Abstract
Objective
To assess the pharmacokinetics of etonogestrel and ethinylestradiol released from a novel combined contraceptive vaginal ring (NuvaRing®) releasing etonogestrel 120µg and ethinylestradiol 15µg per day and compare them with those of a combined oral contraceptive containing desogestrel 150µg/ethinylestradiol 30µg (DSG/EE COC).
Design and setting
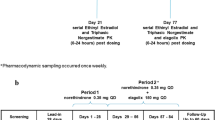
This was a nonblind, randomised, crossover study in 16 healthy women.
Methods
All volunteers received one cycle of DSG/EE COC before being randomised to 1 of 2 treatment groups. The participants in group 1 received 1 cycle of DSG/EE COC, a treatment period with NuvaRing® and an intravenous bolus injection of etonogestrel/ethinylestradiol (150µg/30µg). Those in group 2 received a NuvaRing® treatment period, 1 cycle of DSG/EE COC and the same intravenous bolus injection.
Results and conclusions
After the insertion of NuvaRing®, maximum serum concentrations of etonogestrel and ethinylestradiol were achieved in approximately 1 week. The concentrations subsequently showed a gradual linear decrease in time. The maximum serum concentrations of etonogestrel and ethinylestradiol were approximately 40 and 30%, respectively, of those for the DSG/EE COC. In comparison with the DSG/EE COC, the absolute bioavailability for NuvaRing® was higher for etonogestrel (102.9 vs 79.2%) and similar for ethinylestradiol (55.6 vs 53.8%). Taking the difference in daily doses into account, systemic exposure to etonogestrel was similar for NuvaRing® and the DSG/EE COC, whereas systemic exposure to ethinylestradiol with NuvaRing®was only approximately 50% of that for the DSG/EE COC.







Similar content being viewed by others
References
Mishell DR, Talas M, Parlow AF, et al. Contraception by means of a Silastic vaginal ring impregnated with medroxyprogesterone acetate. Am J Obstet Gynecol 1970; 107: 100–7.
Odlind V. New delivery systems for hormonal contraception. Acta Obstet Gynecol Scand Suppl 1986; 134: 15–20.
de Leede LGJ, Govers CPM, de Nijs H. A multicompartment vaginal ring system for independently adjustable release of contraceptive steroids. Contraception 1986; 34: 589–602.
Newton JR. Classification and comparison of oral contraceptives containing new generation progestogens. Hum Reprod Update 1995; 1: 231–63.
Hammond GL, Bocchinfuso WP, Orava M, et al. Serum distribution of two contraceptive progestins: 3-ketodesogestrel and gestodene. Contraception 1994; 50: 301–18.
Petak SM, Steinberger E. The adrenal gland. In: Goldzieher JW, editor. Pharmacology of the contraceptive steroids. New York: Raven Press, 1994: 233–42.
Acknowledgements
We are grateful to T. Haring, MD (Kendle Clinical Pharmacology Unit, Utrecht, The Netherlands) for her valuable contribution to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Timmer, C.J., Mulders, T.M.T. Pharmacokinetics of Etonogestrel and Ethinylestradiol Released from a Combined Contraceptive Vaginal Ring. Clin Pharmacokinet 39, 233–242 (2000). https://doi.org/10.2165/00003088-200039030-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200039030-00005