Abstract
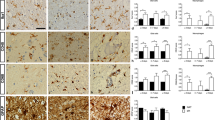
In multiple sclerosis (MS), disruption of the blood-brain barrier might lead to new gadolinium-enhanced lesion formation in the brain and cause acute relapses. Current therapeutic options for acute relapses in MS are limited. The effect of recombinant erythropoietin (rEPO) on cytokine gene expression in TNF-α-treated human brain microvascular endothelial cells was studied. The cells were controls (untreated), exposed for either 6 or 24 h to TNF-α or TNF-α/rEPO. Of the 96 genes studied, interleukin-6 (IL-6), IL-1β, CXCR4, and IL-1α genes were down-regulated when treated with TNF-α/rEPO for 6 h as compared with TNF-α alone. At 24 h, IL-6 and CXCR4 gene expression was 4.24 and 2.98, respectively. Quantitative RT-PCR analysis showed down-regulation by 3.86 and 1.9 for IL-6 and CXCR4 genes, respectively. Our findings suggest that further studies are warranted to evaluate the use of EPO in minimizing acute relapses in MS.
Similar content being viewed by others
References
Agnello D., Bigini P., Villa P., Mennini T., Cerami A., Brines M. L., and Ghezzi P. (2002) Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 952, 128–134.
Beumi M., Cavallaro E., Floccari F., et al. (2003) The pleiotropic effects of erythropoietin in the central nervous system. J. Neuropathol. Exp. Neurol. 62, 228–236.
Blann A. (2000) Endothelial cell activation, injury, damage and dysfunction: separate entities or mutual terms? Blood Coagul. Fibrinolysis 11, 623–630.
Brenneman D. E., Phillips T M., Hauser J., Hill J. M., Spong C. Y., and Gozes I. (2003) Complex array of cytokines released by vasoactive intestinal peptide. Neuropeptides 37, 111–119.
Brown K. A. (2001) Factors modifying the migration of lymphocytes across the blood-brain barrier. Int. Immunopharmacol. 1, 2043–2062.
Chong Z. Z., Kang J. Q., and Maiese K. (2003) Apaf-1, Bcl-xL, cytochrome c, and caspase-9 form the critical elements for cerebral vascular protection by erythropoietin. J. Cereb. Blood Flow Metab. 23, 320–330.
Fauci A. S. (1986) Host factors and the pathogenesis of HIV-induced disease. Nature 384, 529.
Gijbels K., Brocke S., Abrams J. S., and Steinman L. (1995) Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol. Med. 1, 795–805.
Griffin W. S., Sheng J. G., Roberts G. W., and Mrak R. E. (1995) Interleukin-1 expression in different plaque types in Alzheimer’s disease: significance in plaque evolution. J. Neuropathol. Exp. Neurol. 54, 276–281.
Kappos L., Moeri D., Radue E. W., et al. (1999) Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Gadolinium MRI Meta-analysis Group. Lancet 353, 964–969.
Lau L. T. and Yu A. C. H. (2001) Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J. Neurotrauma 18, 351–359.
Martinez-Caceres E. M., Espejo C., and Brieva L. (2002) Expression of chemokine receptors in the different clinical forms of multiple sclerosis. Mult. Scler. 8, 390–395.
Mattson M. P., Barger S. W., Furukawa K., Bruce A. J., Wyss-Coray T., Mark R. J., and Mucke L. (1997) Cellular signaling roles of TGF-α TNF, and αAPP in brain injury responses and Alzheimer’s disease. Brain Res. Rev. 23, 47–61.
Murdoch C., Monk P. N., and Finn A. (1999) CXC Chemokine receptor expression on human endothelial cells. Cytokine 11, 704–712.
Olsen N. V. (2003) Central nervous system frontiers for the use of erythropoietin. Clin. Infect. Dis. 37, S323–330.
Omari K. M. and Dorovini-Zis K. (2003) CD40 expressed by human brain endothelial cells regulates CD4+ T cell adhesion to endothelium. J. Neuroimmunol. 134, 166–178.
Otto V. I., Stahel P. F., Rancan M., Kariya K., Shohami E., Yatsiv I., et al. (2001) Regulation of chemokines and chemokine receptors after experimental closed head injury. Neuroreport 12, 2059–2064.
Petty M. A. and Lo E. H. (2002) Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog. Neurobiol. 68, 311–323.
Pober J. S. and Cotran R. S. (1991) Immunologic interactions of T lymphocytes with vascular endothelium. Adv. Immunol. 50, 261–302.
Proescholdt M. A., Jacobson S., Tresser N., et al. (2002) Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J. Neuropathol. Exp Neurol. 61, 914–925.
Silver N. C., Lai M., Symms M. R., et al. (1998) Serial magnetization transfer imaging to characterize the early evolution of new MS lesions. Neurology 51, 758–764.
Snider J. V., Wechser M. A., and Lossos I. S. (2001) Human disease characterization: real-time quantitative PCR analysis of gene expression. Drug Discov. Today 6, 1062–1067.
Trojano M., Manzari C., and Livrea P (1992) Blood-brain barrier changes in multiple sclerosis [Ital]. J. Neurol. Sci. 13, 55–64.
Wong D., Prameya R., and Dorovini-Zis K. (1999) In vitro adhesion and migration of T lymphocytes across monolayers of human brain microvessel endothelial cells: regulation by ICAM-1, VCAM-1, E-selectin and PECAM-1. J. Neuropathol. Exp. Neurol. 58, 138–152.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avasarala, J.R., Konduru, S.S. Recombinant erythropoietin down-regulates IL-6 and CXCR4 genes in TNF-α-treated primary cultures of human microvascular endothelial cells. J Mol Neurosci 25, 183–189 (2005). https://doi.org/10.1385/JMN:25:2:183
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:25:2:183