Abstract
Kynurenic acid (KYNA) is a tryptophan metabolite and represents the only known endogenous compound acting as an antagonist to excitatory amino acid receptors in the mammalian CNS. Blocking of these receptors in CNS by KYNA affects cardiac function. As it is not known whether human heart is able to synthesize this neuromodulatory amino acid, we investigated the biosynthesizing enzyme of kynurenine aminotransferase (KAT) in the human heart and compared the activity with that of the human brain. The activities of heart and brain KATs were assayed by the conversion of L-kynurenine (L-KYN) to KYNA and quantitated by HPLC with fluorescence detection. Using either pyruvate or 2-oxoglutarate as cosubstrates, heart KAT was found to have a shallow pH optimum between 8 and 9. Highest heart KAT activity was seen in the presence of 2-oxoglutarate, followed by pyruvate, 2-oxoadipate, and 2-oxoisocaproate. Kinetic analyses, performed at pH 8.5, and using various concentrations of L-KYN (from 0.125 to 22.8 mM) in the presence of 2-oxoglutarate (1 and 5 mM) or pyruvate (5 mM) revealed apparent Km values in the millimolar range, for L-KYN 1.5, 27, and 20 mM, respectively. Heart KAT activities were compared with those in human brain KAT I and KAT II showing different pH optima 7.4 and 9.6, respectively. In contrast to brain KAT I, heart KAT activity was not inhibited by an excess of 2 mM L-tryptophan, L-glutamine, or L-phenylalanine at pH 9.6, as well as at pH 8 or 7.4. Our study demonstrates that human heart is capable of synthesizing KYNA from low concentrations of L-KYN selectively. A shallow pH optimum of KAT activity, i.e. between 8.0 and 9.0, pronounced 2-oxoacid specificity, and a lack of sensitivity to inhibition by L-glutamine, L-phenylalanine, and L-tryptophan indicate that the heart KAT system displays enzymatic characteristics different from those of human brain KAT I or KAT II. Fluctuation of L-KYN and 2-oxoacid levels may markedly influence the KYNA synthesis and subsequent KYNA effect on cardiac activity. KYNA synthesis in the human heart suggests a neurophysiologic role. Our studies form the basis for purification and further characterization of KAT protein in human heart as well as for physiologic studies.
Similar content being viewed by others
Main
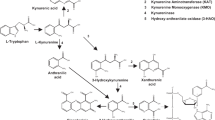
One of the metabolic pathways of greatest biologic interest in tryptophan metabolism is that leading to niacin formation, i.e. the kynurenine pathway (Fig. 1)(1). The kynurenine pathway has also another biologically important function, i.e. the formation of two neuroactive compounds, quinolinic acid with excitatory and KYNA with inhibitory properties(2). KAT (EC 2.6.1.7.), an enzyme of kynurenine pathway of tryptophan metabolism, catalyzes the irreversible transamination reaction between L-KYN and 2-oxoacids, as a cosubstrate, to form KYNA (Fig. 1). In mammals, KAT activity is present in various tissues, such as liver, kidney, small intestine, and brain(3–5). In rat brain, only a single KAT enzyme(5), with the regional heterogeneity of ontogenetic profile(6), is capable of producing KYNA at physiologic (low micromolar) L-KYN concentration. In human brain KYNA is synthesized from L-KYN by the activity of at least two KAT, which were identified and characterized recently(7–9).
Over the last decade of studies upon the function of EAAs it became obvious that they not only play a central role in a wide array of brain processes, such as synaptic plasticity and motor control, but can be involved in the pathogenesis of various neurodegenerative disorders(10). Pharmacologic probes have been applied to subdivide the receptors, which may mediate excitotoxic insults(11). These receptors are linked to ion channels and are named after their model agonists: NMDA, kainate, and AMPA(11). Thus, the pathologic overstimulation of EAA receptors results in neurodegeneration(12). KYNA, the neurobiologically active metabolite of tryptophan, is the only known endogenous compound acting as an antagonist of these ionotropic EAA receptors(2). KYNA has been shown to be neuroprotective in relevant animal experiments, too(13–16). EAA receptors of both the NMDA and the non-NMDA type have been suggested as contributing to the pathogenesis of various neurologic disorders in humans, and a dysfunctional KYNA system has been speculatively linked to the pathogenesis of a number of neurologic diseases, e.g. Huntington's disease, hypoxia-ischemia, or epilepsy(17–20).
In the rat, Carla and co-workers(21) determined KYNA levels in various organs, including heart, in the range of picomoles/g of tissue. Except for the demonstration of a rat KAT mRNA in heart and various organs(22), no information on the biosynthesis of KYNA in the heart is available in literature to our knowledge. The fact that no biosynthetic studies of KYNA are available, along with a pharmacologic observation that KYNA was able to modify responses to EAAs in the heart(23, 24), led us to the investigation of the biosynthetic machinery in the human heart. In human brain, two proteins that are responsible for the formation of KYNA, e.g. KAT I and KAT II, display different enzymatic characteristics(7–9). The purpose of our studies was to test the capability of human cardiac tissue to synthesize KYNA from its bioprecursor L-KYN and to compare the activities of KATs from human brain.
METHODS
Study subjects for KYNA metabolism. For KAT characterization, total ventricular tissue and frontal cortex of one patient was obtained at autopsy. For KAT activity and KYNA levels, measurement of total ventricular tissue of eight patients was used. The specimens were kept frozen at -70°C until investigation within 2 mo. The age and clinical characterization of the subject and postmortem delay are given in the Table 1.
Materials. L-KYN (sulfate salt), pyruvate (sodium salt), 2-oxoglutarate (monosodium salt), 2-oxoisocaproate (sodium salt), pyridoxal 5′-phosphate, and AMP, was obtained from Sigma Chemical Co.. All other chemicals were of the highest commercially available purity.
Tissue preparation. Aliquots of heart tissue were homogenized in a Polytron (Kinematics GmbH, Luzern, Switzerland) in an ice bath in 9 volumes (wt/vol) of 5 mM Tris-acetate buffer, pH 7.4, containing 50 μM pyridoxal 5′-phosphate and 10 mM mercaptoethanol, followed by sonication. The immediately obtained homogenate was divided into two parts. One part was used for the determination of KAT activity and the other was mixed with 0.2 M HCl (vol/vol) and centrifuged for 20 min at 28.000 ×g; the obtained supernatant was used for measurement of KYNA levels.
Determination of KATs. The activities of the KATs were assayed by a modification of the method of Baran et al.(9). Briefly, the reaction mixture contained the homogenate, 2 μM L-KYN, 1 mM 2-oxoacid, 70 μM pyridoxal 5′-phosphate, and 150 mM Tris-acetate (for the range of pH 6.0-8.5) or 150 mM AMP buffer (for the pH range of 9.0-10.6). The reaction was started by the addition of either of the 2-oxoacids (pyruvate, 2-oxoglutarate, 2-oxoisocaproate, 2-oxoisoadipate). After the incubation for 14 h at 37°C, the reaction was terminated by the addition of 14 μL of 50% trichloroacetic acid. Subsequently, 1 mL of 0.1 M HCl was added, and denatured protein was removed by 10-min centrifugation (Eppendorf Microfuge). For KYNA determination the supernatant was applied to a Dowex 50 W cation exchange column(25). KYNA eluted from the column was quantitated by HPLC method, as given below. The blanks were obtained by using tissue heat inactivated for 30 min in a boiling water bath.
Measurement of KYNA. Supernatants of homogenates were applied onto Dowex 50 W cation exchange column prewashed with 0.1 M HCl. Subsequently, the column was washed with 1 mL of 0.1 M HCl and 1 mL of distilled water, and KYNA was eluted with 2 mL of distilled water(25). Eluted KYNA was determined by HPLC with fluorescence detection following the protocols of Shibata(26) in the modification developed by Swartz et al.(27).
The HPLC system used consisted of the following. The pump was an LC from Shimadzu, the fluorescence detector was a Shimadzu RF-535, set at an excitation wavelength of 340 nm and an emission wavelength of 398 nm, and a Shimadzu C-R5A Chromatopac was used for integration. The mobile phase(isocratic system) consisted of 50 mM sodium acetate, 250 mM zinc acetate, and 4% acetonitrile, pH 6.2, and was pumped through a 100 × 4-mm column(HR-80, C-18, particle size 3 μm, InChrom, Austria) at a flow rate of 1.0 mL/min, run at room temperature (23°C). The retention time of KYNA was approximately 5 min with a sensitivity of 150 fmol/injection (signal:noise ratio = 5).
Biochemical characterization. pH dependence of KAT activity was tested between 5.94 and 10.8 in the presence of 1 mM each 2-oxoglutarate and pyruvate. Cosubstrate specificity of KAT was tested at 1 mM concentration of several 2-oxoacids, at pH 7.4, 8.0, and 9.6, using 2 μM and 3 mM L-KYN.
The effect of amino acids on heart KAT and brain KATs was tested using the excess of either 2 mM L-glutamate, L-alanine, L-phenylalanine, L-glutamine, or L-tryptophan in the presence of the cosubstrate pyruvate (1 mM) at pH 7.4, 8.0, and 9.6.
Kinetic analyses. For heart KAT, apparent Km values for L-KYN were determined at different L-KYN concentrations up to 22.8 mM with 1 and 5 mM 2-oxoglutarate or 5 mM pyruvate. Km values for 2-oxoglutarate or pyruvate were determined by varying the concentration of the oxo acids (up to 5 mM) while keeping the L-KYN concentration constant at 5 mM
Protein determination. Protein was measured according to the method of Bradford(28) using a commercially available kit (Bio-Rad) and BSA as a standard.
Data presentation and statistics. All measurement were performed in triplicate. Data are given as means ± SEM. For comparison of groups the t test was applied.
RESULTS
Linearity of assays with time and protein amount. KAT activity measured in heart homogenate was found to be linear with incubation time up to 14 h and when between 0.4 and 0.75 mg of protein was used per incubation (Fig. 2).
pH optimum. A graph illustrating the presence of heart KAT activity along with pH dependence from 5.94 to 10.8 is given in Figure 3. Using either 2-oxoglutarate or pyruvate, heart KAT activity was found to have a shallow pH optimum of about 8.0-9.0.
2-Oxoacid specificity. The 2-oxoacid specificity of heart KAT is documented in the Table 2. Among four 2-oxoacids(pyruvate, 2-oxoglutarate, 2-oxoisocaproate, and 2-oxoadipate) used at pH 7.4, 8, and 9.6 and in the presence of 2 μM or 3 mM L-KYN, heart KAT showed the highest activity with pyruvate at pH 8.0. Lowest heart KAT activity was measured with 2-oxoisocaproate. A 1500-fold increase of L-KYN in the incubation medium enhanced KYNA production between 400- and 700-fold.
Effect of L-amino acids. The effect of several L-amino acids, known substrates for enzymatic transamination, on heart KAT is shown in Table 3. At a concentration of 2 mM L-tryptophan,i.e. 1000 times the concentration of L-KYN, the production of KYNA was significantly increased by 43, 84, and 67% in the heart at pH 7.4, 8.0, and 9.6, respectively. The effect of L-amino acids in the heart was compared with the effect of L-amino acids in the brain, known to contain two KAT proteins, KAT I with a pH optimum 9.6 showing inhibition by L-tryptophan and L-glutamine(7–9) and KAT II with pH optimum at 7.4, showing no inhibition by both L-amino acids(7, 8). In contrast to our findings in the heart, brain KAT activity was strongly inhibited by L-tryptophan at pH 9.6 (KAT I) but not at pH 7.4 (KAT II).
Table 4 lists the effects of different L-tryptophan concentrations(0.125-2.0 mM) on heart KAT activity. Heart activity was increased by approximately 100% in the presence of 2 mM L-tryptophan. Under standard experimental conditions the replacement of 2 μM L-KYN by 2 mM L-tryptophan revealed a comparable rate of KYNA synthesis (Table 4).
Kinetic analyses. The kinetics of heart KAT was assessed by varying the concentration of L-KYN and 2-oxoacids, respectively. Concentration of L-KYN ranged from 0.125 to 22.85 mM and from 0.5 to 22.85 mM, whereas 2-oxoglutarate was kept stable at 1 and 5 mM, respectively. Concentration of L-KYN ranged from 0.5 to 22.85 mM, whereas pyruvate was kept stable at 5 mM. Concentration of 2-oxoglutarate ranged from 0.5 to 10 mM and from 0.0625 to 2.5 mM, whereas L-KYN was kept stable at 5 and 20 mM, respectively. The concentration of pyruvate range from 0.5 to 10 mM and from 0.0625 to 2.5 mM, whereas L-KYN was kept stable at 5 and 20 mM, respectively. Apparent Km and Vmax values were calculated from double reciprocal plots and are presented in Table 5 and Figure 4. Km values for L-KYN were in the 1.5 mM range at fixed 1 mM 2-oxoglutarate and 20 and 27 mM at fixed 5 mM pyruvate and 5 mM 2-oxoglutarate, respectively (Table 5, part A). Variation of concentration of pyruvate or 2-oxoglutarate yieled a curve, which was saturated at about 1.5 mM, and an occurrence of apparent substrate inhibition above of 2 mM. Km values for pyruvate and 2-oxoglutarate at fixed 5 mM concentration of L-KYN were 1.15 and 0.62 mM, respectively (part A). Using a high fixed 20 mM concentration of L-KYN, the Km values for pyruvate and 2-oxoglutarate were similar in a low, 0.21 and 0.69 mM range, respectively (Table 5, part B). Using various 2-oxoglutarate concentrations, Vmax was 17.91 nmol/mg of protein/h in the presence of 20 mM L-KYN.
KAT activity and KYNA levels in human heart tissue. KAT activity and KYNA levels in heart tissue were measured in eight subjects. In the presence of 1 mM pyruvate and 2 μM L-KYN, heart KAT activity was 1.09± 0.20 and 1.57 ± 0.27 pmol/mg of protein/h at pH 7.4 and pH 8.0, respectively. Heart KYNA levels were 67.5 ± 21.36 pmol/mg of protein.
DISCUSSION
As our studies demonstrate, the presence of human heart KYNA levels (67.5 pmol/mg of protein) are comparable to those known in human brain(25), an organ with a well characterized KAT enzyme system(7–9). In human serum, KYNA occurs at a 2 nM concentration(29), which is many fold lower than that of human heart KYNA content found in the low micromolar range. This finding clearly indicates that KYNA determined in heart tissue is not derived from serum but formed in the heart itself. This is indirectly supported by the information that L-KYN is taken up by rat peripheral tissue slices, including heart(30). Moreover, we demonstrated that human total ventricular tissue homogenate is capable of converting L-KYN to KYNA at the very low L-KYN concentration, on the order of 2 μM.
In serum, L-KYN is present in a micromolar range (3.3 μM)(31) and can likely be converted by heart tissue. Recent reports have shown that different enzymes catalyze the transamination between L-KYN and various 2-oxoacids to form KYNA in some peripheral tissues(3, 4) and CNS(5, 7–9). Thus, liver was shown to contain two enzymes, one with pH optimum between 6.0 and 6.5 and a second with pH optimum between 9.0 and 9.5. Although in the human brain extract two enzyme activities could be revealed, one showing pH optimum between 7.4. and 8.0 (KAT II) and a second with pH optimum between 9.0 and 9.6 (KAT I), no information on KAT activities in human heart, however, has been described thus far.
Human heart KAT, active at physiologic pH range, displayed highest KAT activity between pH 8.0 and pH 9.0, using pyruvate as cosubstrate, and between pH 8.45 and pH 9, using 2-oxoglutarate as cosubstrate. Obtained data might indicate a presence of one protein responsible for KYNA formation with comparable activities at physiologic pH values. Further characterizations of heart KAT performed in our study suggest significant biochemical differences to other KATs known so far. For example, in contrast to human brain KAT I, affected dramatically by L-tryptophan, L-glutamine, or L-phenylalanine(7–9), no inhibition of heart KAT activity at any pH used in the experiments was observed. The presence of 2 mM (excess) L-tryptophan in the incubation medium increased the formation of KYNA, comparable to KYNA formation in the presence of only 2 μM L-KYN, thus pointing to a high specificity of heart KAT and the presence of nonspecific transamination.
Increasing the concentration of L-KYN in the incubation medium from 2 μM to 3 mM at different pH values resulted into an increase of KAT activity up to 772-fold. Kinetic analysis of heart KAT at a fixed 1 mM concentration of 2-oxoglutarate revealed the apparent Km value for L-KYN in a low micromolar range. Variation of 2-oxoglutarate and pyruvate yielded, however, a curve which approached saturation at about 1.5 mM and even the occurrence of apparent substrate inhibition above 2 mM. Increase of cosubstrate, e.g. 2-oxoacid concentration, markedly lowered the affinity of the heart KAT, due to the inhibition and subsequent KYNA synthesis. The high substrate specificity of heart KAT in converting L-KYN to KYNA and the lack of susceptibility to inhibition by other amino acids, known as substrates for transamination reactions, suggest that in vivo fluctuation of heart/blood substrates, e.g. 2-oxoacids and/or L-KYN, may significantly influence the formation of KYNA. In this respect the synthesis of KYNA can be significantly altered in disorders of branched chain amino acids and keto acid metabolism, e.g. maple syrup disease and branched chain ketoaciduria(32–34). Accumulation of 2-oxoacids in the organic and lactic acidemia may influence KYNA metabolism with subsequent functional consequences.
The biologic role and effects of KYNA have been documented in the brain. Thus, KYNA is the only known endogenous competitive antagonist of all three NMDA, kainate, and AMPA ionotropic EAA receptors(2, 11, 35). In the last few years it has been demonstrated that KYNA antagonizes in vitro and in vivo the actions of EAA(2) and has neuroprotective and anticonvulsant properties against the neurotoxic action of glutamate agonist quinolinic acid and other neurotoxins(13–16, 36). KYNA has been implicated also in a number of neurologic and psychiatric disorders. In Huntington's disease, brain KYNA levels and KYNA biosynthetic machinery were reduced, probably reflecting neuronal loss(17–19). In Alzheimer disease lowered KAT I and KAT II activities have been measured(37), and reduced KAT I activity but increased KYNA levels have been demonstrated in Down's syndrome(38).
Only very few reports describe a possible functional activity in the heart. Cardiorespiratory effects produced by blockade of EAA receptors after i.v. administration of KYNA have been described by Abrahams and co-workers(24). Recently Lorenzo and co-workers(23) reported specific KYNA activity in isolated rat atria. Thus, in rat atria isolated with their sympathetic fibers, the chronotropic responses to nerve stimulation were reduced in a concentration-dependent manner by 10 μM to 1 mM L-glutamate and by 0.01 to 1.0 μM AMPA. The decreases in the chronotropic response caused by the EAA L-glutamate and by AMPA were prevented by KYNA. Although the knowledge of the biologic function of KYNA metabolism in the human heart is preliminary, accumulated data suggest the functional importance of this well known endogenous brain neuroprotectant. Our finding of a KYNA-synthesizing enzyme system in the heart, an excitable tissue, forms the basis for further biochemical, physiologic, and pharmacologic studies.
Abbreviations
- KYNA:
-
kynurenic acid
- KAT:
-
kynurenine aminotransferase [f]L-KYN, L-kynurenine
- NMDA:
-
N-methyl-D-aspartate
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazole propionate
- AMP:
-
2 amino-2-methyl-1-propanol
- EAA:
-
excitatory amino acid
References
Brown RR 1971 Biochemistry and pathology of tryptophan metabolism and its regulation by amino acids, vitamin B6 and steroid hormone. Am J Clin Nutr 24: 243–247
Stone TW 1993 Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev 45: 309–379
Noguchi T, Minatogawa Y, Okuno E, Nakatani M, Morimoto M, Kido R 1975 Purification and characterization of kynurenine-2-oxoglutarate aminotransferase from the liver, brain and small intestine of rats. Biochem J 151: 399–406
Okuno E, Minatogawa Y, Nakamura M, Kamoda N, Nakanishi J, Makino M, Kido R 1980 Crystallization and characterization of human liver kynurenine-glyoxylate aminotransferase. Biochem J 189: 581–590
Okuno E, Schmidt W, Parks DA, Nakamura M, Schwarcz R 1991 Measurement of rat brain kynurenine aminotransferase at physiological kynurenine concentrations. J Neurochem 57: 533–540
Baran H, Schwarcz R 1993 Regional differences in the ontogenetic pattern of kynurenine aminotransferase in the rat brain. Dev Brain Res 74: 283–286
Okuno E, Nakamura M, Schwarcz R 1991 Two kynurenine aminotransferases in human brain. Brain Res 542: 307–312
Schmidt W, Guidetti P, Okuno E, Schwarcz R 1993 Characterization of human brain kynurenine aminotransferases using[3H]kynurenine as a substrate. Neuroscience 55: 177–184
Baran H, Okuno E, Kido R, Schwarcz R 1994 Purification and characterization of kynurenine aminotransferase I from human brain. J Neurochem 62: 730–738
Schwarcz R, Price DL 1991 The role of excitotoxins in experimental systems and in human neurological disorders. In: Price DL, Thoenen H, Aguayo AJ (eds) Neurodegenerative Disorders: Mechanisms and Prospects for Therapy. John Wiley & Sons Ltd, Chichester, UK, pp 21–34
Watkins JC, Krogsgaard-Larsen P, Honore T 1990 Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci 11: 26–33
Choi DW 1988 Glutamate neurotoxicity and diseases of the nervous system. Neuron 1: 623–634
Andine P, Lehmann A, Ellren K, Wennberg E, Kjellmer I, Nielsen T, Hagberg H 1988 The excitatory amino acid antagonist kynurenic acid administered after hypoxic ischemia in neonatal rats offers neuroprotection. Neurosci Lett 90: 208–212
Perkins MN, Stone TW 1982 An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res 247: 184–187
Beninger RJ, Jhamandas K, Boeghman RJ, El-Defrawy SR 1986 Kynurenic acid-induced protection of neurochemical and behavioural deficits produced by quinolinic acid injections into the nucleus basalis of rats. Neurosci Lett 68: 317–321
Foster AC, Vezzani A, French ED, Schwarcz R 1984 Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett 48: 273–278
Schwarcz R 1992 Excitotoxins, kynurenines and neuropsychiatric diseases: Implications for drug development. In: Schousboe A, Diemer NH, Kofod H (eds) Drug Research Related to Neuroactive Amino Acids(Alfred Benzon Symposium 32). Munksgaard, Copenhagen, pp 287–301
Jauch D, Urbanska EM, Guidetti P, Bird ED, Vonsattel J-PG, Whetsell WO, Schwarcz R 1995 Dysfunction of brain kynurenic acid metabolism in Huntington's disease: focus on kynurenine aminotransferases. J Neurol Sci 130: 39–47
Beal MF, Matson WR, Storey E, Milbury P, Ryan EA, Ogawa T, Bird ED 1992 Kynurenic acid concentrations are reduced in Huntington's disease cerebral cortex. J Neurol Sci 108: 80–87
Feldblum S, Rougier A, Loiseau H, Loiseau P, Cohadon F, Morselli PL, Lloyd KG 1988 Quinolinic-phosphoribosyl transferase activity is decreased in epileptic human brain tissue. Epilepsia 29: 523–529
Carla V, Lombardi G, Beni M, Russi P, Moneti G, Moroni F 1988 Identification and measurement of kynurenic acid in the rat brain and other organs. Anal Biochem 169: 89–94
Mosca M, Cozzi L, Breton J, Speciale C, Okuno E, Schwarcz R, Benatti L 1994 Molecular cloning of rat kynurenine aminotransferase: identity with glutamine transaminase K. FEBS Lett 353: 21–24
Lorenzo PS, Butta NV, Adler-Graschinsky E 1994 Effects of L-glutamate on the responses to nerve stimulation in rat isolated atria. Eur J Pharmacol 258: 253–260
Abrahams TP, Taveira DaSilva AM, Hamosh P, McManigle JE, Gillis RA 1993 Cardiorespiratory effects produced by blockade of excitatory amino acid receptors in cats. Eur J Pharmacol 238: 223–233
Turski WA, Nakamura M, Todd WP, Carpenter BK, Whetsell WO, Schwarcz R 1988 Identification and quantification of kynurenic acid in human brain tissue. Brain Res 454: 164–169
Shibata K 1988 Fluorimetric micro-determination of kynurenic acid, an endogenous blocker of neurotoxicity, by high-performance liquid chromatography. J Chromatogr 430: 376–380
Swartz KJ, Matson WR, MacGarvey U, Ryan EA, Beal MF 1990 Measurement of kynurenic acid in mammalian brain extracts and cerebrospinal fluid by high-performance liquid chromatography with fluorometric and coulometric electrode array detection. Anal Biochem 185: 363–376
Bradford M 1976 A rapid and sensitive method for the quantitation of microgram-quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Halperin JJ, Heyes MP 1992 Neuroactive kynurenines in Lyme borreliosis. Neurology 42: 43–50
Speciale C, Schwarcz R 1990 Uptake of kynurenine into rat brain slices. J Neurochem 54: 156–163
Heyes MP, Saito K, Devinsky O, Nadi NS 1994 Kynurenine pathway metabolites in cerebrospinal fluid and serum in complex partial seizures. Epilepsia 35: 251–259
Taylor J, Robinson BH, Sherwood WG 1978 A defect in branched-chain amino acids metabolism in a patient with congenital lactic acidosis due to dihydrolipoyl dehydrogenase deficiency. Pediatr Res 12: 60–62
Robinson BH, Taylor J, Sherwood WG 1977 Deficiency of dihydrolipoyl dehydrogenase (a component of the pyruvate and-ketoglutarate dehydrogenase complex):A cause of congenital chronic lactic acidosis in infancy. Pediatr Res 11: 1198–1206
Haworth JC, Perry TL, Blass JP, Hansen S, Urquhart N 1976 Lactic acidosis in three sibs due to defects in both pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase complexes. Pediatrics 58: 564–751
Kessler M, Terramani T, Lynch G, Baudry M 1989 A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem 52: 1319–1328
Baran H, Gramer M, Hönack D, Löscher W 1995 Systemic administration of kainate induces marked increases of endogenous kynurenic acid in various brain regions and plasma of rats. Eur J Pharmacol 286: 167–175
Baran H, Cairns N, Singewald N, Lubec G 1995 Kynurenic acid and kynurenine aminotransferases KAT I and KAT II in the brains of patients with DOWN syndrome and Alzheimer's disease. Soc Neurosci Abstr 21: 581.55
Baran H, Cairns N, Lubec B, Lubec G 1996 Increased kynurenic acid levels and decreased brain kynurenine aminotransferase I in patients with Down syndrome. Life Sci 58: 1891–1899
Author information
Authors and Affiliations
Additional information
Supported by a grant of Red Bull Inc. Austria and by Austrian Science Research Fund, No. H0043-MED to H.B.
Rights and permissions
About this article
Cite this article
Baran, H., Amann, G., Lubec, B. et al. Kynurenic Acid and Kynurenine Aminotransferase in Heart. Pediatr Res 41, 404–410 (1997). https://doi.org/10.1203/00006450-199703000-00017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199703000-00017