Summary
Abstract
Celecoxib (Celebrex®), the first cyclo-oxygenase (COX) 2-selective inhibitor (coxib) to be introduced into clinical practice, has been available for almost a decade. It is approved in one or more countries worldwide for the relief of the signs and symptoms of osteoarthritis (OA), rheumatoid arthritis (RA), juvenile rheumatoid arthritis (in patients aged ≥2 years) and ankylosing spondylitis (AS), the management of acute pain in adults, the treatment of primary dysmenorrhoea and the reduction in the number of adenomatous colorectal polyps in familial adenomatous polyposis.
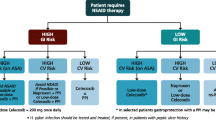
Celecoxib remains an effective and useful altenative to nonselective NSAIDs in the treatment of acute or chronic musculoskeletal pain. In the latter setting, it offers the prospect of improved gastrointestinal (GI) tolerability and, in patients not taking aspirin for cardioprophylaxis, a GI safety advantage. Currently available evidence of an increase in cardiovascular (CV) risk with celecoxib is inconsistent; any increase in risk is likely to be small and similar to that with nonselective NSAIDs. As with all NSAIDs, the potential GI, CV and renal risks of celecoxib must be weighed against the potential benefits in each individual; it is a rational choice for patients at low CV risk who require NSAID therapy, especially those at increased risk of NSAID-induced GI toxicity, but also those unresponsive to, or intolerant of, other NSAIDs. If selected, celecoxib, like all NSAIDs, should be used at the lowest effective dose for the shortest possible duration.
Pharmacological Properties
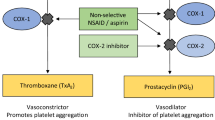
Celecoxib is 30 times more potent at inhibiting COX-2 than COX-1; the analgesic and anti-inflammatory effects of therapeutic dosages of the drug are mediated through COX-2 inhibition. Celecoxib has no effect on platelet function in healthy volunteers; it has qualitatively, if not quantitatively, similar effects on the kidney compared with nonselective NSAIDs.
Celecoxib demonstrates approximate dose-proportionality over the therapeutic dosage range (up to 200mg twice daily). Peak plasma concentrations are attained within 3 hours of an oral dose; steady-state plasma concentrations are achieved within 5 days. Celecoxib is extensively metabolised by the hepatic cytochrome P450 2C9 isoenzyme into inactive metabolites, which are excreted in the urine and faeces. The daily dosage should be reduced by 50% in patients with moderate (Child-Pugh class B) hepatic impairment. Celecoxib has demonstrated potentially significant drug interactions with fluconazole and lithium.
Therapeutic Efficacy
In clinical trials of up to 26 weeks’ duration, celecoxib at dosages between 200 and 800 mg/day was at least as effective as paracetamol (acetaminophen) 4 g/day in treating the signs and symptoms of OA, was generally as effective as, or noninferior to, standard dosages of nonselective NSAIDs (e.g. naproxen, diclofenac, ketoprofen and ibuprofen) in the treatment of OA, RA or AS, and was as effective as other COX-2-selective inhibitors (etoricoxib 30 mg/day and lumiracoxib 100–400 mg/day) in the treatment of OA. Unlike glucosamine 1500 mg/day and/or chondroitin sulfate 1200 mg/day, celecoxib 200 mg/day was significantly more effective than placebo in relieving pain associated with OA of the knee.
Celecoxib was generally similar to nonselective NSAIDs in improving overall health-related quality of life (HR-QOL) in patients with OA or RA flare. However, it demonstrated superiority over paracetamol and nonselective NSAIDs in OA studies assessing patient preference and satisfaction, respectively. Acute pain was significantly reduced within 1–2 days of starting treatment (OA flare); significant responses relative to placebo were apparent within 1 week (AS) or 2 weeks (OA or RA flare) of starting treatment. Continuous, but not on-demand, use of NSAIDs (primarily celecoxib) reduced radiographic progression in a 2-year open-label comparison of these treatment strategies in patients with AS.
The analgesic efficacy of celecoxib 400mg in the postoperative dental pain model was superior to that of placebo and generally similar to that of ibuprofen 400mg. Celecoxib 200mg was at least as effective as hydrocodone/paracetamol 10mg/1000mg after uncomplicated orthopaedic surgery. Perioperative or postoperative administration of celecoxib (400mg followed by 200mg as required or 200mg twice daily) to patients undergoing arthroscopic knee surgery, spinal fusion surgery, laparoscopy or anterior cruciate ligament reconstruction surgery resulted in a significant reduction in postoperative opioid use and/or pain scores relative to placebo.
Celecoxib 400 mg/day was superior to placebo and at least as effective as, or noninferior to, standard dosages of nonselective NSAIDs in the treatment of acute, painful musculoskeletal conditions, namely ankle sprain, lower back pain and shoulder strain/pain.
Gastrointestinal Tolerability and Safety
In large-scale GI clinical outcomes studies (SUCCESS-1 and CLASS), the upper GI tolerability of celecoxib ≤800 mg/day was superior to that of nonselective NSAIDs (naproxen, diclofenac and ibuprofen) in terms of the incidence of individual GI symptoms (abdominal pain, dyspepsia and nausea) and the incidence of symptomatic plus complicated ulcers combined. With regard to the incidence of complicated ulcers alone, a therapeutic dosage of celecoxib 200 or 400 mg/day was superior to nonselective NSAIDs at 12 weeks in SUCCESS-1, whereas a supratherapeutic dosage of celecoxib 800 mg/day was not superior to nonselective NSAIDs, either at 6 or 12–15 months in CLASS. The CLASS results may, however, have been subject to confounding factors, including the higher than anticipated level of concomitant low-dose aspirin use and, beyond 6 months, the higher rate of withdrawal of high GI risk patients (i.e. those with symptomatic ulcers) from the combined nonselective NSAID comparator group. Consistent with this, the annualised incidence of complicated ulcers in the subgroup of patients not taking aspirin was significantly reduced with celecoxib at 6 months, but not at 15 months.
In randomised studies in very high GI risk patients with a recent history of ulcer bleeding, the incidence of recurrent bleeding with celecoxib alone was similar to that with a nonselective NSAID plus a proton-pump inhibitor (PPI), but greater than that with celecoxib combined with a PPI.
Cardiovascular Adverse Event Profile
Currently available evidence of an increased CV risk with celecoxib is inconsistent; any increase in risk is likely to be small and similar to that with nonselective NSAIDs. Specifically, celecoxib 400 mg/day was associated with an increase in long-term CV risk in one placebo-controlled study (APC), but not in two other, similar trials (ADAPT, PreSAP). Similarly, whereas celecoxib 400–800 mg/day was associated with an increase in CV risk based on pooled data from APC and PreSAP, celecoxib at dosages ranging from 200 to 800 mg/day was, in general, not associated with an increase in short- or long-term CV risk relative to placebo and/or nonselective NSAIDs in larger meta-analyses of randomised clinical trials. Consistent with the bulk of the randomised data, numerous observational studies (and meta-analyses) suggest that celecoxib, unlike rofecoxib, does not increase the risk of CV events relative to that associated with the use or non-use of nonselective NSAIDs.
The renovascular tolerability of celecoxib at therapeutic and supratherapeutic dosages was at least as good as that of nonselective NSAIDs; clinically significant blood pressure elevation and/or oedema occurred less frequently with celecoxib (at a low therapeutic dosage) than with rofecoxib (at a high therapeutic dosage).











Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Payne R. Limitations of NSAIDs for pain management: toxicity or lack of efficacy. J Pain 2000 Sep; 1 (3 Suppl.): 14–8
Ardoin SP, Sundy JS. Update on nonsteriodal anti-inflammatory drugs. Curr Opin Rheumatol 2006 May; 18(3): 221–6
Botting RM. Inhibitors of cyclooxygenases: mechanisms, selectivity and uses. J Physiol Pharmacol 2006 Nov; 57 Suppl. 5: 113–24
Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J 2004; 18: 790–804
Fitzgerald GA. COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov 2003 Nov; 2(11): 879–90
Harris RCJr. Cyclooxygenase-2 inhibition and renal physiology. Am J Cardiol 2002 Mar 21; 89(6A): 10–17D
Komers R, Anderson S, Epstein M. Renal and cardiovascular effects of selective cyclooxygenase-2 inhibitors. Am J Kidney Dis 2001; 38(6): 1145–57
Tacconelli S, Capone ML, Patrignani P. Clinical pharmacology of novel selective COX-2 inhibitors. Curr Pharm Des 2004; 10: 589–601
Brune K, Hinz B. Selective cyclooxygenase-2 inhibitors: similarities and differences. Scand J Rheumatol 2004; 33: 1–16
Crofford LJ, Lipsky PE, Brooks P, et al. Basic biology and clinical application of specific cyclooxygenase-2 inhibitors. Arthritis Rheum 2000; 43(1): 4–13
FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 2001 Aug 9; 345(6): 433–42
Clemett D, Goa KL. Celecoxib: a review of its use in osteoarthritis, rheumatoid arthritis and acute pain. Drugs 2000 Apr; 59(4): 957–80
Goldenberg MM. Celecoxib, a selective cyclooxygenase-2 inhibitor for the treatment of rheumatoid arthritis and osteoarthritis. Clin Ther 1999 Sep; 21: 1497–513
Davies NM, McLachlan AJ, Day RO, et al. Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet 2000 Mar; 38(3): 225–42
Celebrex 100 and 200mg: summary of the product characteristics. Pharmacia Limited, Sandwich, England [online]. Available from URL: http://emc.medicines.org.uk/ [Accessed 2007 Apr 6]
Celebrex®: celecoxib capsules prescribing information. G.D. Searle, Chicago (IL) [online]. Available from URL: http://pfizer.com/pfizer/download/uspi_celebrex.pdf [Accessed 2007 Apr 6]
Leese PT, Hubbard RC, Karim A, et al. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol 2000 Feb; 40(2): 124–32
Wilner KD, Rushing M, Waiden C, et al. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J Clin Pharmacol 2002 Sep; 42(9): 1027–30
Widlansky ME, Price DT, Gokce N, et al. Short- and long-term COX-2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension 2003 Sep; 42(3): 310–5
Chenevard R, Hurlimann D, Bechir M, et al. Selective COX-2 inhibition improves endothelial function in coronary artery disease. Circulation 2003 Jan 28; 107(3): 405–9
Frishman WH. Effects of nonsteroidal anti-inflammatory drug therapy on blood pressure and peripheral edema. Am J Cardiol 2002 Mar 21; 89(6A): 18–25D
Werner U, Werner D, Pahl A, et al. Investigation of the pharmacokinetics of celecoxib by liquid chromatographymass spectrometry. Biomed Chromatogr 2002 Feb; 16(1): 56–60
Karim A, Piergies A. Celecoxib steady-state systemic exposure after q24hr (qm or pm) and q12hr dosing: circadian variation in oral absorption. Clin Pharmacol Ther 2002 Feb; 71: 68
Garnett WR. Clinical implications of drug interactions with coxibs. Pharmacotherapy 2001 Oct; 21(10): 1223–32
Karim A, Tolbert D, Piergies A, et al. Celecoxib does not significantly alter the pharmacokinetics or hypoprothrombinemic effect of warfarin in healthy subjects. J Clin Pharmacol 2000 Jun; 40(6): 655–63
Battistella M, Mamdami MM, Juurlink DN. Risk of upper gastrointestinal haemorrhage in warfarin users treated with nonselective NSAIDs or COX-2 inhibitors. Arch Intern Med 2005; 165: 189–92
Sowers JR, White WB, Pitt B, et al. The effects of cyclooxygenase-2 inhibitors and nonsteroidal anti-inflammatory therapy on 24-hour blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Arch Intern Med 2005 Jan 24; 165: 161–8
Whelton A, White WB, Bello AE, et al. Effects of celecoxib and rofecoxib on blood pressure and edema in patients >=65 years of age with systemic hypertension and osteoarthritis. Am J Cardiol 2002 Nov 1; 90(9): 959–63
White WB, Kent J, Taylor A, et al. Effects of celecoxib on ambulatory blood pressure in hypertensive patients on ACE inhibitors. Hypertension 2002 Apr; 39(4): 929–34
Palmer R, Weiss R, Zusman RM, et al. Effects of nabumetone, celecoxib, and ibuprofen on blood pressure control in hypertensive patients on angiotensin converting enzyme inhibitors. Am J Hypertens 2003 Feb; 16(2): 135–9
Geba GP, Weaver AL, Polis AB, et al. Efficacy of rofecoxib, celecoxib, and acetaminophen in osteoarthritis of the knee: a randomized trial. JAMA 2002 Jan 2; 287(1): 64–71
Schnitzer TJ, Weaver AL, Polis AB, et al. Efficacy of rofecoxib, celecoxib, and acetaminophen in patients with osteoarthritis of the knee: a combined analysis of the VACT studies. J Rheumatol 2005 Jun; 32(6): 1093–105
McKenna F, Weaver A, Fiechtner JJ, et al. COX-2 specific inhibitors in the management of osteoarthritis of the knee: a placebo-controlled, randomized, double-blind study. Journal of Clinical Rheumatology 2001 Jun; 7: 151–9
Gibofsky A, Williams GW, McKenna F, et al. Comparing the efficacy of cyclooxygenase 2-specific inhibitors in treating osteoarthritis: appropriate trial design considerations and results of a randomised, placebo-controlled trial. Arthritis Rheum 2003 Nov; 48(11): 3102–11
Birbara C, Ruoff G, Sheldon E, et al. Efficacy and safety of rofecoxib 12.5 mg and celecoxib 200 mg in two similarly designed osteoarthritis studies. Curr Med Res Opin 2006; 22(1): 199–210
Malmstrom K, Fricke JR, Kotey P, et al. A comparison of rofecoxib versus celecoxib in treating pain after dental surgery: a single-center, randomized, double-blind, placebo- and active-comparator-controlled, parallel-group, single-dose study using the dental impaction pain model. Clin Ther 2002 Oct; 24(10): 1549–60
Malmstrom K, Daniels S, Kotey P, et al. Comparison of rofecoxib and celecoxib, two cyclooxygenase-2 inhibitors, in postoperative dental pain: a randomised, placebo- and active-comparator-controlled clinical trial. Clin Ther 1999; 21(10): 1653–63
Merck and Co. Inc. Merck announces voluntary worldwide withdrawal of Vioxx® [online]. 2004 Sep 30
McKenna F, Borenstein D, Wendt H, et al. Celecoxib versus diclofenac in the management of osteoarthritis of the knee. Scand J Rheumatol 2001; 30(1): 11–8
Williams GW, Hubbard RC, Yu SS, et al. Comparison of once-daily and twice-daily administration of celecoxib for the treatment of osteoarthritis of the knee. Clin Ther 2001 Feb; 23(2): 213–27
Williams GW, Ettlinger RE, Ruderman EM, et al. Treatment of osteoarthritis with a once-daily dosing regimen of celecoxib: a randomized, controlled trial. Journal of Clinical Rheumatology 2000; 6(2): 65–74
Martin-Mola E, Munoz-Gomez J, Sanchez-Burson J, et al. Celecoxib is as effective as ibuprofen in treating pain associated with osteoarthritis of the knee [abstract no. SAT0273]. Ann Rheum Dis 2005 Jul; 64 (Suppl. III): 495–6
Rother M, Lavins BJ, Kneer W, et al. Efficacy and safety of epicutaneous ketoprofen in Transfersome® (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial. Ann Rheum Dis 2007 Sep; 66(9): 1178–83
Bensen WG, Fiechtner JJ, McMillen JI, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomised controlled trial. Mayo Clin Proc 1999; 74: 1095–105
Kivitz AJ, Moskowitz RW, Woods E, et al. Comparative efficacy and safety of celecoxib and naproxen in the treatment of osteoarthritis of the hip. J Int Med Res 2001 Nov; 29(6): 467–79
Stengaard-Pedersen K, Ekesbo R, Karvonen A-L, et al. Celecoxib 200mg qd is efficacious in the management of osteoarthritis of the knee or hip regardless of the time of dosing. Rheumatology (Oxford) 2004; 43: 592–5
Singh G, Fort JG, Goldstein JL, et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I Study. Am J Med 2006 Mar; 119(3): 255–66
Schoen E, Moriarty M, Pettitt D, et al. Comparison between celecoxib and traditional NSAIDs in patient satisfaction based on usual rheumatology clinical practice: the SUCCESS-IV study [abstract no. WED-G-561]. Gut 2002 Oct; 51 Suppl. 3: A315
Sheldon E, Beaulieu A, Paster Z, et al. Efficacy and tolerability of lumiracoxib in the treatment of osteoarthritis of the knee: a 13-week, randomized, double-blind comparison with celecoxib and placebo. Clin Ther 2005 Jan; 27(1): 64–77
Fleischmann R, Sheldon E, Maldonado-Cocco J, et al. Lumiracoxib is effective in the treatment of osteoarthritis of the knee: a prospective randomised 13-week study versus placebo and celecoxib. Clin Rheumatol 2005 Aug; 25: 42–53
Lehmann R, Brzosko M, Kopsa P, et al. Efficacy and tolerability of lumiracoxib 100mg once daily in knee osteoarthritis: a 13-week, randomised, double-bind study vs. placebo and celecoxib. Curr Med Res Opin 2005; 21(4): 517–26
Tannenbaum H, Berenbaum F, Reginster J-Y, et al. Lumiracoxib is effective in the treatment of osteoarthritis of the knee: a 13 week, randomised, double-blind study versus placebo and celecoxib. Ann Rheum Dis 2004; 63: 1419–26
Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006 Feb 23; 354(8): 795–808
Luyten FP, Geusens P, Malaise MG. A prospective randomised multicentre study comparing continuous versus intermittent treatment with celecoxib in osteoarthritis of the knee or hip. Ann Rheum Dis 2007 Jan; 66(1): 99–106
Bingham CO3rd, Sebba AI, Rubin BR, et al. Efficacy and safety of etoricoxib 30 mg and celecoxib 200 mg in the treatment of osteoarthritis in two identically designed, randomized, placebo-controlled, non-inferiority studies. Rheumatology (Oxford) 2007 Mar; 46(3): 496–507
Singh G, Goldstein J, Fort J, et al. SUCCESS-1 in osteoarthritis: celecoxib has similar efficacy to the conventional NSAIDs [abstract no. M13]. J Rheumatol 2001 Jul; 28 Suppl. 63: 6
Singh G, Fort JG, Triadafilotoulos G. SUCCESS-1: a global osteoarthritis trial in 13,274 randomized patients. Celecoxib provides similar efficacy to diclofenac and naproxen while providing significantly improved UGI safety. Arthritis Rheum 2001 Sep; 44 Suppl.: S135
Pincus T, Koch G, Lei H, et al. Patient preference for placebo, acetaminophen (paracetamol) or celecoxib efficacy studies: two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Ann Rheum Dis 2004; 63: 931–9
Zhao SZ, Yu SS, Dedhiya SD, et al. Celecoxib improves health-related quality of life in patients with osteoarthritis of the knee [abstract no. 118]. Pharmacotherapy 1999; 19(4): 495
Zhao SZ, Dedhiya SD, Verburg K, et al. Celecoxib 200mg administered once-a-day or in split doses has equal impact on health-related quality of life (HRQOL) of patients with osteoarthritis (OA) [abstract no. 1357]. Arthritis Rheumatism 1999 Sep; 42 Suppl.: S297
Lisse J, Espinoza L, Zhao SZ, et al. Functional status and health-related quality of life of elderly osteoarthritic patients treated with celecoxib. J Gerontol A Biol Sci Med Sci2001 Mar; 56(3): M167–175
Moskowitz RW, Sunshine A, Brugger A, et al. American pain society pain questionnaire and other pain measures in the assessment of osteoarthritis pain: a pooled analysis of three celecoxib pivotal studies. Am J Ther 2003 Jan; 10(1): 12–20
Zhao SZ, McMillen JI, Markenson JA, et al. Evaluation of the functional status aspects of health-related quality of life of patients with osteoarthritis treated with celecoxib. Pharmacotherapy 1999; 19(11): 1269–78
Tindall EA, Sharp JT, Burr A, et al. A 12-month, multicenter, prospective, open-label trial of radiographic analysis of disease progression in osteoarthritis of the knee or hip in patients receiving celecoxib. Clin Ther 2002 Dec; 24(12): 2051–63
Simon LS, Weaver AL, Graham DY, et al. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomised controlled trial. JAMA 1999; 282(20): 1921–8
Geis GS, Hubbard HC, Woods EM, et al. Efficacy and safety of celecoxib, a specific cyclooxygenase-2 (COX-2) inhibitor, in rheumatoid arthritis [abstract no. 854]. Ann Rheum Dis Abstracts 1999 Jun 6, 205
Emery P, Zeidler H, Kvien TK, et al. Celecoxib versus diclofenac in long-term management of rheumatoid arthritis: randomised double-blind comparison. Lancet 1999; 354: 2106–011
Shi W, Wang YM, Li LS, et al. Safety and efficacy of oral nonsteroidal anti-inflammatory drugs in patients with rheumatoid arthritis: a six-month randomised study. Clin Drug Invest 2004; 24(2): 89–101
Zhao SZ, Fiechtner JI, Tindall EA, et al. Evaluation of health-related quality of life of rheumatoid arthritis patients treated with celecoxib. Arthritis Care Res 2000; 13(2): 112–21
Woods EM, Callison DA, Hubbard RC, et al. Response to celecoxib treatment in subgroups of rheumatoid arthritis patients [abstract no. POS-397]. Ann Rheum Dis 2000 Jul; 59 Suppl. 1: 158–9
Dougados M, Behier JM, Jolchine I, et al. Efficacy of celecoxib, a cyclooxygenase 2-specific inhibitor, in the treatment of ankylosing spondylitis: a six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum 2001 Jan; 44(1): 180–5
Sieper J, Klopsch T, Richter M, et al. Comparison of 2 different dosages of celecoxib with diclofenac for the treatment if active ankylosing spondylitis: results of a 12-week randomised double-blind controlled study. Ann Rheum Dis 2007 Jul 6
Kvien TK, Bjorneboe O, Gran JT, et al. Celecoxib and diclofenac have comparable efficacy in ankylosing spondylitis (AS): results from a Norwegian multicenter, 12-week, double-blind, randomized trial [abstract no. SAT0266]. Ann Rheum Dis 2006; 65 (Suppl. 2): 531
Barkhuizen A, Steinfeld S, Robbins J, et al. Celecoxib is efficacious and well tolerated in treating signs and symptoms of ankylosing spondylitis. J Rheumatol 2006; 33: 1805–12
Pfizer Inc. A 12-week, randomized, double-blind, parallel-group study of 2 doses of celecoxib compared to diclofenac in patients with ankylosing spondylitis [online]. Available from URL: http://www.clinicalstudyresults.org [Accessed 2007 Sep 24]
Wanders A, van der Heijde D, Landewe R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomised clinical trial. Arthritis Rheum 3944; 52(6): 1756–65
Ekman EF, Wahba M, Ancona F. Analgesic efficacy of perioperative celecoxib in ambulatory arthroscopic knee surgery: a double-blind, placebo-controlled study. Arthroscopy 2006 Jun; 22(6): 635–42
Ekman EF, Berger M, Bhadra P. Letter to the editor. Arthroscopy 2006 Jul; 22(7): 804
Doyle G, Jayawardena S, Ashraf E, et al. Efficacy and tolerability of nonprescription ibuprofen versus celecoxib for dental pain. J Clin Pharmacol 2002 Aug; 42(8): 912–9
Krishnaswami S, Kowalski K, Cheung R. Analgesic efficacy of celecoxib 400mg in postsurgical dental pain [abstract no. A327]. Anesthesiology 2006; 105: A327
Gimbel JS, Brugger A, Zhao W, et al. Efficacy and tolerability of celecoxib versus hydrocodone/acetaminophen in the treatment of pain after ambulatory orthopedic surgery in adults. Clin Ther 2001 Feb; 23(2): 228–41
Reuben SS, Ekman EF. The effect of cyclooxygenase-2 inhibition on analgesia and spinal fusion. J Bone Joint Surg Am 2005 Mar; 87(3): 536–42
White PF, Sacan O, Tufanogullari B, et al. Effect of short-term postoperative celecoxib administration on patient outcome after outpatient laparoscopic surgery. Can J Anesth 2007; 54(5): 342–8
Reuben SS, Ekman EF. The effect of initiating a preventive multimodal analgesic regimen on long-term patient outcomes for outpatient anterior cruciate ligament reconstruction surgery. Anesth Analg 2007; 105: 228–32
Petri M, Hufman SL, Waser G, et al. Celecoxib effectively treats patients with acute shoulder tendinitis/bursitis. J Rheumatol 2004; 31(8): 1614–20
Petrella R, Ekman EF, Schuller R, et al. The efficacy of celecoxib, a COX-2 specific inhibitor, and naproxen in the management of acute ankle sprain: results of a double-blind, randomized controlled trial. Clin J Sports Med 2004; 14(4): 225–31
Bertin P, Behier J, Noel E, et al. Celecoxib is as efficacious as naproxen in the management of acute shoulder pain. J Int Med Res 2003; 31: 102–12
Ekman EF, Fiechtner JJ, Levy S, et al. Efficacy of celecoxib versus ibuprofen in the treatment of acute pain: a multicenter, double-blind, randomized controlled trial in acute ankle sprain. Am J Orthop 2002 Aug; 31(8): 445–51
Nadarajah A, Abrahan L, Lau FL, et al. Efficacy and tolerability of celecoxib compared with diclofenac slow release in the treatment of acute ankle sprain in an Asian population. Singapore Med J 2006 Jun; 47(6): 534–42
Yepes JP, Ekman E, Levy SD, et al. Efficacy of celecoxib versus diclofenac in the treatment of pain associated with acute ankle sprain: a multicenter, doubleblind, randomized, control trial [abstract no. 113]. J Clin Rheumatol 2002 Jun; 8 Suppl.: 77
Ralha L, Oliveira L, Chahade W, et al. Efficacy and tolerability of celecoxib 200mg bid versus diclofenac 75mg bid in acute low back pain [abstract no. P025]. APLAR Journal of Rheumatology 2006; 9 (Suppl. 1): A29–30
Moore RA, Derry S, Makinson GT, et al. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports. Arthritis Res Ther 2005; 7(3): R644–665
Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000 Sep 13; 284(10): 1247–55
Goldstein J, Bjarnason I, Spalding W, et al. Long-term NSAIDs but not COX-2 specific inhibitors are associated with anemia [abstract no. 16]. Gastroenterology 2004 Apr; 126 Suppl. 2: A–1
Wiholm BE. Identification of sulfonamide-like adverse drug reactions to celecoxib in the World Health Organization database. Curr Med Res Opin 2001; 17(3): 210–6
Shapiro LE, Knowles SR, Weber E, et al. Safety of celecoxib in individuals allergic to sulfonamide: a pilot study. Drug Saf 2003; 26(3): 187–95
Knowles S, Shapiro L, Shear NH. Should celecoxib be contrain-dicated in patients who are allergic to sulfonamides? Revisiting the meaning of ‘sulfa’ allergy. Drug Saf 2001; 24(4): 239–47
Woessner KM, Simon RA, Stevenson DD. The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum 2002 Aug; 46(8): 2201–6
Sanchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A, et al. Safety of etoricoxib, a new cyclooxygenase 2 inhibitor, in patients with nonsteroidal anti-inflammatory drug-induced urticaria and angioedema. Ann Allergy Asthma Immunol 2005; 95: 154–8
Quiralte J, Delgado J, S de San Pedro B, et al. Safety of the new selective cyclooxygenase type 2 inhibitors rofecoxib and celecoxib in patients with anaphylactoid reactions to nonsteroidal anti-inflammatory drugs. Ann Allergy Asthma Immunol 2004; 93: 360–4
Roll A, Wuthrich B, Schmid-Grendelmeier P, et al. Tolerance to celecoxib in patients with a history of adverse reactions to nonsteroidal anti-inflammatory drugs. Swiss Med Wkly 2006 Oct 28; 136(43–44): 684–90
Patterson R, Bello AE, Lefkowith J. Immunologic tolerability profile of celecoxib. Clin Ther 1999; 21: 2065–79
Singh G, Goldstein J, Bello AE, et al. The effect of low-dose aspirin therapy on the incidence of UGI symptoms among patients receiving celecoxib or conventional NSAIDS: analysis of the SUCCESS-1 and CLASS trials [abstract no. 67]. J Clin Rheum 2002 Jun; 8 Suppl.: S62
Goldstein JL, CLASS Investigators. Gastrointestinal event rates in the CLASS study: 6-month vs longer-term follow-up analyses [abstract no. T1504]. Gastroenterology 2002 Apr; 122 Suppl. 1: A469
Ashcroft DM, Chapman SR, Clark WK, et al. Upper gastroduodenal ulceration in arthritis patients treated with celecoxib. Ann Pharmacother 2001 Jul; 35(7–8): 829–34
Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 2002 Sep 21; 325(7365): 619–23
Lanas A, Schieman J. Low-dose aspirin and upper gastrointestinal damage: epidemiology, prevention and treatment. Curr Med Res Opin 2007 Jan; 23(1): 163–73
Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective COX-2 inhibitors, traditional non-aspirin NSAIDs, aspirin, and combinations. Gut 2006; 55(12): 1731–8
Silverstein F, Simon L, Faich G. Reporting of 6-month vs 12-month data in a clinical trial of celecoxib. Reply. JAMA 2001 Nov 21; 286: 2399–400
Chan FK, Wong VW, Suen BY, et al. Combination of a cyclooxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomised trial. Lancet 2007 May 12; 369(9573): 1621–6
Chan FK, Hung LC, Suen BY, et al. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med 2002 Dec 26; 347(26): 2104–10
Lai K-C, Chu K-M, Hui W-M, et al. Celecoxib compared with lansoprazole and naproxen to prevent gastrointestinal ulcer complications. Am J Med 2005; 118: 1271–8
Rahme E, Barkun AN, Toubouti Y, et al. Do proton-pump inhibitors confer additional gastrointestinal protection in patients given celecoxib? Arthritis Rheum 2007 May 25; 57(5): 748–55
Mamdani M, Rochon PA, Juurlink DN, et al. Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. BMJ 2002 Sep 21; 325(7365): 624
Goldstein JL, Cryer B, Amer F, et al. Celecoxib plus aspirin versus naproxen and lansoprazole plus aspirin: a randomized, double-blind, endoscopic trial. Clin Gastroenterol Hepatol 2007 Oct; 5(10): 1167–74
ADAPT Research Group. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer’s disease anti-inflammatory prevention trial (ADAPT). PLoS Clin Trials 2006 Nov 17; 1(7): e33
Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med 2006 Aug 31; 355(9): 873–84
Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 2006 Aug 31; 355(9): 885–95
Solomon SD, McMurray JJV, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 2005 Mar 17; 352(11): 1071–80
Solomon SD, Pfeffer MA, McMurray JJ, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation 2006 Sep 5; 114(10): 1028–35
White WB, Faich G, Whelton A, et al. Comparison of thromboembolic events in patients treated with celecoxib, a cyclooxygenase-2 specific inhibitor, versus ibuprofen or diclofenac. Am J Cardiol 2002 Feb 15; 89(4): 425–30
White WB, West CR, Borer JS, et al. Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol 2007 Jan 1; 99(1): 91–8
Pfizer Inc.. A double-blind, randomized, placebo-controlled, comparative study of celecoxib (SC-58635) for the inhibition of progression of Alzheimer’s disease [online]. Available from URL: http://www.clinicalstudyresults.org/documents/company-study_75_0.pdf [Accessed 2007 May 15]
Caldwell B, Aldington S, Weatherall M, et al. Risk of cardiovascular events and celecoxib: a systematic review and meta-analysis. J R Soc Med 2006 Mar; 99(3): 132–40
Brophy JM. Celecoxib and cardiovascular risks. Expert Opin Drug Saf 2005; 4(6): 1005–15
White WB, Faich G, Borer JS, et al. Cardiovascular thrombotic events in arthritis trials of the cyclooxygenase-2 inhibitor celecoxib. Am J Cardiol 2003 Aug 15; 92(4): 411–8
Whelton A, Maurath CJ, Verburg KM, et al. Renal safety and tolerability of celecoxib, a novel cyclooxygenase-2 inhibitor. Am J Ther 2000; 7: 159–75
Aw T-J, Haas SJ, Liew D, et al. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch Intern Med 2005; 165: 1–7
Whelton A, Fort JG, Puma JA. Cyclooxygenase-2-specific inhibitors and cardiorenal function: a randomized, controlled trial of celecoxib and rofecoxib in older hypertensive osteoarthritis patients. Am J Ther 2001 Mar; 8(2): 85–95
Hernandez-Diaz S, Varas-Lorenzo C, Garcia Rodriguez LA. Non-steroidal antiinflammatory drugs and the risk of acute myocardial infarction. Basic Clin Pharmacol Toxicol 2006 Mar; 98(3): 266–74
McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase. JAMA 2006 Oct 4; 296(13): 1633–44
Andersohn F, Suissa S, Garbe E. Use of first- and second-generation cyclooxygenase-2-selective nonsteroidal antiinflammatory drugs and risk of acute myocardial infarction. Circulation 2006 Apr 25; 113(16): 1950–7
Bak S, Andersen M, Tsiropoulos I, et al. Risk of stroke associated with nonsteroidal anti-inflammatory drugs: a nested case-control study. Stroke 2003 Feb; 34(2): 379–86
Fischer LM, Schlienger RG, Matter CM, et al. Current use of nonsteroidal antiinflammatory drugs and the risk of acute myocardial infarction. Pharmacotherapy 2005 Apr; 25(4): 503–10
Garcia Rodriguez LA, Varas-Lorenzo C, Maguire A, et al. Nonsteroidal antiinflammatory drugs and the risk of myocardial infarction in the general population. Circulation 2004 Jun 22; 109(24): 3000–6
Garcia Rodriguez LA, Varas C, Patrono C. Differential effects of aspirin and non-aspirin nonsteroidal antiinflammatory drugs in the primary prevention of myocardial infarction in postmenopausal women. Epidemiology 2000 Jul; 11(4): 382–7
Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase-2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet 2005 Feb 5; 365: 475–81
Helin-Salmivaara A, Virtanen A, Vesalainen R, et al. NSAID use and the risk of hospitalisation for first myocardial infarction in the general population: a nationwide case-control study from Finland. Eur Heart J 2006 Jul; 27(14): 1657–63
Hippisley-Cox J, Coupland C, Logan R, et al. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005 Dec 3; 331(7528): 1310–6
Johnsen SP, Larsson H, Tarone RE, et al. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Intern Med 2005 May 9; 165(9): 978–84
Kimmel SE, Berlin JA, Reilly M, et al. Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann Intern Med 2005; 142: 157–64
Kimmel SE, Berlin JA, Reilly M, et al. The effects of nonselective non-aspirin non-steroidal anti-inflammatory medications on the risk of nonfatal myocardial infarction and their interaction with aspirin. J Am Coll Cardiol 2004 Mar 17; 43(6): 985–90
Levesque LE, Brophy JM, Zhang B. The risk for myocardial infarction with cyclooxygenase-2 inhibitors: a population study of elderly adults. Ann Intern Med 2005; 142: 481–9
McGettigan P, Han P, Henry D. Cyclooxygenase-2 inhibitors and coronary occlusion — exploring dose-response relationships. Br J Clin Pharmacol 2006 Sep; 62(3): 358–65
Schlienger RG, Jick H, Meier CR. Use of nonsteroidal anti-inflammatory drugs and the risk of first-time acute myocardial infarction. Br J Clin Pharmacol 2002 Sep; 54(3): 327–32
Singh G, Mithal A, Triadafilopoulos G. Both selective COX-2 inhibitors and non-selective NSAIDs increase the risk of acute myocardial infarction in patients with arthritis: selectivity is with the patient not the drug class. Ann Rheum Dis 2005; 64 Suppl. III: 85
Solomon DH, Glynn RJ, Levin R, et al. Nonsteroidal anti-inflammatory drug use and acute myocardial infarction. Arch Intern Med 2002 May 27; 162(10): 1099–104
Solomon DH, Schneeweiss S, Glynn RJ, et al. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation 2004 May 4; 109(17): 2068–73
Sturkenboom MCJM, Dieleman JVK,. Cardiovascular events during use of COX-2 selective and non-selective NSAIDs. Pharmacoepidemiol Drug Saf2005; 14: S57
Watson DJ, Rhodes T, Cai B, et al. Lower risk of thromboembolic cardiovascular events with naproxen among patients with rheumatoid arthritis. Arch Intern Med 2002 May 27; 162(10): 1105–10
Curtis JP, Wang Y, Portnay EL, et al. Aspirin, ibuprofen, and mortality after myocardial infarction: retrospective cohort study. BMJ 2003 Dec 6; 327(7427): 1322–3
Gislason GH, Jacobsen S, Rasmussen JN, et al. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation 2006 Jun 27; 113(25): 2906–13
MacDonald TM, Wei L. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet 2003 Feb 15; 361(9357): 573–4
Mamdani M, Rochon P, Juurlink DN, et al. Effect of selective cyclooxygenase 2 inhibitors and naproxen on short-term risk of acute myocardial infarction in the elderly. Arch Intern Med 2003 Feb 24; 163(4): 481–6
Rahme E, Watson DJ, Kong SX, et al. Association between nonnaproxen NSAIDs, COX-2 inhibitors and hospitalization for acute myocardial infarction among the elderly: a retrospective cohort study. Pharmacoepidemiol Drug Saf 2007 May; 16(5): 493–503
Ray WA, Stein CM, Daugherty JR, et al. COX-2 selective nonsteroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet 2002 Oct 5; 360(9339): 1071–3
Ray WA, Stein CM, Hall K, et al. Non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease: an observational cohort study. Lancet 2002 Jan 12; 359(9301): 118–23
Spalding WM, Reeves MJ, Whelton A. Thromboembolic cardiovascular risk among arthritis patients using cyclooxygenase-2-selective inhibitor or nonselective cyclooxygenase inhibitor nonsteroidal anti-inflammatory drugs. Am J Ther 2007 Jan; 14(1): 3–12
Velentgas P, West W, Cannuscio CC, et al. Cardiovascular risk of selective cyclooxygenase-2 inhibitors and other non-aspirin non-steroidal anti-inflammatory medications. Pharmacoepidemiol Drug Saf 2006 Sep; 15(9): 641–52
Mamdami M, Juurlink DN, Lee DS, et al. Cyclo-oxygenase-2 inhibitors versus non-selective non-steroidal anti-inflammatory drugs and congestive heart failure outcomes in elderly patients: a population-based cohort study. Lancet 2004 May 29; 363(9423): 1751–6
Hudson M, Rahme E, Richard H, et al. Risk of congestive heart failure with nonsteroidal antiinflammatory drugs and selective cyclooxygenase 2 inhibitors: a class effect? Arthritis Rheum 2007 Mar 29; 57(3): 516–23
Solomon DH, Schneeweiss S, Levin R, et al. Relationship between COX-2 specific inhibitors and hypertension. Hypertension 2004 Aug; 44(2): 140–5
Wolfe F, Zhao S, Pettitt D. Blood pressure déstabilisation and edema among 8538 users of celecoxib, rofecoxib, and non-selective nonsteroidal antiinflammatory drugs (NSAID) and nonusers of NSAID receiving ordinary care. J Rheumatol 2004 Jun; 31(6): 1143–51
Wang J, Mullins CD, Mamdani M, et al. New diagnosis of hypertension among celecoxib and nonselective NSAID users: a population-based cohort study. Ann Pharmacother 2007 Jun; 41(6): 937–43
Bresalier RS, Sandier RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005 Mar 17; 352(11): 1092–102
Kurth T, Glynn RJ, Walker AM, et al. Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal antiinflammatory drugs. Circulation 2003 Sep 9; 108(10): 1191–5
Pfizer Inc. Celebrex® hepatic safety: US medical information letter [online]. 2007
Maddrey WC, Maurath CJ, Verburg KM, et al. The hepatic safety and tolerability of the novel cyclooxygenase (COX)-2 inhibitor celecoxib. Am J Ther 2000 May; 7(3): 153–8
U.S. FDA. Analysis and recommendations for agency action regarding nonsteroidal anti-inflammatory drugs and cardiovascular risk. J Pain Palliat Care Pharmacother 2005; 19(4): 83–97
Cheer S, Goa K. Parecoxib (parecoxib sodium). Drugs 2001; 61(8): 1133–41
Brooks PM. The burden of musculoskeletal disease — a global perspective. Clin Rheumatol 2006 Nov; 25(6): 778–81
Jordan KM, Arden NK, Doherty M, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committtee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003; 62: 1145–55
Doan T, Massarotti E. Rheumatoid arthritis; an overview of new and emerging therapies. J Clin Pharm 2005 Jul; 45: 751–62
Wolfe F, Michaud K, Burke TA, et al. Longer use of COX-2-specific inhibitors compared to nonspecific nonteroidal antiinflammatory drugs: a longitudinal study of 3639 patients in community practice. J Rheumatol 2004; 31: 355–8
Harley C, Wagner S. Persistence with COX-2 inhibitors in managed care: an analysis of claims data. Manag Care Interface 2003 Oct; 16(10): 38–45
Moride Y, Ducruet T, Rochon S, et al. Persistency of use of COX-2-specific inhibitors and non-specific non-steroidal anti-inflammatory drugs (NSAIDs) in Quebec. Rheumatology (Oxford) 2003 Nov; 42 (Suppl. 3): iii17–22
Zhao SZ, Wentworth C, Burke TA, et al. Drug switching patterns among patients with rheumatoid arthritis and osteoarthritis using COX-2 specific inhibitors and non-specific NSAIDs. Pharmacoepidemiol Drug Saf 2004 May; 13(5): 277–87
Laine L. Gastrointestinal bleeding with low-dose aspirin — what’s the risk? Aliment Pharmacol Ther 2006 Sep 15; 24(6): 897–908
Schnitzer TJ, Burmester GR, Mysler E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004 Aug 21; 364(9435): 665–74
Wright JM. The double-edged sword of COX-2 selective NSAIDs. CMAJ 2002 Nov 12; 167(10): 1131–7
Rahme E, Nedjar H. Risks and benefits of COX-2 inhibitors vs non-selective NSAIDs: does their cardiovascular risk exceed their gastrointestinal benefit? A retrospective cohort study. Rheumatology (Oxford) 2007 Mar; 46(3): 435–8
Schneeweiss S, Solomon DH, Wang PS, et al. Simultaneous assessment of short-term gastrointestinal benefits and cardiovascular risks of selective cyclooxygenase 2 inhibitors and nonselective nonsteroidal antiinflammatory drugs: An instrumental variable analysis. Arthritis Rheum 2006 Oct 30; 54(11): 3390–8
Moore RA, Derry S, McQuay HJ. Cyclo-oxygenase-2 selective inhibitors and nonsteroidal anti-inflammatory drugs: balancing gastrointestinal and cardiovascular risk. BMC Musculoskelet Disord 2007 Aug 3; 8: 73
Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR study group. N Engl J Med 2000 Nov 23; 343(21): 1520–8
Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 2005 Mar 17; 352(11): 1081–91
EMEA. European Medicines Agency concludes action on COX-2 inhibitors [online]. Available from URL: http://www.emea.eu.int/pdfs/human/press/pr/20776605en.pdf [Accessed 2007 Jun 29]
Furberg CD. Decisions by regulatory agencies: are they evidence-based? Trials 2007 Apr; 8: 13
EMEA. Public CHMP assessment report for medicinal products containing non-selective non steroidal anti-inflammatory drugs (NSAIDs) [online]. Available from URL: http://www.emea.europa.eu/pdfs/human/opiniongen/44213006en.pdf [Accessed 2007 Jun 29]
Kearney PM, Baigent C, Godwin J, et al. Do selective cyclooxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? meta-analysis of randomised trials. BMJ 2006 Jun 3; 332: 1302–8
Chen LC, Ashcroft DM. Risk of myocardial infarction associated with selective COX-2 inhibitors: meta-analysis of randomised controlled trials. Pharmacoepidemiol Drug Saf 2007 Jul; 16(7): 762–72
Sciulli MG, Capone ML, Tacconelli S, et al. The future of traditional nonsteroidal antiinflammatory drugs and cyclooxygenase-2 inhibitors in the treatment of inflammation and pain. Pharmacological Reports 2005; 57 Suppl.: 66–85
Zarraga IGE, Schwarz ER. Coxibs and heart disease: what have we learned and what else we need to know. J Am Coll Cardiol 2007; 49(1): 1–14
Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage 2007 Aug 24
Sibilia J, Dereay G, Montalescot G. What do we know about the cardiovascular toxicity of the NSAIDs? (in French]Presse Med 2006 Sep; 35 (9 Spec No. 1): 1S11–23
Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000 Sep; 43 (9): 1905–15
Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum 2002 Feb; 46 (2): 328–46
Zhang W, Doherty M, Arden N., et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committtee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2005; 64: 669–81
Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a task force of the EULAR Standing Committtee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2007; 66: 377–88
Combe B, Landewe R, Lukas C, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committtee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2007; 66: 34–45
Schnitzer TJ. Update on guidelines for the treatment of chronic musculoskeletal pain. Clin Rheumatol 2006; 25 Suppl. 1: S22–29
Maillard M, Burnier M. Comparative cardiovascular safety of traditional nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf 2006; 5(1): 83–94
Scheiman JM, Fendrick AM. Summing the risk of NSAID therapy [letter]. Lancet 2007 May 12; 369: 1580–1
Juulink D. Commentary. ACP J Club 2005 Jul; 143(1): 2–3
Chen JT, Pucino F, Resman-Targoff BH. Celecoxib versus a non-selective NSAID plus proton-pump inhibitor: what are the considerations? J Pain Palliat Care Pharmacother 2006; 20(4): 11–32
Wilcox CM, Allison J, Benzuly K, et al. Consensus development conference on the use of nonsteroidal anti-inflammatory agents, including cyclooxygenase-2 enzyme inhibitors and aspirin. Clin Gastroenterol Hepatol 2006; 4 (1082–89)
Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs. An update for clinicians: a scientific statement for the American Heart Association. Circulation 2007; 115: 1634–42
GI-Reasons — a trial of GI safety of celecoxib compared with non-selective nonsteroidal antiinflammatory drugs (NSAIDs) I [online]. Available from URL: http://clinicaltrials.gov/ct/show/NCT00373685 [Accessed 2007 Sep 24]
PRECISION: prospective randomized evaluation of celecoxib integrated safety vs ibuprofen or naproxen [online]. Available from URL: http://clinicaltrials.gov/ct/show/NCT00346216 [Accessed 2007 Jun 29]
The standard care versus celecoxib outcome trial [online]. Available from URL: http://www.clinicaltrials.gov/ct/show/NCT00447759 [Accessed 2007 Sep 24]
Study of celecoxib or diclofenac and omeprazole for gastrointestinal (GI) safety in high GI risk patients with arthritis [online]. Available from URL: http://www.clinicaltrials.gov/ct/show/NCT00141102 [Accessed 2007 Sep 24]
Cryer B. COX-2-specific inhibitor or proton pump inhibitor plus traditional NSAID: is either approach sufficient for patients at highest risk of NSAID-induced ulcers? Gastroenterology 2004; 127: 1256–62
Loyd M, Rublee DA, Jacobs P. An economic model of long-term use of celecoxib in patients with osteoarthritis (in ENG]. BMC Gastroenterol 2007 Jul 4; 7(1): 25
Australian Government Department of Health and Aging Therapeutic Goods Administration. Medicines regulator cancels registration of antiinflammatory drug, lumiracoxib (Prexige) [online]. Available from URL: http://www.tga.gov.au/media/2007/070811-lumiracoxib.htm [Accessed 2007 Oct 1]
Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol 2006; 33 Suppl. 78: 4–11
Zochling J, van der Heijde D, Burgos-Vargas R, et al. ASAS/ EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2006; 65: 442–52
Rawal N. Analgesia for day-case surgery. Br J Anaesth 2001; 87(1): 73–87
Reuben SS. Update on the role of nonsteroidal anti-inflammatory drugs and coxibs in the management of acute pain. Curr Opin Anaesthesiol 2007 Oct; 20(5): 440–50
Romsing J, Moiniche S. A systematic review of COX-2 inhibitors compared with traditional NSAIDs, or different COX-2 inhibitors for post-operative pain. Acta Anaesthesiol Scand 2004; 48: 525–46
Gajraj NM. COX-2 inhibitors celecoxib and parecoxib: valuable options for postoperative pain management. Curr Top Med Chem 2007; 7(3): 235–49
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: N.M. Davies, Faculty of Pharmacy, University of Sydney, Sydney, New South Wales, Australia; M. Dougados, Faculté de Médecine, Université Paris-Descartes, Paris, France; L. Graudins, School of Women and Children’s Health, SCH, Randwick, New South Wales, Australia; R.C. Harris, Department of Medicine, Vanderbilt University School of Medicine, Nashville, Tennessee, USA; S. Knowles, Clinical Pharmacology, Sunnybrook & Women’s Health Sciences Centre, Toronto, Canada; K.C. Lee, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China; F. McKenna, Rheumatic Diseases Unit, Trafford General Hospital, Manchester, England; W.B. White, Section of Hypertension and Vascular Diseases, University of Connecticut, Farmington, Connecticut, USA.
Data Selection
Sources: Medical literature published in any language since 2000 on celecoxib, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline and AdisBase search terms were ‘celecoxib’ or ‘SC-58635’. EMBASE search terms were ‘celecoxib’. Searches were last updated 24 Oct 2007.
Selection: Studies in patients with osteoarthritis, rheumatoid arthrtis, ankylosing spondylitis or acute pain who received celecoxib. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Celecoxib, cyclo-oxygenase-2 inhibitor, pharmacodynamics, pharmacokinetics, therapeutic use, osteoarthritis, rheumatoid arthritis, acute pain, gastrointestinal tolerability, safety.
Rights and permissions
About this article
Cite this article
Frampton, J.E., Keating, G.M. Celecoxib. Drugs 67, 2433–2474 (2007). https://doi.org/10.2165/00003495-200767160-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200767160-00008