond phase 1. Curr Opin Microbiol 2004; 7: 466–76
Sams-Dodd F. Target-based drug discovery: is something wrong? Drug Discov Today 2005; 10: 139–47
Xia Y, Yu H, Jansen R, et al. Analyzing cellular biochemistry in terms of molecular networks. Annu Rev Biochem 2004; 73: 1051–87
Yamamoto K, Fujii R, Toyofuku Y, et al. The KDEL receptor mediates a retrieval mechanism that contributes to quality control at the endoplasmic reticulum. EMBO J 2001;20: 3082–91
Morphy R, Kay C, Rankovic Z. From magic bullets to designed multiple ligands. Drug Discov Today 2004; 9: 641–51
De Backer MD, Ilyina T, Ma XJ, et al. Genomic profiling of the response of Candida albicans to itraconazole treatment using a DNA microarray. Antimicrob Agents Chemother 2001; 45: 1660–70
Agarwal AK, Rogers PD, Baerson SR, et al. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifungal agents in Saccharomyces cerevisiae. J Biol Chem 2003; 278: 34998–5015
Monk BC, Cannon RD. Genomic pathways to antifungal discovery. Curr Drug Targets Infect Disord 2002; 2: 309–29
Bayles KW. The bactericidal action of penicillin: new clues to an unsolved mystery. Trends Microbiol 2000; 8: 274–8
Denning DW. Echinocandin antifungal drugs. Lancet 2003; 362: 1142–51
Monk BC, Perlin DS. Fungal plasma membrane proton pumps as promising new antifungal targets. Crit Rev Microbiol 1994; 20: 209–23
Weston SA, Camble R, Colls J, et al. Crystal structure of the anti-fungal target N-myristoyl transferase. Nat Struct Biol 1998; 5: 213–21
Sanglard D, Billie J. Current understanding of the modes of action and resistance mechanisms to conventional and emerging agents for the treatment of Candida infections. In: Calderone R, editor. Candida and candidiasis. Washington, DC: ASM Press, 2002: 349–83
Lipinski CA, Lombardo F, Dominy BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 2001; 46: 3–26
Hopkins AL, Groom CR. The draggable genome. Nat Rev Drug Discov 2002; 1: 727–30
Potoski J. Timely synthetic support for medicinal chemists. Drug Discov Today 2005; 10: 115–20
National Cancer Institute/National Institute of Health. Developmental therapeutics program [online]. Available from URL: http://dtp.nci.nih.gov/index.html [Accessed 2005 Jun 3]
Joseph SJ, Hugenholtz P, Sangwan P, et al. Laboratory cultivation of widespread and previously uncultured soil bacteria. Appl Environ Microbiol 2003; 69: 7210–5
Kaeberlein T, Lewis K, Epstein SS. Isolating ‘uncultivable’ microorganisms in pure culture in a simulated natural environment. Science 2002; 296: 1127–9
Zengler K, Toledo G, Rappe M, et al. Cultivating the uncultured. Proc Natl Acad Sci U S A 2002; 99: 15681–6
Handelsman J. Metagenomics: application of genomics to uncultured microorganisms. Microbiol Mol Biol Rev 2004; 68: 669–85
Schloss PD, Handelsman J. Biotechnological prospects from metagenomics. Curr Opin Biotechnol 2003; 14: 303–10
McDaniel R, Ebert-Khosla S, Hopwood DA, et al. Rational design of aromatic polyketide natural products by recombinant assembly of enzymatic subunits. Nature 1995; 375: 549–54
Maggio ET, Shenderovich M, Kagan R, et al. Structural pharmacogenomics, drug resistance and the design of anti-infective super-drugs. Drug Discov Today 2002; 7: 1214–20
Poole K. Mechanisms of bacterial biocide and antibiotic resistance. J Appl Microbiol 2002; 92 Suppl.: 55S–64S
Sanglard D. Resistance of human fungal pathogens to antifungal drugs. Curr Opin Microbiol 2002; 5: 379–85
Post FA, Willcox PA, Mathema B, et al. Genetic polymorphism in mycobacterium tuberculosis isolates from patients with chronic multidrug-resistant tuberculosis. J Infect Dis 2004; 190: 99–106
White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 1998; 11: 382–402
White TC, Holleman S, Dy F, et al. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother 2002; 46: 1704–13
Guillemard V, Saragovi HU. Novel approaches for targeted cancer therapy. Curr Cancer Drug Targets 2004; 4: 313–26
Arap W, Kolonin MG, Trepel M, et al. Steps toward mapping the human vasculature by phage display. Nat Med 2002; 8: 121–7
Oh P, Li Y, Yu J, et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature 2004; 429: 629–35
Cutler JE. N-glycosylation of yeast, with emphasis on Candida albicans. Med Mycol 2001; 39Suppl. 1: 75–86
Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol 2003; 11: 272–9
Chauhan N, Li D, Calderone R, et al. The cell wall of Candida species. In: Calderone R, editor. Candida and candidiasis. Washington, DC: ASM Press, 2002: 159–75
Malinska K, Malinsky J, Opekarova M, et al. Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol Biol Cell 2003; 14: 4427–36
Bagnat M, Keranen S, Shevchenko A, et al. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci U S A 2000; 97: 3254–9
Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 2003; 55: 27–55
Green DW. The bacterial cell wall as a source of antibacterial targets. Expert Opin Ther Targets 2002; 6: 1–19
Miyagi T, Wada T, Yamaguchi K, et al. Sialidase and malignancy: a mini review. Glycoconj J 2004; 20: 189–98
Sachs G, Shin JM, Briving C, et al. The pharmacology of the gastric acid pump: the H+, K+-ATPase. Annu Rev Pharmacol Toxicol 1995; 35: 277–305
Parderu P, Park S, Perlin DS. Capsofungin uptake is mediated by a high-affinity transporter in Candida albicans. Antimicrob Agents Chemother 2004; 48: 3845–9
Sawyers C. Targeted cancer therapy. Nature 2004; 432: 294–7
Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2003; 2: 114–22
Drenkard E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect 2003; 5: 1213–9
Kuhn DM, Ghannoum MA. Candida biofilms: antifungal resistance and emerging therapeutic options. Curr Opin Investig Drugs 2004; 5: 186–97
Friedrich C, Scott MG, Karunaratne N, et al. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob Agents Chemother 1999; 43: 1542–8
Ginsburg I. Bactericidal cationic peptides can also function as bacteriolysis-inducing agents mimicking beta-lactam antibiotics? It is enigmatic why this concept is consistently disregarded. Med Hypotheses 2004; 62: 367–74
Hancock RE, Patrzykat A. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr Drug Targets Infect Disord 2002; 2: 79–83
Reddy KV, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 2004; 24: 536–47
Niimi K, Harding DR, Parshot R, et al. Chemosensitization of fluconazole resistance in Saccharomyces cerevisiae and pathogenic fungi by a D-octapeptide derivative. Antimicrob Agents Chemother 2004; 48: 1256–71
Monk BC, Niimi K, Lin S, et al. Surface-active fungicidal D-peptide inhibitors of the plasma membrane proton pump that block azole resistance. Antimicrob Agents Chemother 2005; 49: 57–70
Shadidi M, Sioud M. Selective targeting of cancer cells using synthetic peptides. Drug Resist Updat 2003; 6: 363–71
Tucker GC. Alpha v integrin inhibitors and cancer therapy. Curr Opin Investig Drugs 2003; 4: 722–31
Moffitt MC, Neilan BA. The expansion of mechanistic and organismic diversity associated with non-ribosomal peptides. FEMS Microbiol Lett 2000; 191: 159–67
Papagianni M. Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnol Adv 2003; 21: 465–99
Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002; 415: 389–95
Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol 2000; 8: 402–10
Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci U S A 2000; 97: 8856–61
Yeaman MR, Yount NY. Code among chaos: immunorelativity and the AEGIS model of antimicrobial peptides. ASM News 2005; 71: 21–7
Yount NY, Yeaman MR. Multidimensional signatures in antimicrobial peptides. Proc Natl Acad Sci U S A 2004; 101: 7363–8
De Lucca AJ, Walsh TJ. Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob Agents Chemother 1999; 43: 1–11
van’t Hof W, Veerman EC, Heimerhorst EJ, et al. Antimicrobial peptides: properties and applicability. Biol Chem 2001; 382: 597–619
Hancock RE, Chappie DS. Peptide antibiotics. Antimicrob Agents Chemother 1999; 43: 1317–23
Andreu D, Ubach J, Boman A, et al. Shortened cecropin A-melittin hybrids: significant size reduction retains potent antibiotic activity. FEBS Lett 1992; 296: 190–4
Merrifield EL, Mitchell SA, Ubach J, et al. D-enantiomers of 15-residue cecropin A-melittin hybrids. Int J Pept Protein Res 1995; 46: 214–20
Merrifield RB, Juvvadi P, Andreu D, et al. Retro and retroenantio analogs of cecropin-melittin hybrids. Proc Natl Acad Sci U S A 1995; 92: 3449–53
Wade D, Andreu D, Mitchell SA, et al. Antibacterial peptides designed as analogs or hybrids of cecropins and melittin. Int J Pept Protein Res 1992; 40: 429–36
Fernandez-Lopez S, Kim HS, Choi EC, et al. Antibacterial agents based on the cyclic D,L-alpha-peptide architecture. Nature 2001; 412: 452–5
Thanou M, Florea BI, Langemeyer MW, et al. N-trimethylated chitosan chloride (TMC) improves the intestinal permeation of the peptide drug buserelin in vitro (Caco-2 cells) and in vivo (rats). Pharm Res 2000; 17: 27–31
Chung HH, Harms G, Seong CM, et al. Dendritic oligoguanidines as intracellular translocators. Biopolymers 2004; 76: 83–96
Ruissen AL, Groenink J, Heimerhorst EJ, et al. Effects of histatin 5 and derived peptides on Candida albicans. Biochem J 2001; 356: 361–8
Ulvatne H, Samuelsen O, Haukland HH, et al. Lactoferricin B inhibits bacterial macromolecular synthesis in Escherichia coli and Bacillus subtilis. FEMS Microbiol Lett 2004; 237: 377–84
Li XS, Reddy MS, Baev D, et al. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J Biol Chem 2003; 278: 28553–61
Hamamoto K, Kida Y, Zhang Y, et al. Antimicrobial activity and stability to proteolysis of small linear cationic peptides with D-amino acid substitutions. Microbiol Immunol 2002; 46: 741–9
Palsdottir H, Hunte C. Lipids in membrane protein structures. Biochim Biophys Acta 2004; 1666: 2–18
Hancock RE. Peptide antibiotics. Lancet 1997; 349: 418–22
Peschel A, Otto M, Jack RW, et al. Inactivation of the dit operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 1999; 274: 8405–10
Ganz T, Lehrer RI. Antibiotic peptides from higher eukaryotes: biology and applications. Mol Med Today 1999; 5: 292–7
Check W. Innate immunity depends on toll-like receptors. ASM News 2004; 70: 317–22
Mattsby-Baltzer I, Roseanu A, Motas C, et al. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr Res 1996; 40: 257–62
Shinoda I, Takase M, Fukuwatari Y, et al. Effects of lactoferrin and lactoferricin on the release of interleukin 8 from human polymorphonuclear leukocytes. Biosci Biotechnol Biochem 1996; 60: 521–3
Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532–5
Serrano R, Kielland-Brandt MC, Fink GR. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature 1986; 319: 689–93
Ostresh JM, Blondelle SE, Dorner B, et al. Generation and use of nonsupport-bound peptide and peptidomimetic combinatorial libraries. Methods Enzymol 1996; 267: 220–34
Balzi E, Wang M, Leterme S, et al. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcriptional regulator PDR1. J Biol Chem 1994; 269: 2206–14
Kolaczkowski M, van der Rest M, Cybularz-Kolaczkowski A, et al. Anticancer drugs, ionophoric peptides, and steroids as substrates of the yeast multidrug transporter Pdr5p. J Biol Chem 1996; 271: 31543–8
Decottignies A, Rogers B, Kowlaczkowski M, et al. The pleiotropic drug ABC transporters from Saccharomyces cerevisiae. In: Paulsen IT, Lewis K, editors. Microbial multidrug efflux. Wymondham: Horizon Scientific Press, 2002: 157–76
Ko KH, Lee CJ, Shin CY, et al. Inhibition of mucin release from airway goblet cells by polycationic peptides. Am J Physiol 1999; 277: L811–15
Lee CJ, Paik SH, Ko KH, et al. Effects of polycationic peptides on mucin release from airway goblet cells: relationship between polymer size and activity. Inflamm Res 2002; 51: 490–4
Bosshart H, Heinzelmann M. Endotoxin-neutralizing effects of histidine-rich peptides. FEBS Lett 2003; 553: 135–40
Steiner DF. The proprotein convertases. Curr Opin Chem Biol 1998; 2: 31–9
Molloy SS, Anderson ED, Jean F, et al. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol 1999; 9: 28–35
Cameron A, Appel J, Houghten RA, et al. Polyarginines are potent furin inhibitors. J Biol Chem 2000; 275: 36741–9
Kacprzak MM, Peinado JR, Than ME, et al. Inhibition of furin by polyarginine-containing peptides: nanomolar inhibition by nona-D-arginine. J Biol Chem 2004; 279: 36788–94
Mattner F, Fleitmann JK, Lingnau K, et al. Vaccination with poly-L-arginine as immunostimulant for peptide vaccines: induction of potent and long-lasting T-cell responses against cancer antigens. Cancer Res 2002; 62: 1477–80
Mitchell DJ, Kim DT, Steinman L, et al. Polyarginine enters cells more efficiently than other polycationic homopolymers. J Pept Res 2000; 56: 318–25
Rothbard JB, Kreider E, VanDeusen CL, et al. Arginine-rich molecular transporters for drug delivery: role of backbone spacing in cellular uptake. J Med Chem 2002; 45: 3612–8
Luedtke NW, Carmichael P, Tor Y. Cellular uptake of aminoglycosides, guanidinoglycosides, and poly-arginine. J Am Chem Soc 2003; 125: 12374–5
Kabouridis PS. Biological applications of protein transduction technology. Trends Biotechnol 2003; 21: 498–503
Lundberg P, Langel U. A brief introduction to cell-penetrating peptides. J Mol Recognit 2003; 16: 227–33
Midoux P, Kichler A, Boutin V, et al. Membrane permeabilization and efficient gene transfer by a peptide containing several histidines. Bioconjug Chem 1998; 9: 260–7
Temsamani J, Vidal P. The use of cell-penetrating peptides for drug delivery. Drug Discov Today 2004; 9: 1012–9
Fuchs SM, Raines RT. Pathway for polyarginine entry into mammalian cells. Biochemistry 2004; 43: 2438–44
Nah JW, Yu L, Han SO, et al. Artery wall binding peptide-poly(ethylene glycol)-grafted-poly(L-lysine)-based gene delivery to artery wall cells. J Control Release 2002; 78: 273–84
Sakharov DV, Jie AF, Bekkers ME, et al. Polylysine as a vehicle for extracellular matrix-targeted local drug delivery, providing high accumulation and long-term retention within the vascular wall. Arterioscler Thromb Vasc Biol 2001; 21: 943–8
Sakharov DV, Jie AF, Filippov DV, et al. Binding and retention of polycationic peptides and dendrimers in the vascular wall. FEBS Lett 2003; 537: 6–10
Mai JC, Mi Z, Kim SH, et al. A proapoptotic peptide for the treatment of solid tumors. Cancer Res 2001; 61: 7709–12
Pauletti GM, Okumu FW, Borchardt RT. Effect of size and charge on the passive diffusion of peptides across Caco-2 cell monolayers via the paracellular pathway. Pharm Res 1997; 14: 164–8
Landon LA, Deutscher SL. Combinatorial discovery of tumor targeting peptides using phage display. J Cell Biochem 2003; 90: 509–17
Trepel M, Arap W, Pasqualini R. In vivo phage display and vascular heterogeneity: implications for targeted medicine. Curr Opin Chem Biol 2002; 6: 399–404
Shadidi M, Sioud M. Identification of novel carrier peptides for the specific delivery of therapeutics into cancer cells. FASEB J 2003; 17: 256–8
He HT, Xu CR, Song X, et al. Syntheses of cyclic prodrugs of RGD peptidomimetics with various macrocyclic ring sizes: evaluation of physicochemical, transport and antithrombic properties. J Pept Res 2003; 61: 331–42
Kelleman A, Mattern RH, Pierschbacher MD, et al. Incorporation of thioether building blocks into an alphavbeta3-specific RGD peptide: synthesis and biological activity. Biopolymers 2003; 71: 686–95
Rust WL, Carper SW, Plopper GE. The promise of integrins as effective targets for anticancer agents. J Biomed Biotechnol 2002; 2: 124–30
Fujii H, Nishikawa N, Komazawa H, e. A new pseudo-peptide of Arg-Gly-Asp (RGD) with inhibitory effect on tumor metastasis and enzymatic degradation of extracellular matrix. Clin Exp Metastasis 1998; 16: 94–104
Tsuchiya Y, Sawada S, Tsukada K, et al. A new pseudo-peptide of Arg-Gly-Asp (RGD) inhibits intrahepatic metastasis of orthotopically implanted murine hepatocellular carcinoma. Int J Oncol 2002; 20: 319–24
Fujimoto K, Iwasaki C, Arai C, et al. Control of cell death by the smart polymeric vehicle. Biomacromolecules 2000; 1: 515–8
Lestini BJ, Sagnella SM, Xu Z, et al. Surface modification of liposomes for selective cell targeting in cardiovascular drug delivery. J Control Release 2002; 78: 235–47
Xu CR, He HT, Song X, et al. Synthesis and comparison of physiochemical, transport and antithrombic properties of a cyclic prodrug and the parent RGD peptidomimetic. Tetrahedron 2003; 59: 2861–9
Chen Y, Xu X, Hong S, et al. RGD-Tachyplesin inhibits tumor growth. Cancer Res 2001; 61: 2434–8
Yokoyama Y, Ramakrishnan S. Addition of integrin binding sequence to a mutant human endostatin improves inhibition of tumor growth. Int J Cancer 2004; 111: 839–48
Marquez M, Nilsson S, Lennartsson L, et al. Charge-dependent targeting: results in six tumor cell lines. Anticancer Res 2004; 24: 1347–51
Litovchick A, Evdokimov AG, Lapidot A. Aminoglycoside-arginine conjugates that bind TAR RNA: synthesis, characterization, and antiviral activity. Biochemistry 2000; 39: 2838–52
Eubank TD, Biswas R, Jovanovic M, et al. Inhibition of bacterial RNase P by aminoglycoside-arginine conjugates. FEBS Lett 2002; 511: 107–12
Li Q, Lee JY, Castillo R, et al. NB2001, a novel antibacterial agent with broad-spectrum activity and enhanced potency against beta-lactamase-producing strains. Antimicrob Agents Chemother 2002; 46: 1262–8
Stone GW, Zhang Q, Castillo R, et al. Mechanism of action of NB2001 and NB2030, novel antibacterial agents activated by beta-lactamases. Antimicrob Agents Chemother 2004; 48: 477–83
Jiang T, Olson ES, Nguyen QT, et al. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A 2004; 101: 17867–72
Cortez-Retamozo V, Backmann N, Senter PD, et al. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res 2004; 64: 2853–7
Stijlemans B, Conrath K, Cortez-Retamozo V, et al. Efficient targeting of conserved cryptic epitopes of infectious agents by single domain antibodies: African trypanosomes as paradigm. J Biol Chem 2004; 279: 1256–61
Grant GA, editor. Synthetic peptides: a user’s guide. 2nd ed. Oxford, UK: Oxford University Press, 2002
Jois SD, Hughes R, Siahaan TJ. Comparison of the solution conformations of a cell-adhesive peptide LBE and its reverse sequence EBL. J Biomol Struct Dyn 1999; 17: 429–44
Hugo CP, Pichler RP, Schulze-Lohoff E, et al. Thrombospondin peptides are potent inhibitors of mesangial and glomerular endothelial cell proliferation in vitro and in vivo. Kidney Int 1999; 55: 2236–49
Guo NH, Krutzsch HC, Inman JK, et al. Antiproliferative and antitumor activities of D-reverse peptides derived from the second type-1 repeat of thrombospondin-1. J Pept Res 1997; 50: 210–21
Fenniri H. Combinatorial chemistry: a practical approach. Oxford, UK: Oxford University Press, 2000
Glattli AL, Seebach D, van Gunsteren WF. Do valine side chains have an influence on the folding behavior of β-substituted β-peptides? Helv Chim Acta 2004; 87: 2487–506
Lind R, Greenhow D, Perry S, et al. Comparative metabolism of α- and β-peptides in the insect Heliothis virescens and in plant cells of black mexican sweet maize. Chem Biodivers 2004; 1(9): 1391–400
Seebach D, Beck AK, Bierbaum DJ, et al. The world of β- and γ-peptides comprised of homologated proteinogenic amino acids and other components. Chem Biodivers 2004; 1: 1111–239
Biltresse S, Attolini M, Dive G, et al. Novel RGD-like molecules based on the tyrosine template: design, synthesis, and biological evaluation on isolated integrins alpha(V)beta/alpha(IIb)beta(3) and in cellular adhesion tests. Bioorg Med Chem 2004; 12: 5379–93
Acknowledgments
The authors wish to acknowledge Ms Amanda King and Mr Karl Shaffer for their assistance in literature searches. Dr Roger Lins is thanked for proof-reading this manuscript.
No sources of funding were used in the preparation of this article.
The authors have no conflicts of interest directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monk, B.C., Harding, D.R.K. Peptide Motifs for Cell-Surface Intervention. BioDrugs 19, 261–278 (2005). https://doi.org/10.2165/00063030-200519040-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200519040-00005