Abstract
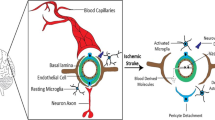
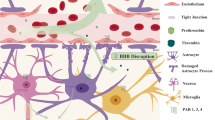
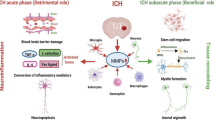
Proteases, such as tissue plasminogen activator, thrombin, metalloproteinases, and cathepsins, have complex functions in the mammalian brain under both normal and pathological conditions. Some of these proteases are expressed by neuronal cells, whereas others are made by the immunocompetent, macrophage-like cells of the brain: the microglia. This article reviews the physiological and pathological functions of these proteinases in the brain as well as recent findings linking extracellular proteases with neuronal cell death in ischemic or hemorrhagic stroke. Better understanding of protease expression and signaling, microglial activation, and their relationship with neuronal cell death during stroke injury could contribute to the development of relevant inhibitors as novel neuroprotective agents for treating ischemic stroke and intracerebral hemorrhage.
Similar content being viewed by others
References
Qureshi AI, Suri MF, Shatla AA, et al. Intraarterial recombinant tissue plasminogen activator for ischemic stroke:an accelerating dosing regimen. Neurosurgery 2000;47:473–476, discussion 477–479.
Lapchak P. Hemorrhagic transformation following ischemic stroke:significance, causes, and relationship to therapy and treatment. Curr Neurol Neurosci Rep 2002;2:38–43.
Giansily-Blaizot M, Aguilar-Martinez P, Briquel ME, et al. Two novel cases of cerebral haemorrhages at the neonatal period associated with inherited factor VII deficiency, one of them revealing a new nonsense mutation (Ser52Stop). Blood Coagul Fibrinolysis 2003;14:217–220.
Greenberg SM, Cho HS, O’Donnell HC, et al. Plasma beta-amyloid peptide, transforming growth factor-beta1, and risk for cerebral amyloid angiopathy. Ann NY Acad Sci 2000;903:144–149.
Jamshidi J, Izumoto S, Yoshimine T, Maruno M. Central neurocytoma presenting with intratumoral hemorrhage. Neurosurg Rev 2001;24:48–52.
Barnes B, Cawley CM, Barrow DL. Intracerebral hemorrhage secondary to vascular lesions. Neurosurg Clin N Am 2002;13:289–297.
Rincon F, Mayer SA. Novel therapies for intracerebral hemorrhage. Curr Op Crit Care 2004;10:94–100.
Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Ann Neurol 1986;19:105–111.
Schwartz M, Shaked I, Fisher J, Mizrahi T, Schori H. Protective autoimmunity against the enemy within:fighting glutamate toxicity. Trends Neurosci 2003;26:297–302.
Qureshi AI, Suarez JI, Yahia AM, et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage:an in vivo microdialysis study. Crit Care Med 2003;31:1482–1489.
Matsushita K, Meng W, Wang X, et al. Evidence for apoptosis after intercerebral hemorrhage in rat striatum. J Cereb Blood Flow Metab 2000;20:396–404.
Qureshi AI, Suri MF, Ostrow PT, et al. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery 2003;52:1041–1047, discussion 1047,1048.
Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J. Nuclear factor-kappa B and cell death after experimental intracerebral hemorrhage in rats. Stroke 1999;30:2472–2477, discussion 2477,2478.
Castillo J, Davalos A, Alvarez-Sabin J, et al. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology 2002;58:624–629.
Power C, Henry S, Del Bigio MR, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol 2003;53:731–742.
Xue M, Balasubramaniam J, Del Bigio M. Brain inflammation following intracerebral hemorrhage. Curr Neuropharmacol 2003;1:325–332.
Thiex R, Kuker W, Muller HD, et al. The long-term effect of recombinant tissue-plasminogen-activator (rt-PA) on edema formation in a large-animal model of intracerebral hemorrhage. Neurol Res 2003;25:254–262.
Thiex R, Mayfrank L, Rohde V, Gilsbach JM, Tsirka SA. The role of endogenous versus exogenous tPA on edema formation in murine ICH. Exp Neurol 2004;189:25–32.
Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996;19:312–318.
Koeppen AH, Dickson AC, McEvoy JA. The cellular reactions to experimental intracerebral hemorrhage. J Neurol0.0 Sci 1995;134(Suppl):102–112.
Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res 2000;871:57–65.
Turner CP, Panter SS, Sharp FR. Anti-oxidants prevent focal rat brain injury as assessed by induction of heat shock proteins (HSP70,HO-1/HSP32,HSP47) following subarachnoid injections of lysed blood. Brain Res Mol Brain Res 1999;65:87–102.
Mayne M, Ni W, Yan HJ, et al. Antisense oligodeoxynucleotide inhibition of tumor necrosis factor-alpha expression is neuroprotective after intracerebral hemorrhage. Stroke 2001;32:240–248.
Imamura N, Hida H, Aihara N, et al. Neurodegeneration of substantia nigra accompanied with macrophage/microglia infiltration after intrastriatal hemorrhage. Neurosci Res 2003;46:289–298.
Wang J, Rogove AD, Tsirka AE, Tsirka SE. Protective role of MIF in an animal model of intracerebral hemorrhage. Ann Neurol 2003;54:655–664.
Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J. Neurosci 1997;17:543–552.
Yepes M, Sandkvist M, Coleman TA, et al. Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent. J Clin Invest 2002;109:1571–1578.
Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin induced neuronal degeneration and seizure are mediated by tissue-type plasminogen activator. Nature 1995;377:340–344.
Mataga N, Nagai N, Hensch TK. Permissive proteolytic activity for visual cortical plasticity. Proc Natl Acad Sci 2002;99:7717–7721.
Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nature Neurosci 2003;6:168–174.
Tsirka SE, Bugge TH, Degen JL, Strickland S. Neuronal death in the CNS demonstrates a non-fibrin substrate for plasmin. Proc Natl Acad Sci USA 1997;94:9779–9781.
Wu YP, Siao CJ, Lu W, et al. The tPA/plasmin extracellular proteolytic system regulates seizure-induced hippocampal mossy fiber outgrowth through a proteoglycan substrate. J Cell Biol 2000;148:1295–1304.
Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nature Med 1998;4:228–232.
Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction:a gene targeting and gene transfer study in mice. Circulation 1999;99:2440–2444.
Sappino AP, Madani R, Huarte J, et al. Extracellular proteolysis in the adult murine brain. J Clin Invest 1993;92:679–685.
Seeds NW, Verrall S, Friedman G, et al. Plasminogen activators and their interaction with the extracellular matrix in neural development, plasticity and regeneration. Sem Neurosci 1996;8:405–412.
Seeds NW, Basham ME, Haffke SP. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proc Natl Acad Sci USA 1999;96:14,118–14,123.
Basham ME, Seeds NW. Plasminogen expression in the neonatal and adult mouse brain. J Neurochem 2001;77:318–325.
Rogove AD, Siao C, Keyt B, Strickland S, Tsirka SE. Activation of microglia reveals a non-proteolytic cytokine function for tissue plasminogen activator in the central nervous system. J Cell Sci 1999;112:4007–4016.
Siao CJ, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J Neurosci 2002;22:3352–3358.
Seeds NW, Basham ME, Ferguson JE. Absence of tissue plasminogen activator gene or activity impairs mouse cerebellar motor learning. J Neurosci 2003;23:7368–7375.
Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron 1998;21:813–825.
Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest 2003;112:1533–1540.
Meschia JF, Miller DA, Brott TG. Thrombolytic treatment of acute ischemic stroke. Mayo Clin Proc 2002;77:542–551.
Zivin JA. Thrombolytic stroke therapy:past, present, and future. Neurology 1999;53:14–19.
Tsirka SE. Clinical implications of the involvement of tPA in neuronal cell death. J Mol Med 1997;75:341–347.
Figueroa BE, Keep RF, Betz AL, Hoff JT. Plasminogen activators potentiate thrombin-induced brain injury. Stroke 1998;29:1202–1207.
Zhao BQ, Ikeda Y, Ihara H, et al. Essential role of endogenous tissue plasminogen activator through matrix metalloproteinase 9 induction and expression on heparin-produced cerebral hemorrhage after cerebral ischemia in mice. Blood 2004;103:2610–2616.
Liberatore GT, Samson A, Bladin C, Schleuning WD, Medcalf RL. Vampire bat salivary plasminogen activator (desmoteplase):a unique fibrinolytic enzyme that does not promote neurodegeneration. Stroke 2003;34:537–543.
Dihanich M, Kaser M, Reinhard E, Cunningham D, Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron 1991;6:575–581.
Weinstein JR, Gold SJ, Cunningham DD, Gall CM. Cellular localization of thrombin receptor mRNA in rat brain:expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. J Neurosci 1995;15:2906–2919.
Niclou S, Suidan HS, Brown-Luedi M, Monard D. Expression of the thrombin receptor mRNA in rat brain. Cell Mol Biol (Noisy-legrand) 1994;40:421–428.
Ryu J, Pyo H, Jou I, Joe E. Thrombin induces NO release from cultured rat microglia via protein kinase C, mitogen-activated protein kinase, and NF-kappa B. J Biol Chem 200;275:29,955–29,999.
Coughlin S. Thrombin signalling and protease-activated receptors. Nature 2000;407:258–264.
Reiter R, Derhaschnig U, Spiel A, et al. Regulation of protease-activated receptor 1 (PAR1) on platelets and responsiveness to thrombin receptor activating peptide (TRAP) during systemic inflammation in humans. Thromb Haemost 2003;90:898–903.
Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 2002;296:1880–1882.
Sorensen SD, Nicole O, Peavy RD, et al. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol 2003;64:1199–1209.
Ellis CA, Malik AB, Gilchrist A, et al. Thrombin induces proteinase-activated receptor-1 gene expression in endothelial cells via activation of Gi-linked Ras/mitogen-activated protein kinase pathway. J Biol Chem 1999;274:13,718–13,727.
Brandes RP, Viedt C, Nguyen K, et al. Thrombin-induced MCP-1 expression involves activation of the p22phox-containing NADPH oxidase in human vascular smooth muscle cells. Thromb Haemost 2001;85:1104–1110.
Hawkins RL, Seeds NW. Protease inhibitors influence the direction of neurite outgrowth. Dev Brain Res 1989;45:203–209.
Jalink K, Moolenaar WH. Thrombin receptor activation causes rapid neural cell rounding and neurite retraction independent of classic second messengers. J Cell Biol 1992;118:411–419.
Suidan HS, Stone SR, Hemmings BA, Monard D. Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron 1992;8:363–375.
de La Houssaye BA, Mikule K, Nikolic D, Pfenninger KH. Thrombin-induced growth cCone collapse: involvement of phospholipase A2 and eicosanoid Generation. J Neurosci 1999;19:10,843–10,855.
Gill JS, Pitts K, Rusnak FM, Owen WG, Windebank AJ. Thrombin induced inhibition of neurite outgrowth from dorsal root ganglion neurons. Brain Res 1998;797:321–327.
Festoff BW, Suo Z, Citron BA. Plasticity and stabilization of neuromuscular and CNS synapses:interactions between thrombin protease signaling pathways and tissue transglutaminase. Int Rev Cytol 2001;211:153–177.
Suo Z, Wu M, Ameenuddin S, et al. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J Neurochem 2002;80:655–666.
Suo Z, Wu M, Citron BA, Gao C, Festoff BW. Persistent protease-activated receptor 4 signaling mediates thrombin-induced microglial activation. J Biol Chem 2003;278:31,177–31,183.
Gingrich MB, Traynelis SF. Serine proteases and brain damage—is there a link? Trends Neurosci 2000;23:399–407.
Lee KR, Betz AL, Kim S, Keep RF, Hoff JT. The role of the coagulation cascade in brain edema formation after intracerebral hemorrhage. Acta Neurochir (Wien) 1996;138:396–400, discussion 400,401.
Nishino A, Suzuki M, Ohtani H, et al. Thrombin may contribute to the pathophysiology of central nervous system injury. J Neurotrauma 1993;10:167–179.
Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc Natl Acad Sci USA 2000;97:2264–2269.
Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J Neurosci 1997;17:5316–5326.
Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage:effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke 2001;32:2932–2938.
Smirnova IV, Zhang SX, Citron BA, Arnold PM, Festoff BW. Thrombin is an extracellular signal that activates intracellular death protease pathways inducing apoptosis in model motor neurons. J Neurobiol 1998;36:64–80.
Xi G, Wagner KR, Keep RF, et al. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke 1998;29:2580–2586.
Kitaoka T, Hua Y, Xi G, Hoff JT, Keep RF. Delayed argatroban treatment reduces edema in a rat model of intracerebral hemorrhage. Stroke 2002;33:3012–3018.
Hamada R, Matsuoka H. Antithrombin therapy for intracerebral hemorrhage. Stroke 2000;31:794–795.
LaMonte MP, Nash ML, Wang DZ, et al. Argatroban anti-coagulation in patients with acute ischemic stroke (ARGIS-1): a randomized, placebo-controlled safety study. Stroke 2004;35:1677–1682.
Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases:biologic activity and clinical implications. J Clin Oncol 2000;18:1135–1149.
Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 1996;98:2572–2579.
Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci 2001;2:502–511.
Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 2002;115:3719–3727.
Watanabe N, Ikeda U. Matrix metalloproteinases and atherosclerosis. Curr Atheroscler Rep 2004;6:112–120.
Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia 2002;39:279–291.
Rosenberg GA, Cunningham LA, Wallace J, et al. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain:activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res 2001;893:104–112.
Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats:inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 1998;29:1020–1030.
McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol 2001;13:534–540.
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2: 161–174.
Rosenberg GA. Matrix metalloproteinases in brain injury. J Neurotrauma 1995;12:833–842.
Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol 1998;274:R1203–1211.
Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation:role for plasminogen activators and matrix metalloproteinases. J Neurosci Res 2002;69:1–9.
Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol 2003;28:229–244.
Rosenberg GA, Dencoff JE, McGuire PG, Liotta LA, Stetler-Stevenson WG. Injury-induced 92-kilodalton gelatinase and urokinase expression in rat brain. Lab Invest 1994;71:417–422.
Rosenberg GA, Navratil M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology 1997;48:921–926.
Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke 2000;31:3034–3040.
Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke 2003;34:2025–2030.
Lee JM, Yin KJ, Hsin I, et al. Matrix metalloproteinase-9 and spontaneous hemorrhage in an animal model of cerebral amyloid angiopathy. Ann Neurol 2003;54:379–382.
Jung SS, Zhang W, Van Nostrand WE. Pathogenic A beta induces the expression and activation of matrix metalloproteinase-2 in human cerebrovascular smooth muscle cells. J Neurochem 2003;85:1208–1215.
Petanceska S, Canoll P, Devi LA. Expression of rat cathepsin S in phagocytic cells. J Biol Chem 1996;271:4403–4409.
Ryan RE, Sloane BF, Sameni M, Wood PL. Microglial cathepsin B:an immunological examination of cellular and secreted species. J Neurochem 1995;65:1035–1045.
Sastradipura DF, Nakanishi H, Tsukuba T, et al. Identification of cellular compartments involved in processing of cathepsin E in primary cultures of rat microglia. J Neurochem 1998;70:2045–2056.
Gresser O, Weber E, Hellwig A, Riese S, Regnier-Vigouroux A. Immunocompetent astrocytes and microglia display major differences in the processing of the invariant chain and in the expression of active cathepsin L and cathepsin S. Eur J Immunol 2001;31:1813–1824.
Santambrogio L, Belyanskaya SL, Fischer FR, et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA 2001;98:6295–6300.
Nishioku T, Hashimoto K, Yamashita K, et al. Involvement of cathepsin E in exogenous antigen processing in primary cultured murine microglia. J Biol Chem 2002;277:4816–4822.
Kingham PJ, Pocock JM. Microglial secreted cathepsin B induces neuronal apoptosis. J Neurochem 2001;76:1475–1484.
Gan L, Ye S, Chu A, et al. Identification of cathepsin B as a mediator of neuronal death induced by Abeta-activated microglial cells using a functional genomics approach. J Biol Chem 2004;279:5565–5572.
Schotte P, Van Criekinge W, Van de Craen M, et al. Cathepsin B-mediated activation of the proinflammatory caspase-11. Biochem Biophys Res Commun 1998;51:379–387.
Vancompernolle K, Van Herreweghe F, Pynaert G, et al. Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett 1998;38:150–158.
Stoka V, Turk B, Schendel SL, et al. Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J Biol Chem 2001;276:3149–3157.
Guicciardi ME, Deussing J, Miyoshi H, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest 2000;106:1127–1137.
Bednarski E, Ribak CE, Lynch G. Suppression of cathepsins B and L causes a proliferation of lysosomes and the formation of meganeurites in hippocampus. J Neurosci 1997;17:4006–4021.
Chen M, Conn KJ, Festoff BW. A receptor for cathepsin G:alpha 1-antichymotrypsin complexes on mouse spinal cord astrocytes. Neurology 1993;43:1223–1227.
Kingham PJ, Pocock JM. Microglial apoptosis induced by chromogranin A is mediated by mitochondrial depolarisation and the permeability transition but not by cytochrome c release. J Neurochem 2000;74:1452–1462.
Kohda Y, Yamashima T, Sakuda K, et al. Dynamic changes of cathepsins B and L expression in the monkey hippocampus after transient ischemia. Biochem Biophys Res Commun 1996;228:616–622.
Gray J, Haran MM, Schneider K, et al. Evidence that inhibition of cathepsin-B contributes to the neuroprotective properties of caspase inhibitor Tyr-Val-Ala-Asp-chloromethyl ketone. J Biol Chem 2001;276:32,750–32,755.
Seyfried D, Han Y, Zheng Z, et al. Cathepsin B and middle cerebral artery occlusion in the rat. Neurosurg 1997;87:716–723.
Yoshida M, Yamashima T, Zhao L, et al. Primate neurons show different vulnerability to transient ischemia and response to cathepsin inhibition. Acta Neuropathol (Berl) 2002;104:267–272.
Zujovic V, Taupin V. Use of cocultured cell systems to elucidate chemokine-dependent neuronal/microglial interactions:control of microglial activation. Methods 2003;29:345–350.
Pearson HE, Payne BR, Cunningham TJ. Microglial invasion and activation in response to naturally occurring neuronal degeneration in the ganglion cell layer of the postnatal cat retina. Brain Res Dev Brain Res 1993;76:249–255.
Rodriguez RW, Gomide VC, Chadi G. Astroglial and microglial activation in the wistar rat ventral tegmental area after a single striatal injection of 6-hydroxydopamine. Int J Neurosci 2004;114:197–216.
Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci 1993;13:455–466.
Flavin MP, Coughlin K, Ho LT. Soluble macrophage factors trigger apoptosis in cultured hippocampal neurons. Neuroscience 1997;80:437–448.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Tsirka, S.E. Contribution of extracellular proteolysis and microglia to intracerebral hemorrhage. Neurocrit Care 3, 77–85 (2005). https://doi.org/10.1385/NCC:3:1:077
Issue Date:
DOI: https://doi.org/10.1385/NCC:3:1:077