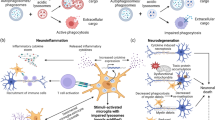
Abstract
In the central nervous system (CNS), apoptosis plays an important role during development and is a primary pathogenic mechanism in several adult neurodegenerative diseases. A main feature of apoptotic cell death is the efficient and fast removal of dying cells by macrophages and nonprofessional phagocytes, without eliciting inflammation in the surrounding tissue. Apoptotic cells undergo several membrane changes, including the externalization of so-called “eat me” signals whose cognate receptors are present on professional phagocytes. Among these signals, the aminophospholipid phosphatidylserine (PS) appears to have a crucial and unique role in preventing the classical pro-inflammatory activation of macrophages, thus ensuring the silent and safe removal of apoptotic cells. Although extensively studied in the peripheral organs, the process of recognition and removal of apoptotic cells in the brain has only recently begun to be unraveled. Here, we summarize the evidence suggesting that upon interaction with PS-expressing apoptotic neurons, microglia may no longer promote the inflammatory cascade, but rather facilitate the elimination of damaged neurons through antiinflammatory and neuroprotective functions. We propose that the anti-inflammatory microglial phenotype induced through the activation of the specific PS receptor (PtdSerR), expressed by resting and activated microglial cells, could be relevant to the final outcome of neurodegenerative diseases, in which apoptosis seems to play a crucial role.
Similar content being viewed by others
References
Perry V. H., Andersson P. B., and Gordon S. (1993) Macrophages and inflammation in the central nervous system. Trends Neurosci. 16, 268–273.
Perry V. H., Bolton S. J., Anthony D. C., and Betmouni S. (1998) The contribution of inflammation to acute and chronic neurodegeneration. Res. Immunol. 149, 721–725.
Kreutzberg G. W. (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318.
Streit W. J. (2002) Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40, 133–139.
Minghetti L. and Levi G. (1998) Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog. Neurobiol. 54, 99–125.
Zhang J. and Rivest S. (2001) Anti-inflammatory effects of prostaglandin E2 in the central nervous system in response to brain injury and circulating lipopolysaccharide. J. Neurochem. 76, 855–864.
Mirza B., Hadberg H., Thomsen P., and Moos T. (2000) The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience 95, 425–432.
Perry V. H., Cunningham C., and Boche D. (2002) Atypical inflammation in the central nervous system in prion disease. Curr. Opin. Neurol. 15, 349–354.
Minghetti L., Greco A., Cardone F., et al. (2000) Increased brain synthesis of prostaglandin E2 and F2-isoprostane in human and experimental transmissible spongiform encephalopathies. J. Neuropathol. Exp. Neurol. 59, 866–871.
Minghetti L., Cardone F., Greco A., et al. (2002) Increased CSF levels of prostaglandin E(2) in variant Creutzfeldt-Jakob disease. Neurology 58, 127–129.
Bellamy C. O., Malcomson R. D., Harrison D. J., and Wyllie A. H. (1995) Cell death in health and disease: the biology and regulation of apoptosis. Semin. Cancer Biol. 6, 3–16.
Savill J., Dransfield I., Gregory C., and Haslett C. (2002) A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2, 965–975.
Peiser L. and Gordon S. (2001) The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect. 3, 149–159.
Sambrano G. R. and Steinberg D. (1995) Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine. Proc. Natl. Acad. Sci. USA 92, 1396–1400.
Gershov D., Kim S., Brot N., and Elkon K. B. (2000) C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J. Exp. Med. 192, 1353–1364.
Nauta A. J., Daha M. R., Kooten C., and Roos A. (2003) Recognition and clearance of apoptotic cells: a role for complement and pentraxins. Trends Immunol. 24, 148–154.
Gregory C. D. (2000) CD14-dependent clearance of apoptotic cells: relevance to the immune system. Curr. Opin. Immunol. 12, 27–34.
Savill J., Fadok V., Henson P., and Haslett C. (1993) Phagocyte recognition of cells undergoing apoptosis. Immunol. Today 14, 131–136.
Henson P. M., Bratton D. L., and Fadok V. A. (2001) Apoptotic cell removal. Curr. Biol. 11, R795–805.
Mozzi R., Buratta S., and Goracci G. (2003) Metabolism and functions of phosphatidylserine in mammalian brain. Neurochem. Res. 28, 195–214.
Schlegel R. A. and Williamson P. (2001) Phosphatidylserine, a death knell. Cell Death Differ. 8, 551–563.
Fadok V. A., Xue D., and Henson P. (2001) If phosphatidylserine is the death knell, a new phosphatidylserine-specific receptor is the bell ringer. Cell Death Differ. 8, 582–587.
Hoffmann P. R., de Cathelineau A. M., Ogden C. A., et al. (2001) Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell. Biol. 155, 649–659.
Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., and Henson P. M. (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokines production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 4, 890–898.
Yuan J. and Yankner B. A. (2000) Apoptosis in the nervous system. Nature 407, 802–809.
Gray F., Chretien F., Adle-Biassette H., et al. (1999) Neuronal apoptosis in Creutzfeldt-Jakob disease. J. Neuropathol. Exp. Neurol. 58, 321–328.
Friedlander R. M. (2003) Apoptosis and caspases in neurodegenerative diseases. N. Engl. J. Med. 348, 1365–1375.
Smith M. E. (2001) Phagocytic properties of microglia in vitro: implications for a role in multiple sclerosis and EAE. Microsc. Res. Tech. 54, 81–94.
Webster S. D., Park, M., Fonseca M. I., and Tenner A. J. (2000) Structural and functional evidence for microglial expression of C1qR(P), the C1q receptor that enhances phagocytosis. J. Leukoc. Biol. 67, 109–116.
van Beek J., Elward K., Gasque P. (2003) Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann. NY Acad. Sci. 992, 56–71.
Bamberger M. E., and Landreth G. E. (2001) Microglial interaction with beta-amyloid: implications for the pathogenesis of Alzheimer’s disease. Microsc. Res. Tech. 15, 59–70.
Husemann J., Loike J. D., Anankov R., Febbraio M., and Silverstein S. C. (2002) Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia 40, 195–205.
Witting A., Muller P., Herrmann A., Kettenmann H., and Nolte C. (2000) Phagocytic clearance of apoptotic neurons by Microglia/Brain macrophages in vitro: involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J. Neurochem. 75, 1060–1070.
Marzolo M. P., von Bernhardi R., and Inestrosa N. C. (1999) Mannose receptor is present in a functional state in rat microglial cells. J. Neurosci. Res. 58, 387–395.
Linehan S. A., Martinez-Pomares L., Stahl P. D., and Gordon S. (1999) Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: In situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J. Exp. Med. 189, 1961–1972.
Zimmer H., Riese S., and Regnier-Vigouroux A. (2003) Functional characterization of mannose receptor expressed by immunocompetent mouse microglia. Glia 42, 89–100.
Walton M., Sirimanne E., Reutelingsperger C., Williams C., Gluckman P., and Dragunow M. (1997) Annexin V labels apoptotic neurons following hypoxia-ischemia. Neuroreport 8, 3871–3875.
Rimon G., Bazenet C. E., Philpott K. L., and Rubin L. L. (1997) Increased surface phosphatidylserine is an early marker of neuronal apoptosis. J. Neurosci. Res. 48, 563–570.
Das P., Estephan R., and Banerjee P. (2003) Apoptosis is associated with an inhibition of aminophospholipid translocase (APTL) in CNS-derived HN2–5 and HOG cells and phosphatidylserine is a recognition molecule in microglial uptake of the apoptotic HN2–5 cells. Life Science 72, 2617–2627.
Maiese K. and Vincent A. M. (2000) Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J. Neurosci. Res. 59, 568–580.
Adayev T., Estephan R., Meserole S., Mazza B., Yurkow E. J., and Banerjee P. (1998) Externalization of phosphatidylserine may not be an early signal of apoptosis in neuronal cells, but only the phosphatidylserine-displaying apoptotic cells are phagocytosed by microglia. J. Neurochem. 71, 1854–1864.
Hisatomi T., Sakamoto T., Sonoda K. H., et al. (2003) Clearance of apoptotic photoreceptors: elimination of apoptotic debris into the subretinal space and macrophage-mediated phagocytosis via phosphatidylserine receptor and integrin alphavbeta3. Am. J. Pathol. 162, 1869–1879.
Chang G. H., Barbaro N. M., and Pieper R. O. (2000) Phosphatidylserine-dependent phagocytosis of apoptotic glioma cells by normal human microglia, astrocytes, and glioma cells. Neuro. Oncol. 2, 174–183.
De Simone R., Ajmone-Cat M. A., Nicolini A., and Minghetti L. (2002) Expression of phosphatidylserine receptor and down-regulation of pro-inflammatory molecule production by its natural ligand in rat microglial cultures. J. Neuropathol. Exp. Neurol. 61, 237–244.
Fadok V. A., Bratton D. L., Rose D. M., Pearson A., Ezekewitz R. A., and Henson P. M. (2000) A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405, 85–90.
Chan A., Seguin R., Magnus T., Papadimitriou C., Toyka K. V., Antel J. P., and Gold R. (2003) Phagocytosis of apoptotic inflammatory cells by microglia and its therapeutic implications: Termination of CNS autoimmune inflammation and modulation by interferon-beta. Glia 43, 231–242.
De Simone R., Ajmone-Cat M. A., Tirassa P., and Minghetti L. (2003) Apoptotic PC12 cells exposing phosphatidylserine promote the production of anti-inflammatory and neuroprotective molecules by microglial cells. J. Neuropathol. Exp. Neurol. 62, 208–216.
Schutte B., Nuydens R., Geerts H., and Ramaekers F. (1998) Annexin V binding assay as a tool to measure apoptosis in differentiated neuronal cells. J. Neurosci. Methods 86, 63–69.
Levi G., Minghetti L., and Aloisi F. (1998) Regulation of prostanoid synthesis in microglial cells and effects of prostaglandin E2 on microglial functions. Biochimie 80, 899–904.
Akaike A., Kaneko S., Tamura Y., Nakata N., Shiomi H., Ushikubi F., and Narumiya S. (1994) Prostaglandin E2 protects cultured cortical neurons against N-methyl-d-aspartate receptor-mediated glutamate cytotoxicity. Brain Res. 14, 237–243.
Kim E. J., Kwon K. J., Park J. Y., Lee S. H., Moon C. H., and Baik E. J. (2002) Neuroprotective effects of prostaglandin E2 or cAMP against microglial and neuronal free radical mediated toxicity associated with inflammation. J. Neurosci. Res. 70, 97–107.
Thery C., Dobbertin A., and Mallat M. (1994) Downregulation of in vitro neurotoxicity of brain macrophages by prostaglandin E2 and a beta-adrenergic agonist. Glia 11, 383–386.
Bezzi P., Carmignoto G., Pasti L., et al. (1998) Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 15, 281–285.
Fiebich B. L., Schleicher S., Spleiss O., Czygan M., and Hull M. (2001) Mechanisms of prostaglandin E2-induced interleukin-6 release in astrocytes: possible involvement of EP4-like receptors, p38 mitogen-activated protein kinase and protein kinase C. J. Neurochem. 5, 950–958.
Takadera T., Yumoto H., Tozuka Y., and Ohyashiki T. (2002) Prostaglandin E(2) induces caspase-dependent apoptosis in rat cortical cells. Neurosci. Lett. 317, 61–64.
Khoury S. J., Hancock W. W., and Weiner H. L. (1992) Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E. J. Exp. Med. 176, 1355–1364.
Walsh D. T., Perry V. H., and Minghetti L. (2000) Cyclooxygenase-2 is highly expressed in microglial-like cells in a murine model of prion disease. Glia 29, 392–396.
Roberts A. B. and Sporn M. B. (1993) Physiological actions and clinical applications of transforming growth factor-beta (TGF-β). Growth Factors. 8, 1–9.
Unsicker K. and Strelau J. (2000) Functions of transforming growth factor-beta isoforms in the nervous system. Cues based on localization and experimental in vitro and in vivo evidence. Eur. J. Biochem. 267, 6972–6975.
Krupinski J., Kumar P., Kumar S., and Kaluza J. (1996) Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke 27, 852–857.
Mattson M. P., Barger S. W., Furukawa K., et al. (1997) Cellular signaling roles of TGF-β, TNF-α and β APP in brain injury responses and Alzheimer’s disease. Brain Res. Rev. 23, 47–61.
Baker C. A., Lu Z. Y., Zaitsev I., and Manuelidis L. (1999) Microglial activation varies in different models of Creutzfeldt-Jakob disease. J. Virol. 73, 5089–5097.
Cunningham C., Boche D., and Perry V. H. (2002) Transforming growth factor-β1, the dominant cytokine in murine prion disease: influence on inflammatory cytokine synthesis and alteration of vascular extracellular matrix. Neuropathol. Appl. Neurobiol. 28, 107–119.
Elkabes S., Di Cicco-Bloom E. M., and Black I. B. (1996) Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J. Neurosci. 16, 2508–2521.
Sofroniew M. V., Howe C. L., and Mobley W. C. (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 24, 1217–1281.
Lindholm D., Heumann R., Meyer M., and Thoenen H. (1987) Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 330, 658–659.
Crutcher K. A., Scott S. A., Liang S., Everson W. V., and Weingartner J. (1993) Detection of NGF-like activity in human brain tissue: increased levels in Alzheimer’s disease. J. Neurosci. 13, 2540–2550.
Polazzi E., Gianni T., and Contestabile A. (2001) Microglial cells protect cerebellar granule neurons from apoptosis: evidence for reciprocal signaling. Glia 36, 271–280.
Bruce-Keller A. J. (1999) Microglial-neuronal interactions in synaptic damage and recovery. J. Neurosci. Res. 58, 191–201.
Chamak B. and Mallat M. (1991) Fibronectin and laminin regulate the in vitro differentiation of microglial cells. Neuroscience 4, 513–527.
Reyes-Reyes M., Mora N., Gonzalez G., and Rosales C. (2002) beta1 and beta2 integrins activate different signalling pathways in monocytes. Biochem. J. 363, 273–280.
Moore K. J., El Khoury J., Medeiros L. A., Terada K., Geula C., Luster A. D., and Freeman M. W. (2002) A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J. Biol. Chem. 277, 47,373–47,379.
Leverrier Y. and Ridley A. J. (2001) Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr. Biol. 11, 195–199.
Caron E. and Hall A. (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282, 1717–1721.
Todt J. C., Hu B., Punturieri A., Sonstein J., Polak T., and Curtis J. L. (2002) Activation of protein kinase C βII by the stereo-specific phosphatidylserine receptor is required for phagocytosis of apoptotic thymocytes by resident murine tissue macrophages. J. Biol. Chem. 277, 35,906–35,914.
Ajmone-Cat M. A., De Simone R., Nicolini A., and Minghetti L. (2003) Effects of phosphatidylserine on p38 mitogen activated protein kinase, cyclic AMP responding element binding protein and nuclear factor-kappaB activation in resting and activated microglial cells. J. Neurochem. 84, 413–416.
Pahl H. L. (1999) Activators and target genes of Rel/NF-κB transcription factors. Oncogene 18, 6853–6866.
O’Neill L. A. and Kaltschmidt C. (1997) NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 20, 252–258.
McDonald P. P., Fadok V. A., Bratton D., and Henson P. M. (1999) Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-β in macrophages that have ingested apoptotic cells. J. Immunol. 163, 6164–6172.
Hu B., Punturieri A., Todt J., Sonstein J., Polak T., and Curtis J. L. (2002) Recognition and phagocytosis of apoptotic T cells by resident murine tissue macrophages require multiple signal transduction events. J. Leukoc. Biol. 71, 881–889.
Mayr B. and Montminy M. (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell. Biol. 2, 599–609.
Koistinaho M. and Koistinaho J. (2002) Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia 40, 175–183.
Ono K. and Han J. (2000) The p38 signal transduction pathway: activation and function. Cell Signal. 12, 1–13.
Nagata K., Ohashi K., Nakano T., Arita H., Zong C, Hanafusa H., and Mizuno K. (1996) Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J. Biol. Chem. 271, 30,022–30,027.
Nakano T., Ishimoto Y., Kishino J., Umeda M., Inoue K., Nagata K., et al. (1997) Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J. Biol. Chem. 272, 29,411–29,414.
Scott R. S., McMahon E. J., Pop S. M., et al. (2001) Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411, 207–211.
Camenisch T. D., Koller B. H., Earp H. S., and Matsushima G. K. (1999) A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J. Immunol. 162, 3498–3503.
Azuma Y., Inami Y., and Matsumoto K. (2002) Alterations in cell surface phosphatidylserine and sugar chains during apoptosis and their time-dependent role in phagocytosis by macrophages. Biol. Pharm. Bull. 25, 1277–1281.
Kagan V. E., Borisenko G. G., Serinkan B. F., et al. (2003) Appetizing rancidity of apoptotic cells for macrophages: oxidation, externalization, and recognition of phosphatidylserine. Am. J. Physiol. Lung. Cell. Mol. Physiol. 285, L1–17.
Kagan V. E., Gleiss B., Tyurina Y. Y., et al. (2002) A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J. Immunol. 169, 487–499.
Vincent A. M. and Maiese K. (1999) Direct temporal analysis of apoptosis induction in living adherent neurons. J. Histochem. Cytochem. 47, 661–672.
Polazzi E. and Contestabile A. (2002) Reciprocal interactions between microglia and neurons: from survival to neuropathology. Rev. Neurosci. 13, 221–242.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Simone, R., Ajmone-Cat, M.A. & Minghetti, L. Atypical antiinflammatory activation of microglia induced by apoptotic neurons. Mol Neurobiol 29, 197–212 (2004). https://doi.org/10.1385/MN:29:2:197
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/MN:29:2:197