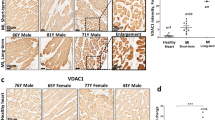
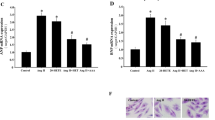
Abstract
The roles of aldosterone in the progression of heart failure have not been fully elucidated. This study examined whether aldosterone nongenomically activates reactive oxygen species (ROS) production, causing myocyte apoptosis. Addition of aldosterone to neonatal rat cardiac myocytes caused the activation of NADPH oxidase and intracellular ROS production in a dose-dependent manner (10−9−10−7 mol/L). NADPH oxidase activation was evident as soon as 5 min after aldosterone treatment. Neither an inhibitor for nuclear transcription (actinomycin D) nor an inhibitor of new protein synthesis (cycloheximide) blocked this rapid activation, and specific binding of aldosterone to plasma membrane fraction was inhibited by eplerenone, suggesting a nongenomic mechanism. Aldosterone did not affect the mRNA or protein levels of NOX2, which is a major subunit of NADPH oxidase in myocytes, after 48 h. Nuclear staining with DAPI showed that aldosterone (10−7 mol/L) increased the myocyte apoptosis (2.3 fold, p<0.001), coincident with the activation of caspase-3 (1.4 fold, p<0.05), compared with the serum-deprived control after 48 h. Aldosterone also induced phosphorylation of apoptosis signal–regulating kinase 1 (ASK1). These effects of aldosterone on myocyte ROS accumulation, ASK1 activation, and apoptosis were abolished by eplerenone, a mineralocorticoid receptor (MR) antagonist, apocynin, an inhibitor of NADPH oxidase activation, and tempol, a free radical scavenger, but by neither RU486, a glucocorticoid receptor antagonist, nor butylated hydroxyanisol (BHA), a mitochondrial ROS scavenger. In conclusion, aldosterone-mediated ROS production is blocked by eplerenone and induced by the nongenomic activation of NADPH oxidase, leading to myocyte apoptosis associated with ASK1 activation. These proapoptotic actions of aldosterone may play a role in the progression of heart failure.
Similar content being viewed by others
Article PDF
References
Silvestre JS, Heymes C, Oubenaissa A, et al: Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation 1999; 99: 2694–2701.
Silvestre JS, Robert V, Heymes C, et al: Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem 1998; 273: 4883–4891.
Barnett CA, Pritchett EL : Detection of corticosteroid type I binding sites in heart. Mol Cell Endocrinol 1988; 56: 191–198.
Lazar G, Pagano M, Agarwal MK : Purification and characterization of the activated mineralocorticoid receptor from rat myocardium. Biochim Biophys Acta 1990; 1033: 41–48.
Lombes M, Alfaidy N, Eugene E, Lessana A, Farman N, Bonvalet JP : Prerequisite for cardiac aldosterone action. Mineralocorticoid receptor and 11 beta-hydroxysteroid dehydrogenase in the human heart. Circulation 1995; 92: 175–182.
Pitt B, Zannad F, Remme WJ, et al: The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717.
Pitt B, Remme W, Zannad F, et al: Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321.
Mizuno Y, Yoshimura M, Yasue H, et al: Aldosterone production is activated in failing ventricle in humans. Circulation 2001; 103: 72–77.
Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L : Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation 1990; 82: 1730–1736.
Jorde UP, Vittorio T, Katz SD, Colombo PC, Latif F, Le Jemtel TH : Elevated plasma aldosterone levels despite complete inhibition of the vascular angiotensin-converting enzyme in chronic heart failure. Circulation 2002; 106: 1055–1057.
Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT : Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol 2002; 161: 1773–1781.
Nakano S, Kobayashi N, Yoshida K, Ohno T, Matsuoka H : Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extracellular signal–regulated kinases, NAD(P)H oxidase/lectin–like oxidized low-density lipoprotein receptor-1, and Rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens Res 2005; 28: 925–936.
Yoshida K, Kim-Mitsuyama S, Wake R, et al: Excess aldosterone under normal salt diet induces cardiac hypertrophy and infiltration via oxidative stress. Hypertens Res 2005; 28: 447–455.
Chai W, Garrelds IM, Arulmani U, Schoemaker RG, Lamers JM, Danser AH : Genomic and nongenomic effects of aldosterone in the rat heart: why is spironolactone cardioprotective? Br J Pharmacol 2005; 145: 664–671.
Singal PK, Khaper N, Farahmand F, Bello-Klein A : Oxidative stress in congestive heart failure. Curr Cardiol Rep 2000; 2: 206–211.
Ide T, Tsutsui H, Kinugawa S, et al: Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res 1999; 85: 357–363.
Belch JJ, Bridges AB, Scott N, Chopra M : Oxygen free radicals and congestive heart failure. Br Heart J 1991; 65: 245–248.
Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A : Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation 1998; 97: 1536–1539.
von Harsdorf R, Li PF, Dietz R : Signaling pathways in reactive oxygen species–induced cardiomyocyte apoptosis. Circulation 1999; 99: 2934–2941.
Wencker D, Chandra M, Nguyen K, et al: A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest 2003; 111: 1497–1504.
Griendling KK, Sorescu D, Ushio-Fukai M : NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 2000; 86: 494–501.
Heymes C, Bendall JK, Ratajczak P, et al: Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 2003; 41: 2164–2171.
Li JM, Gall NP, Grieve DJ, Chen M, Shah AM : Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 2002; 40: 477–484.
Miyata K, Rahman M, Shokoji T, et al: Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol 2005; 16: 2906–2912.
Callera GE, Montezano AC, Yogi A, et al: c-Src–dependent nongenomic signaling responses to aldosterone are increased in vascular myocytes from spontaneously hypertensive rats. Hypertension 2005; 46: 1032–1038.
Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM : Aldosterone mediates angiotensin II–induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 2006; 20: 1546–1548.
Mano A, Tatsumi T, Shiraishi J, et al: Aldosterone directly induces myocyte apoptosis through calcineurin-dependent pathways. Circulation 2004; 110: 317–323.
Shiraishi J, Tatsumi T, Keira N, et al: Important role of energy-dependent mitochondrial pathways in cultured rat cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 2001; 281: H1637–H1647.
Matoba S, Tatsumi T, Keira N, et al: Cardioprotective effect of angiotensin-converting enzyme inhibition against hypoxia/reoxygenation injury in cultured rat cardiac myocytes. Circulation 1999; 99: 817–822.
Mohazzab-H KM, Kaminski PM, Wolin MS : Lactate and P O2 modulate superoxide anion production in bovine cardiac myocytes: potential role of NADH oxidase. Circulation 1997; 96: 614–620.
Zhang M, Kho AL, Anilkumar N, et al: Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation 2006; 113: 1235–1243.
Nakamura S, Yoshimura M, Nakayama M, et al: Possible association of heart failure status with synthetic balance between aldosterone and dehydroepiandrosterone in human heart. Circulation 2004; 110: 1787–1793.
Chai W, Garrelds IM, de Vries R, Batenburg WW, van Kats JP, Danser AH : Nongenomic effects of aldosterone in the human heart: interaction with angiotensin II. Hypertension 2005; 46: 701–706.
Kuster GM, Pimentel DR, Adachi T, et al: Alpha-adrenergic receptor–stimulated hypertrophy in adult rat ventricular myocytes is mediated via thioredoxin-1–sensitive oxidative modification of thiols on Ras. Circulation 2005; 111: 1192–1198.
Turchin A, Guo CZ, Adler GK, Ricchiuti V, Kohane IS, Williams GH : Effect of acute aldosterone administration on gene expression profile in the heart. Endocrinology 2006; 147: 3183–3189.
Sergeant S, McPhail LC : Opsonized zymosan stimulates the redistribution of protein kinase C isoforms in human neutrophils. J Immunol 1997; 159: 2877–2885.
Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M : Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood 2005; 106: 1067–1075.
Tsubouchi H, Inoguchi T, Sonta T, et al: Statin attenuates high glucose–induced and diabetes-induced oxidative stress in vitro and in vivo evaluated by electron spin resonance measurement. Free Radic Biol Med 2005; 39: 444–452.
Sato A, Liu JP, Funder JW : Aldosterone rapidly represses protein kinase C activity in neonatal rat cardiomyocytes in vitro. Endocrinology 1997; 138: 3410–3416.
Ichijo H, Nishida E, Irie K, et al: Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997; 275: 90–94.
Saitoh M, Nishitoh H, Fujii M, et al: Mammalian thioredoxin is a direct inhibitor of apoptosis signal–regulating kinase (ASK) 1. EMBO J 1998; 17: 2596–2606.
Hatai T, Matsuzawa A, Inoshita S, et al: Execution of apoptosis signal–regulating kinase 1 (ASK1)−induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem 2000; 275: 26576–26581.
Yamaguchi O, Higuchi Y, Hirotani S, et al: Targeted deletion of apoptosis signal–regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci U S A 2003; 100: 15883–15888.
Watanabe T, Otsu K, Takeda T, et al: Apoptosis signal–regulating kinase 1 is involved not only in apoptosis but also in non-apoptotic cardiomyocyte death. Biochem Biophys Res Commun 2005; 333: 562–567.
Funder JW : Aldosterone, mineralocorticoid receptors and vascular inflammation. Mol Cell Endocrinol 2004; 217: 263–269.
Takeda Y : Pleiotropic actions of aldosterone and the effects of eplerenone, a selective mineralocorticoid receptor antagonist. Hypertens Res 2004; 27: 781–789.
Thompson A, Han VK, Yang K : Differential expression of 11beta-hydroxysteroid dehydrogenase types 1 and 2 mRNA and glucocorticoid receptor protein during mouse embryonic development. J Steroid Biochem Mol Biol 2004; 88: 367–375.
Reini SA, Wood CE, Jensen E, Keller-Wood M : Increased maternal cortisol in late-gestation ewes decreases fetal cardiac expression of 11beta-HSD2 mRNA and the ratio of AT1 to AT2 receptor mRNA. Am J Physiol Regul Integr Comp Physiol 2006; 291: R1708–R1716.
Qin W, Rudolph AE, Bond BR, et al: Transgenic model of aldosterone-driven cardiac hypertrophy and heart failure. Circ Res 2003; 93: 69–76.
Kitagawa H, Yanagisawa J, Fuse H, et al: Ligand-selective potentiation of rat mineralocorticoid receptor activation function 1 by a CBP-containing histone acetyltransferase complex. Mol Cell Biol 2002; 22: 3698–3706.
Frantz S, Brandes RP, Hu K, et al: Left ventricular remodeling after myocardial infarction in mice with targeted deletion of the NADPH oxidase subunit gp91PHOX. Basic Res Cardiol 2006; 101: 127–132.
Doerries C, Grote K, Hilfiker-Kleiner D, et al: Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res 2007; 100: 894–903.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayashi, H., Kobara, M., Abe, M. et al. Aldosterone Nongenomically Produces NADPH Oxidase–Dependent Reactive Oxygen Species and Induces Myocyte Apoptosis. Hypertens Res 31, 363–375 (2008). https://doi.org/10.1291/hypres.31.363
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1291/hypres.31.363