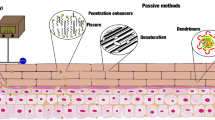
Abstract
Transdermal delivery of peptides and proteins avoids the disadvantages associated with the invasive parenteral route of administration and other alternative routes such as the pulmonary and nasal routes. Since proteins have a large size and are hydrophilic in nature, they cannot permeate passively across the skin due to the stratum corneum which allows the transport of only small lipophilic drug molecules. Enhancement techniques such as chemical enhancers, iontophoresis, microneedles, electroporation, sonophoresis, thermal ablation, laser ablation, radiofrequency ablation and noninvasive jet injectors aid in the delivery of proteins by overcoming the skin barrier in different ways. In this review, these enhancement techniques that can enable the transdermal delivery of proteins are discussed, including a discussion of mechanisms, sterility requirements, and commercial development of products. Combination of enhancement techniques may result in a synergistic effect allowing increased protein delivery and these are also discussed.



Similar content being viewed by others
REFERENCES
Antosova Z, Mackova M, Kral V, Macek T. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 2009;27(11):628–35.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–8.
van de Weert M, Jorgensen L, Horn Moeller E, Frokjaer S. Factors of importance for a successful delivery system for proteins. Expert Opin Drug Deliv. 2005;2(6):1029–37.
Steinstrasser I, Merkle HP. Dermal metabolism of topically applied drugs: pathways and models reconsidered. Pharm Acta Helv. 1995;70(1):3–24.
Tauber U. Drug metabolism in the skin: advantages and disadvantages. In: Hadgraft J, Guy RH, editors. Transdermal drug delivery: developmental issues and research initiatives. New York: Marcel Dekker; 1989. p. 99–112.
Magnusson BM, Runn P. Effect of penetration enhancers on the permeation of the thyrotropin releasing hormone analogue pGlu-3-methyl-His-Pro amide through human epidermis. Int J Pharm. 1999;178(2):149–59.
Chen Y, Shen Y, Guo X, Zhang C, Yang W, Ma M, et al. Transdermal protein delivery by a coadministered peptide identified via phage display. Nat Biotechnol. 2006;24(4):455–60.
Frankenburg S, Grinberg I, Bazak Z, Fingerut L, Pitcovski J, Gorodetsky R, et al. Immunological activation following transcutaneous delivery of HR-gp100 protein. Vaccine. 2007;25(23):4564–70.
Foldvari M, Baca-Estrada ME, He Z, Hu J, Attah-Poku S, King M. Dermal and transdermal delivery of protein pharmaceuticals: lipid-based delivery systems for interferon alpha. Biotechnol Appl Biochem. 1999;30(Pt 2):129–37.
Caccetta R, Blanchfield JT, Harrison J, Toth I, Benson HAE. Epidermal penetration of a therapeutic peptide by lipid conjugation; stereo-selective peptide availability of a topical distereomeric lipopeptide. Int J Pept Res Ther. 2006;12:327–33.
Jain S, Jain P, Umamaheshwari RB, Jain NK. Transfersomes—a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev Ind Pharm. 2003;29(9):1013–26.
Abla N, Naik A, Guy RH, Kalia YN. Contributions of electromigration and electroosmosis to peptide iontophoresis across intact and impaired skin. J Control Release. 2005;108(2–3):319–30.
Green PG. Iontophoretic delivery of peptide drugs. J Control Release. 1996;41:33–48.
Lau DT, Sharkey JW, Petryk L, Mancuso FA, Yu Z, Tse FL. Effect of current magnitude and drug concentration on iontophoretic delivery of octreotide acetate (Sandostatin) in the rabbit. Pharm Res. 1994;11(12):1742–6.
Medi BM, Singh J. Electronically facilitated transdermal delivery of human parathyroid hormone (1–34). Int J Pharm. 2003;263(1–2):25–33.
Prausnitz MR, Lee CR, Liu CH, Pang JC, Singh TP, Langer R, et al. Transdermal transport efficiency during skin electroporation and iontophoresis. J Control Release. 1996;38:205–17.
Miller LL, Kolaskie CJ, Smith GA, Rivier J. Transdermal iontophoresis of gonadotropin releasing hormone (LHRH) and two analogues. J Pharm Sci. 1990;79(6):490–3.
Schuetz YB, Naik A, Guy RH, Vuaridel E, Kalia YN. Transdermal iontophoretic delivery of triptorelin in vitro. J Pharm Sci. 2005;94(10):2175–82.
Nestor Jr JJ, Ho TL, Simpson RA, Horner BL, Jones GH, McRae GI, et al. Synthesis and biological activity of some very hydrophobic superagonist analogues of luteinizing hormone-releasing hormone. J Med Chem. 1982;25(7):795–801.
Pikal MJ. Penetration enhancement of peptide and protein drugs by electrochemical means: transdermal iontophoresis. In: Lee VHL, Hashida M, Mizushima Y, editors. Trends and future perspectives in peptide and protein drug delivery. Chur: Harwood Academic Publishers GmbH; 1995.
Vemulapalli V, Banga AK, Friden PM. Optimization of iontophoretic parameters for the transdermal delivery of methotrexate. Drug Deliv. 2008;15(7):437–42.
Langkjaer L, Brange J, Grodsky GM, Guy RH. Iontophoresis of monomeric insulin analogues in vitro: effects of insulin charge and skin pretreatment. J Control Release. 1998;51(1):47–56.
Hoogstraate AJ, Srinivasan V, Sims SM, Higuchi WI. Iontophoretic enhancement of peptides: behaviour of leuprolide versus model permeants. J Control Release. 1994;31:41–7.
Banga AK. Therapeutic peptides and proteins. 2nd ed. New York: Taylor & Francis; 2006.
Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Invest Dermatol. 2006;126(5):1080–7.
Kalluri H, Banga AK. Microneedles and transdermal drug delivery. J Drug Del Sci Tech. 2009;19(5):303–10.
Li G, Badkar A, Kalluri H, Banga AK. Microchannels created by sugar and metal microneedles: characterization by microscopy, macromolecular flux and other techniques. J Pharm Sci. 2010;99(4):1931–41.
Verbaan FJ, Bal SM, van den Berg DJ, Groenink WH, Verpoorten H, Luttge R, et al. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J Control Release. 2007;117(2):238–45.
Wu XM, Todo H, Sugibayashi K. Enhancement of skin permeation of high molecular compounds by a combination of microneedle pretreatment and iontophoresis. J Control Release. 2007;118(2):189–95.
Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci USA. 2008;105(6):2058–63.
Lin W, Cormier M, Samiee A, Griffin A, Johnson B, Teng CL, et al. Transdermal delivery of antisense oligonucleotides with microprojection patch (Macroflux) technology. Pharm Res. 2001;18(12):1789–93.
Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm Res. 2008;25(1):104–13.
Chabri F, Bouris K, Jones T, Barrow D, Hann A, Allender C, et al. Microfabricated silicon microneedles for nonviral cutaneous gene delivery. Br J Dermatol. 2004;150(5):869–77.
Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24(7):1369–80.
Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97(3):503–11.
Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24(10):1653–64.
Xie Y, Xu B, Gao Y. Controlled transdermal delivery of model drug compounds by MEMS microneedle array. Nanomedicine. 2005;1(2):184–90.
Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19(1):63–70.
Ito Y, Hagiwara E, Saeki A, Sugioka N, Takada K. Feasibility of microneedles for percutaneous absorption of insulin. Eur J Pharm Sci. 2006;29(1):82–8.
Sullivan SP, Murthy N, Prausnitz MR. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv Mater. 2008;20:933–8.
Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23(5):1008–19.
Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–24.
Roxhed N, Griss P, Stemme G. Membrane-sealed hollow microneedles and related administration schemes for transdermal drug delivery. Biomed Microdevices. 2008;10(2):271–9.
Paik SJ, Byun S, Lim JM, Park Y, Lee A, Chung S, et al. In-plane single-crystal-silicon microneedles for minimally invasive microfluid systems. Sens Actuators A. 2004;114:276–84.
Martanto W, Moore JS, Kashlan O, Kamath R, Wang PM, O’Neal JM, et al. Microinfusion using hollow microneedles. Pharm Res. 2006;23(1):104–13.
Nordquist L, Roxhed N, Griss P, Stemme G. Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: an initial comparison with subcutaneous administration. Pharm Res. 2007;24(7):1381–8.
Teo MA, Shearwood C, Ng KC, Lu J, Moochhala S. In vitro and in vivo characterization of MEMS microneedles. Biomed Microdevices. 2005;7(1):47–52.
Martanto W, Moore JS, Couse T, Prausnitz MR. Mechanism of fluid infusion during microneedle insertion and retraction. J Control Release. 2006;112(3):357–61.
Ameri M, Wang X, Maa YF. Effect of irradiation on parathyroid hormone PTH(1–34) coated on a novel transdermal microprojection delivery system to produce a sterile product—adhesive compatibility. J Pharm Sci. 2010;99(4):2123–34.
Kalluri H, Banga AK. Formation and Closure of Microchannels in Skin Following Microporation. Pharm Res [serial on the Internet]. 2010: Available from: http://www.ncbi.nlm.nih.gov/pubmed/20354766.
Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices. 2009;11(1):35–47.
Gupta J. Microneedles for transdermal drug delivery in human subjects. Atlanta: Georgia Institute of Technology; 2009.
Donnelly RF, Singh TR, Tunney MM, Morrow DI, McCarron PA, O’Mahony C, et al. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm Res. 2009;26(11):2513–22.
Prausnitz MR. Do high-voltage pulses cause changes in skin structure? J Control Release. 1996;40:321–6.
Edwards DA, Prausnitz MR, Langer R, Weaver JC. Analysis of enhanced transdermal transport by skin electroporation. J Control Release. 1995;34:211–21.
Zhao YL, Murthy SN, Manjili MH, Guan LJ, Sen A, Hui SW. Induction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization. Vaccine. 2006;24(9):1282–90.
Chang SL, Hofmann GA, Zhang L, Deftos LJ, Banga AK. The effect of electroporation on iontophoretic transdermal delivery of calcium regulating hormones. J Control Release. 2000;66(2–3):127–33.
Prausnitz MR, Pliquett U, Langer R, Weaver JC. Rapid temporal control of transdermal drug delivery by electroporation. Pharm Res. 1994;11(12):1834–7.
Vanbever R, Lecouturier N, Preat V. Transdermal delivery of metoprolol by electroporation. Pharm Res. 1994;11(11):1657–62.
Prausnitz MR, Edelman ER, Gimm JA, Langer R, Weaver JC. Transdermal delivery of heparin by skin electroporation. Biotechnology (NY). 1995;13(11):1205–9.
Prausnitz MR, Bose VG, Langer R, Weaver JC. Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. Proc Natl Acad Sci USA. 1993;90(22):10504–8.
Therapeutics A. PassPort Patch. 2010; Available from: http://www.alteatherapeutics.com/. Accessed June 2010
Banga AK. Microporation applications for enhancing drug delivery. Expert Opin Drug Deliv. 2009;6(4):343–54.
TransPharma. ViaDerm system. 2010; Available from: http://www.transpharma-medical.com/viaderm_system.html. Accessed June 2010
Levin G, Gershonowitz A, Sacks H, Stern M, Sherman A, Rudaev S, et al. Transdermal delivery of human growth hormone through RF-microchannels. Pharm Res. 2005;22(4):550–5.
Mitragotri S, Kost J. Low-frequency sonophoresis: a review. Adv Drug Deliv Rev. 2004;56(5):589–601.
Luis J, Park EJ, Meyer RJ, Smith NB. Rectangular cymbal arrays for improved ultrasonic transdermal insulin delivery. J Acoust Soc Am. 2007;122(4):2022–30.
Lee S, Snyder B, Newnham RE, Smith NB. Noninvasive ultrasonic transdermal insulin delivery in rabbits using the light-weight cymbal array. Diabetes Technol Ther. 2004;6(6):808–15.
Smith NB, Lee S, Shung KK. Ultrasound-mediated transdermal in vivo transport of insulin with low-profile cymbal arrays. Ultrasound Med Biol. 2003;29(8):1205–10.
Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269(5225):850–3.
Tachibana K, Tachibana S. Use of ultrasound to enhance the local anesthetic effect of topically applied aqueous lidocaine. Anesthesiology. 1993;78:1091–6.
Mitragotri S, Kost J. Transdermal delivery of heparin and low-molecular weight heparin using low-frequency ultrasound. Pharm Res. 2001;18(8):1151–6.
El-Kamel AH, Al-Fagih IM, Alsarra IA. Effect of sonophoresis and chemical enhancers on testosterone transdermal delivery from solid lipid microparticles: an in vitro study. Curr Drug Deliv. 2008;5(1):20–6.
Tezel A, Dokka S, Kelly S, Hardee GE, Mitragotri S. Topical delivery of anti-sense oligonucleotides using low-frequency sonophoresis. Pharm Res. 2004;21(12):2219–25.
Mitragotri S, Blankschtein D, Langer R. An explanation for the variation of the sonophoretic transdermal transport enhancement from drug to drug. J Pharm Sci. 1997;86(10):1190–2.
Banga AK. New technologies to allow transdermal delivery of therapeutic proteins and small water-soluble drugs. Am J Drug Deliv. 2006;4(4):221–30.
Biosolutions P. P.L.E.A.S.E. platform. 2010; Available from: http://www.pantec-biosolutions.com/. Accessed June 2010
Abbey N. Laser Assisted Drug Delivery (LAD). 2010; Available from: www.norwoodabbey.com. Accessed June 2010
Koh JL, Harrison D, Swanson V, Norvell DC, Coomber DC. A comparison of laser-assisted drug delivery at two output energies for enhancing the delivery of topically applied LMX-4 cream prior to venipuncture. Anesth Analg. 2007;104(4):847–9.
Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov. 2006;5(7):543–8.
Dean HJ, Fuller D, Osorio JE. Powder and particle-mediated approaches for delivery of DNA and protein vaccines into the epidermis. Comp Immunol Microbiol Infect Dis. 2003;26(5–6):373–88.
Chen D, Payne LG. Targeting epidermal Langerhans cells by epidermal powder immunization. Cell Res. 2002;12(2):97–104.
Osorio JE, Zuleger CL, Burger M, Chu Q, Payne LG, Chen D. Immune responses to hepatitis B surface antigen following epidermal powder immunization. Immunol Cell Biol. 2003;81(1):52–8.
Kendall M, Rishworth S, Carter F, Mitchell T. Effects of relative humidity and ambient temperature on the ballistic delivery of micro-particles to excised porcine skin. J Invest Dermatol. 2004;122(3):739–46.
Pillai O, Panchagnula R. Transdermal delivery of insulin from poloxamer gel: ex vivo and in vivo skin permeation studies in rat using iontophoresis and chemical enhancers. J Control Release. 2003;89(1):127–40.
Badkar AV, Smith AM, Eppstein JA, Banga AK. Transdermal delivery of interferon alpha-2B using microporation and iontophoresis in hairless rats. Pharm Res. 2007;24(7):1389–95.
Katikaneni S, Badkar A, Nema S, Banga AK. Molecular charge mediated transport of a 13 kD protein across microporated skin. Int J Pharm. 2009;378(1–2):93–100.
Vemulapalli V, Yang Y, Friden PM, Banga AK. Synergistic effect of iontophoresis and soluble microneedles for transdermal delivery of methotrexate. J Pharm Pharmacol. 2008;60(1):27–33.
Bommannan DB, Tamada J, Leung L, Potts RO. Effect of electroporation on transdermal iontophoretic delivery of luteinizing hormone releasing hormone (LHRH) in vitro. Pharm Res. 1994;11(12):1809–14.
Riviere JE, Monteiro-Riviere NA, Rogers RA, Bommannan D, Tamada JA, Potts RO. Pulsatile transdermal delivery of LHRH using electroporation: drug delivery and skin toxicology. J Control Release. 1995;36:229–33.
Mutalik S, Parekh HS, Davies NM, Udupa N. A combined approach of chemical enhancers and sonophoresis for the transdermal delivery of tizanidine hydrochloride. Drug Deliv. 2009;16(2):82–91.
Kost J, Pliquett U, Mitragotri S, Yamamoto A, Langer R, Weaver J. Synergistic effect of electric field and ultrasound on transdermal transport. Pharm Res. 1996;13(4):633–8.
Le L, Kost J, Mitragotri S. Combined effect of low-frequency ultrasound and iontophoresis: applications for transdermal heparin delivery. Pharm Res. 2000;17(9):1151–4.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Lavinia Lewis, Jim Agalloco, Bill Lambert, Russell Madsen, and Mark Staples
Rights and permissions
About this article
Cite this article
Kalluri, H., Banga, A.K. Transdermal Delivery of Proteins. AAPS PharmSciTech 12, 431–441 (2011). https://doi.org/10.1208/s12249-011-9601-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-011-9601-6