Abstract
It has long been widely acknowledged that ultraviolet (UV) light is an environment risk factor that can lead to cancer, particularly skin cancer. However, it is worth noting that UV radiation holds potential for cancer treatment as a relatively high-energy electromagnetic wave. With the help of nanomaterials, the role of UV radiation has caught increasing attention in cancer treatment. In this review, we briefly summarized types of UV-induced cancers, including malignant melanoma, squamous cell carcinoma, basal cell carcinoma, Merkel cell carcinoma. Importantly, we discussed the primary mechanisms underlying UV carcinogenesis, including mutations by DNA damage, immunosuppression, inflammation and epigenetic alterations. Historically limited by its shallow penetration depth, the introduction of nanomaterials has dramatically transformed the utilization of UV light in cancer treatment. The direct effect of UV light itself generally leads to the suppression of cancer cell growth and the initiation of apoptosis and ferroptosis. It can also be utilized to activate photosensitizers for reactive oxygen species (ROS) production, sensitize radiotherapy and achieve controlled drug release. Finally, we comprehensively weigh the significant risks and limitations associated with the therapeutic use of UV radiation. And the contradictory effect of UV exposure in promoting and inhibiting tumor has been discussed. This review provides clues for potential clinical therapy as well as future study directions in the UV radiation field. The precise delivery and control of UV light or nanomaterials and the wavelength as well as dose effects of UV light are needed for a thorough understanding of UV radiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the information from the World Health Organization (WHO), close to 20 million new cases of cancer occurred in the year 2022 paralleled by 9.7 million deaths due to it [1]. In recent years, the incidence of skin cancer, especially squamous cell carcinoma, has been reported to be increased significantly. Due to long-term exposure to the external environment, the skin is the most vulnerable organ of the human body after ultraviolet (UV) irradiation and it is reported that 90% of skin cancers are related to UV radiation damage. The significance of UV light or radiation as a pivotal contributor to cancer development is extensively acknowledged [2,3,4]. UV light is an electromagnetic wave separated as long-wave UVA (315–400 nm), mid-wave UVB (280–315 nm), and short-wave UVC (100–280 nm) [5]. The majority of the UV radiation reaching earth's surface is UVA, with a smaller proportion being UVB. In contrast, UVC is predominantly absorbed by the ozone layer located in the stratosphere (Fig. 1). UVA, accounting for 90%-95% of the total UV, has a high penetration ability. The radiation exposure extends into the papillary layer of the dermis, influencing cellular elements within the dermis and the subcutaneous region, such as fibroblasts, endothelial cells of blood vessels, and Langerhans cells, and stimulates the activity of matrix metalloproteinases (MMPs), leading to the breakdown of collagen and elastin fibers, which causes visible photoaging damage such as skin relaxation, sagging, and abnormal increase of wrinkles [6]. The content of UVB is low (5%~10% of the total UV) but it has high energy, which is mainly absorbed by the epidermis. The effects of UVB includes stratum corneum thickening, epidermal hyperplasia, melanocytes proliferation, induced DNA mutations and chromosomal variants in keratinocytes, which leads to cell carcinogenesis [7]. The shorter the wavelength of UV light, the higher its energy content. Usually, the production of vitamin D and endorphins could be stimulated mainly by UVB light [8, 9]. Nonetheless, prolonged and excessive exposure to UV light can have adverse effects on the skin, such as atrophy, dyspigmentation, wrinkles formation, and an elevated risk of malignant transformation. Previous research often portrays UV light as a harmful factor and therefore has proposed prevention and treatment strategies based on current understanding [2, 10,11,12,13].
The relationship between UV and skin. The three types of UV rays, primarily UVA and UVB, penetrate to different depths in the skin tissue. UVA can reach the dermis layer of the skin, while UVB is limited to the epidermis and the uppermost layer of the dermis. In normal, UVB boosts vitamin D3 and endorphin synthesis. However, excessive UV exposure can lead to the formation of cancers including melanoma, basal cell carcinoma, squamous cell carcinoma, and Meckel cell carcinoma. The box shows the different cell sources of these cancers
However, every coin has two sides. Sunlight has been applied to treat a variety of diseases since BC. Finsen won the Nobel Prize in Physiology and Medicine in 1903 for his success in treating disease with concentrated light irradiation, especially in treating cutaneous tuberculosis. In the 20th century, Goeckerman began to use high pressure mercury lamp combined with external application of coal tar to treat psoriasis and achieved good results, which was the beginning of modern phototherapy of UV radiation and also intriguingly presents a potential for its application in the treatment of cancer directly or indirectly [14].
Although the therapeutic use of UV light on cancer was often limited by its poor penetration depth in the past, these drawbacks are largely circumvented for the development of nanomaterials at present [15,16,17,18]. UV light itself usually functions by inhibiting cancer cell proliferation, inducing their apoptosis or ferroptosis, enhancing radiotherapy sensitivity, triggering chemical reactions to enable controlled-drug release and facilitating the creation of reactive oxygen species (ROS) in photodynamic therapy (PDT) [19,20,21,22,23]. On the other hand, UV light can serve as a stimulant to activate certain particles or molecules that produce novel effects and destroy cancer cells [24,25,26,27,28]. With the widespread appearance and promotion of nanomaterials, the role of UV radiation has caught increasing attention in cancer treatment.
In this review, we briefly introduce UV-induced cancers and mechanisms of carcinogenesis. Furthermore, we focus on summarizing the current applications of UV radiation, both in itself and as a stimulant in the treatment of cancer. In addition, given UV radiation's well-established role as a carcinogen, we comprehensively weigh the significant risks and limitations associated with the therapeutic use of UV radiation including potential long-term effects, the challenges of targeted delivery, and the risk of inducing secondary cancers. And the contradictory effect of UV exposure in promoting and inhibiting cancer has been discussed. It will assist researchers in understanding the dual relationship between UV radiation and cancer and developing more favorable cancer treatment strategies based on UV radiation.
Photocarcinogenesis: UV-induced cancers
A growing amount of research indicates that UV radiation exposure is linked to the emergence and growth of cancers in several body areas [4, 29,30,31,32,33,34]. Specially, skin cancer, encompassing both malignant melanoma and non-melanoma skin cancers (NMSCs), exhibits the strongest correlation with UV exposure (Fig. 1). Conditions are predominantly observed in individuals with lighter skin pigmentation and are especially prevalent in regions that receive frequent solar exposure, such as the head and neck [29, 35,36,37,38].
Malignant melanoma
Malignant melanoma is famous for its challenging responsiveness to therapeutic approaches and its propensity for metastatic dissemination with the ability to occur in different parts of the body [39,40,41]. Superficial spreading melanoma (SSM), a melanoma subtype closely linked to cumulative solar damage, initially presents its pathogenic effects within the superficial epidermal layers. Nevertheless, this neoplasm is distinguished by its rapid propensity to invade the dermis, subsequently infiltrates the lymphatic pathways and extends to distant organs [41, 42]. A positive association has been consistently observed between annual UV exposure and the incidence of melanoma. The substantial surge in melanoma cases affecting the trunk and upper limbs is likely a consequence of the growing prevalence of desultory, high-intensity recreational UV radiation exposure, such as sunbathing and the use of indoor tanning facilities [43].
Non-melanoma skin cancers
In the realm of NMSCs, squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) reign supreme, accounting for approximately 20 to 25 % and 75 to 80 % of cases respectively. Among the remaining cases, Merkel cell carcinoma (MCC) constitutes a rare yet significant proportion [44]. Despite the higher incidence rates of BCC and SCC, their impact on life-threatening potential is significantly less when compared to malignant melanoma. Investigations have revealed a significant link correlating the risk factors of BCC and SCC with various parameters of UV exposure including dosage, intensity, and the specific mode of radiation, underscoring the critical role of UV in the etiology of these malignancies. Furthermore, individual factors such as fair skin pigmentation often amplify one's susceptibility to UV radiation, which contribute to an individual's vulnerability to NMSCs [45, 46].
Squamous cell carcinoma
SCC arises from the squamous cells that form the outermost layers of the epidermis namely the epidermis's protective shell [45]. UVB radiation stands out as the paramount contributor to the etiology of cutaneous squamous cell carcinoma (cSCC) among environmental factors and accumulated total solar UV radiation exposure has been positively associated with an elevated incidence of cSCC, which has a significant impact on the molecular pathogenesis [47, 48]. Additionally, UV could perturb extracellular matrix (ECM) components such as metalloproteinases and collagen to promote the progression of cSCC [49]. It also synergizes with certain cutaneous human papillomavirus (HPV) types potentiating UVR-mediated damage at an initial stage of cSCC carcinogenesis [50]. Exposure to UV can cause mutations in individual keratinocytes and give these cells a survival advantage. With environmental selection, such keratinocytes gradually form many subclones in clinically normal appearing skin and can gradually develop polyclonal cSCC as genetic or epigenetic changes accumulate [51, 52]. Meanwhile, the progression of Actinic Keratosis (AK) is predominantly attributed to UV exposure, which is characterized by the presence of atypical cellular alterations within the epidermal keratinocytes and considered as a significant precursor lesion for SCC [53].
Basal cell carcinoma
BCC is the most common type of skin cancer in humans, originating from the cells that constitute the basal layer of the epidermis with clinical presentation associated with its subtype. UV radiation is primarily responsible for causing BCC, and the time gap between UV exposure and the onset of BCC can span several decades. Cumulative exposure to environmental UV radiation is associated with a rise in both the relative and absolute risks of BCC, peaking in the head and neck region, whereas intermittent intense UV exposure during childhood and adolescence such as outdoor activities is correlated with a significant increase in subsequent BCC risk in this age group [54]. Comparing those with little occupational UV exposure to those with high levels, the latter group had a significantly higher BCC risk [55].
Merkel cell carcinoma
Merkel cell carcinoma (MCC) emerges as a neuroendocrine skin cancer characterized by its rarity and preferential occurrence on the head and neck with high aggressiveness and mortality as well as a worse prognosis [56]. Recent evidence suggests that MCC is more likely to originate from dermal fibroblasts, epidermal keratinocytes, or immature totipotent stem cells that undergo neuroendocrine differentiation during the process of malignant transformation [57, 58]. UV light and human Merkel cell polyomavirus (MCPyV) are thought to be the main cause. Chronic exposure to sunlight can result in two main biological effects [59]. One is UV‑mediated DNA damage with mutational signatures that are characteristic of UV exposure, which is mainly found in MCPyV− MCCs in countries with high UV exposure. UV radiation specifically promotes the development of MCC by activating the Wnt signaling pathway and inducing the expression of MMPs. Furthermore, it contributes to the accumulation of mutations in genes critical for cell cycle regulation and neuroendocrine differentiation, such as TP53, RB1, AKT1, BCL-2, and c-MYC, thereby facilitating the promotion of carcinogenesis in MCC [57, 58]. On the other hand, UV radiation is implicated in the enhancement of MCPyV replication and its subsequent integration into the host genome through its induction of immunosuppression, thereby contributing to the pathogenesis of MCC [60].
Mechanism of carcinogenesis by UV
UV has been increasingly studied for its carcinogenic mechanisms because of its widespread impact and distribution. UV radiation has been shown to directly instigate DNA damage and indirectly produce ROS, which subsequently result in mutations. Concurrently, UV radiation possesses the capacity to modify the functions of immune cells through the mediation of inflammatory factors and pro-inflammatory agents. Epigenetic changes and inflammation are also involved in the malignant transformation process. More complicated, these primary mechanisms are known to interplay with one another (Fig. 2). This intricate interplay between UV radiation and skin biology emphasizes the crucial need for a deeper exploration of these mechanisms to guide the development of potent preventative and therapeutic measures.
Summary of the dual relationship between UV and tumor. Genetic mutations, immunosuppression, inflammation, epigenetic alterations, and their interplays are the main mechanisms and pathways for UV-induced cancer, which demonstrates role of UV in carcinogenesis. In addition, properties of UV light including inducing apoptosis, oxygen independent, matching photosensitizer, enough energy, catalytic property serve as the basis for its role in tumor therapy, which indicates its potential for tumor treatment
Mutations: directly or indirectly DNA damage
Direct DNA damage
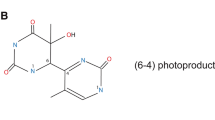
The bulk of direct DNA damage stem predominantly from UVB radiation, which produces multiple types of DNA damage such as DNA strand breaks, DNA adducts, DNA–protein crosslinks [61, 62]. Among the photoproducts after UV exposure, cyclobutane pyrimidine dimer (CPDs) and pyrimidine (6-4) pyrimidone photoproducts (6-4PPs) are the two most important (Fig. 3). The former arises from a [2+2] cycloaddition of the C5-C6 double bonds of adjacent pyrimidine bases and the latter arises from a [2+2] cycloaddition involving the C5-C6 double bond of the 5 '-end pyrimidine and the C4 carbonyl group of the 3′-end thymine [63]. However, the greatest interest with respect to UV light carcinogenesis has been in CPDs, which are the major DNA photoproducts formed following UV light and repaired in human skin primarily by nucleotide excision repair (NER) [62, 64,65,66]. Tommasi et al. has found methylation of DNA bases significantly increases their vulnerability to UV-induced lesions. When cells are irradiated with natural sunlight, CPDs formation significantly increased due to the presence of 5-methylcytosine bases [67]. It is reported that Poly(ADP-ribosyl)ation (PARylation) can occur in DDB1 through PARP1 thereby inhibiting DNA damage repair when DNA is damaged, which may be an important mechanism of DNA repair dysregulation under UV exposure [68].
UV-photoproducts and base mismatch. UV directly, or indirectly through ROS lead to the formation of DNA photoproducts. For example, based on thymine and guanine, DNA photoproducts that may lead to the formation of mutations mainly are (A) cyclobutane pyrimidine dimers (CPDs), (B) pyrimidine (6–4) pyrimidone photoproducts (6-4PPs), and (C) 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxo-dG). Specially, 8-oxo-dG can mismatch with adenine during DNA replication and result in G > T transversion during subsequent replication
Indirect DNA damage—Oxidative stress
Meanwhile, both types of UV radiation are considered to elicit epidermal cells damage and eventually induce cancer for genome damage caused by oxidative stress (the primary mechanism for UVA) [64, 69,70,71]. Chromophores resident within skin cells including DNA, tryptophan, and urocanic acid, act as receptors for UV photons and absorb them to generate ROS. After that, cell signaling pathways are activated through a key mediator namely fyn, a key constituent of the Src family of protein tyrosine kinases with pivotal role in modulation of signal transduction pathways [72]. These ROS possess the capacity to inflict oxidative damage upon proteins and DNA, thereby predisposing to base mispairing and the induction of genetic mutations. Furthermore, these ROS can significantly augment the glucose metabolism in melanoma cells [73, 74]. A notable consequence of DNA's exposure to ROS is the formation of oxidized guanosine namely 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxo-dG) (Fig. 3). It is recognized by 8-oxoguanine DNA glycosylase (OGG1), repaired by base excision repair (BER) and generated after UV exposure, which will pair with adenine during DNA replication resulting in G:C→T: A transversion mutations [75,76,77] (Fig. 3). When UVB-induced CPDs and 6-4PPs are incorrectly resolved or UVA-induced oxidation lesions are not repaired timely, mutations including C: G → T: A transition, CC:GG → TT: AA tandem mutations or A: T → T: A transversion will form [78,79,80]. These mutations called “UV signature mutations” could be detected in a variety of skin cancers and are used to identify UV-induced DNA damage [66, 81]. While ROS can indeed damage DNA, an effect that should not be ignored is the oxidation of critical intracellular proteins including nucleotide excision repair (NER) proteins, thereby increasing the possibility of mutations in genetic materials caused by UVB [82,83,84]. Interestingly, reducing oxidative stress levels can prevent UV-induced cellular oxidative death and carcinogenesis [85, 86].
Mutation genes
Finally, these changes will accumulate over time, which could cause high-mutational background in skin cells and overwhelms DNA repair mechanisms and further potentially contribute to the emergence as well as development of skin cancer [71, 87, 88]. Malignant melanoma, resulting from the intricate interaction of developmental signals and genetic events, is further aggravated by UV radiation [89,90,91]. Exposed to UV light, the MC1R-PTEN axis in melanocytes is considered an important response center. MC1R has been found to promote the repair of UV-induced DNA damage whereas MC1R variants exhibit defects in their interaction with PTEN upon UV exposure so that they fail to inhibit the PI3K/ AKT signaling pathway driving the carcinogenic transformation of melanoma [92]. In addition, homeostatic processes can be disturbed by some genetic events after UV exposure. Melanomagenesis can occur as a result of melanocyte stem cells (MCSCs) proliferation and migration to the epidermis where they differentiate into melanocytes mediated by the overexpression of High Mobility Group AT-Hook 2 (HMGA2) after UVB exposure [93]. Beyond that, BRAF, NRAS, and CDKN2A are the major mutation genes related to the occurrence of melanoma [94,95,96]. TP53/Trp53 gene frequently subjected to mutational events in skin carcinogenesis is hypothesized to constitute an early molecular vulnerability in the UV-radiation-driven carcinogenic cascade [65, 88, 97, 98]. It has been found that the UV-induced mutations of TP53 or PTCH where “UV signature mutations” usually exist have a potential acceleration of BRAF(V600E)-driven melanomagenesis and are strongly associated with the development of BCC [46, 99,100,101,102,103]. Keratinocytes carrying TP53 mutations can gain a growth advantage by resisting apoptosis, which allows them to eventually give rise to AK and SCC by clonal amplification. Sunlight-induced production of CPDs and the resulting decrease in DNA repair proficiency conspire to significantly modulate the TP53 mutation spectrum observed in skin malignancies [101, 104, 105]. Analysis of the mutational landscape across 13 oncogenic genes within a cohort of 57 BCCs revealed a high frequency of genetic alterations. Predominant mutations observed in PTCH1 and TP53 genes that were mutated in 71.9% and 45.6% of the BCCs respectively consistent with UV-induced fingerprint mutations characterized by C > T and CC > TT transitions [106]. Simultaneously, a multitude of well-documented melanoma driver mutations and other frequently observed mutations are found at sites of UV-induced CPDs in melanocytes [107]. According to a recent study by Gabriel et al., UV radiation facilitates blastic plasmacytoid dendritic cell neoplasm (BPDCN) by allowing plasmacytoid dendritic cells (pDCs) carrying potential pathogenic genes TET2 to acquire additional genetic mutations. Concurrently, BPDCN tumor cells or precancerous cells in patient skin significantly enrich for genetic mutation signatures associated with UV exposure [108].
Immunosuppression
Launch immunosuppressive molecular events
The immunosuppressive effects of UV exposure begin with early molecular events that induce downstream biological effects. The chromophores mainly including trans-UCA(urocanic acid) in the stratum corneum and nucleotide bases in epidermal keratinocytes or immune cells (such as Langerhans cells) can absorb energy and induce the corresponding cellular damage pathway when exposed to UV light [109]. After UVB reaches the skin, platelet-activating factors (PAFs) and platelet-activating factor receptor (PAF-R) agonists can generate to bind with PAF-R for the generation of UVB-mediated ROS and subcellular microvesicle particles (MVP) carrying the mediators with the ability of mediating systemic immunosuppression and thereby enhancing tumor growth are released [110]. Mast cells can be called from the skin to the draining lymph nodes by chemokine signaling due to PAF activation of their epigenetic alterations, where they secrete immunogenic cytokines and inhibit germinal center formation [111]. CPDs still play an important role in a series of cellular and molecular events related to immunosuppression. The reduction of CPDs in the epidermis could prevent the suppression of delayed and contact hypersensitivity reactions and systemic immunity caused by UV light [112]. The immunosuppressive mechanism of other molecule such as Tryptophan and 7-dehydrocholesterol has also been gradually clarified [109, 113, 114].
Downregulate the functionality of immune cells
In addition, UV exposure can suppress immune cell functions including immune response, induce them to exhibit an immunosuppressive state and expansion of regulatory T cells (Treg). Excessive UV irradiation, based on molecular perturbations and genetic lesions, impairs the homeostatic functionality of cutaneous immune cells. This includes a diminution in the phagocytic and chemotactic proficiency of neutrophils, alongside a decrement in their adhesive properties, an escalation in the apoptosis of peripheral blood mononuclear cells (PBMCs), and a marked attenuation of the antigen-presenting efficacy of human Langerhans and dermal dendritic cells (DCs) [115, 116]. UV light exposure has been shown to dysregulate T cell migration patterns by interfering with Sphingosine-1-phosphate (S1P) signaling and causes systemic immunosuppression contributing to the development of skin cancer [117]. Following UVB irradiation, CD11b+Langerin- DCs manifest an upregulation of CD86 expression accompanied by an enhancement of tolerogenic gene transcription linked to the functionality and proliferative capacity of Tregs [118]. UVB exposure also induces the skin infiltration of Gr-1+CD11b+ myeloid cell often referred to as myeloid-derived suppressor cells (MDSCs) that usually play an immunosuppressive role in inflammation and tumor microenvironment [119].
Stimulate the production of immunosuppressive factors
Meanwhile, production of factors with immunosuppressive effects can also be promoted by UV exposure. Chronic exposure to UV radiation induces the activation of select skin immune cell subsets such as retinoic acid receptor-related orphan receptor gamma t (RORγt)–differentiated innate lymphoid cells (ILCs) and T lymphocytes, resulting in a perturbation of local cytokine landscape characterized by an enrichment of IL-22 and IL-17A associated with the proliferation of mutated keratinocytes (KCs) [120]. A variety of mediators with immunosuppressive effects such as PGE2, cytokines including IL-6, 8, 10 and tumor necrosis factor-alpha (TNF-α) could be produced when PAFs and PAF-like ligands bind with PAF-R and induce downstream immunosuppressive biological effects [111].
Inflammation
Production of inflammatory mediators
Chronic inflammation is acknowledged as a pivotal factor that propels the transformation of epidermal cells and their progression towards malignancy [121, 122]. Prolonged or excessive exposure to UV radiation can lead to a state of chronic inflammation in the skin. This is due to the induction of DNA damage and alterations in the ECM by UV [123, 124]. Following UV radiation, many inflammatory mediators (such as inflammation-associated cytokines, chemokines, prostanoid-synthesizing enzyme COX-2 and lipid mediators) are released from cells residing in the skin, which is likely to be mediated by the UV-induced release of self-RNAs as a damage-associated molecular pattern dependent on Toll-like receptor 3 (TLR3) and Toll-like receptor adaptor molecule 1 (TRIF) from keratinocytes [104, 125, 126]. These mediators can activate the STAT3 and NF-κB signaling pathways closely related to carcinogenesis and development, thereby promoting tumor proliferation and anti-apoptotic capabilities [127,128,129]. Besides, the activation of the TGFβ1/SMAD3 signaling cascade by chronic UV exposure instigates photo-inflammation processes and bolsters malignant biological behaviors of skin cancer cells [130].
Activation of inflammasomes
UVB can also disrupt protein synthesis and induce DNA damage within cells, causing ribosomes to collide with other molecules in the cytoplasm and producing ribotoxic stress response (RSR), which initiates an activation of the NLRP1 inflammasomes facilitating the maturation and subsequent excretion of key pro-inflammatory cytokines (such as IL-1β, IL-18) that promote the development of skin cancer by enhancing inflammation and immune suppression [131, 132]. Further, long-term chronic NLRP1-dependent inflammation may increase a patient's risk of developing SCCs [133, 134].
Interestingly, male mice exhibited greater sensitivity to UV exposure, manifesting more severe inflammatory damages and an earlier onset of skin cancers [135]. This phenomenon suggested that gender may influence carcinogenesis due to different levels of UV-induced inflammatory response. From the different aspects, suppressing UV-induced inflammation was also accompanied by suppressing skin photocarcinogenesis [136]. Nonsteroidal anti-inflammatory drugs such as aspirin(ASA) are capable of delaying SCC onset induced by chronic UVB [11]. Genetic ablation of TNF-α in transgenic mouse models attenuated the progression of SCC subsequent to repetitive UVR treatment [137]. Collectively, these findings highlight the pivotal role of UV-induced inflammation in cancer development, emphasizing the need for further research to elucidate its mechanisms and potential therapeutic targets.
Epigenetic alterations
Cancer initiation and progression are caused not solely by UV-induced mutations in significant genes but also by the subsequent alterations in epigenetics, which result in their abnormal expression patterns conducive to cancer development and aggressive behavior. Increasing research has found accumulation of epigenetic changes can drive cancer development as well as progression and alterations in the epigenome are capable of inducing the emergence of cancer apart from genetic mutations [138,139,140]. Under constant exposure of UV radiation, the cutaneous layer demonstrates a propensity for epigenetic shifts, such as the methylation of genomic DNA, histone acetylation and the posttranscriptional governance of noncoding RNAs, which leads to skin cancer [141,142,143,144,145,146,147,148].
Methylated modification
UV radiation-induced alterations in DNA methylation encompasses both hypomethylation and hypermethylation affecting genomic stability, which could lead to the silencing of tumor suppressor genes or the upregulation of oncogenes [146]. The methylation signatures observed in AK exhibit canonical characteristics of oncogenic methylation patterns and demonstrate a certain resemblance to the epigenetic profiles of cSCC, which mainly showed typical features of stem cell methylomes [149]. Meanwhile, analysis of transcriptomic and epigenomic changes during different stages of UVB-induced skin cancer has found that differentially DNA methylated regions mostly located in the distal intergenic regions and the promoters varied over time. These changes in methylation levels were associated with different stages of skin cancer development and were more pronounced in the early stages [150]. Epigenetic modification of cytosine can also promote the formation of UV-indued CPDs, resulting in mutagenesis effects. For instance, cytosine methylation promotes increase of UVB-induced CPD formation nearly 2-fold relative to the unmethylated [151]. The highest levels of CPDs produced under UVB radiation near sequences containing 5-carboxylcytosine (5caC) produced by DNA methyltransferases and 5mC oxidases at CpG dinucleotides [152].
Histone modification
UV-induced epigenetic carcinogenesis also involves the regulation of chromatin structure through histone changes. Yao et al. found UV decreased global H3K27ac characterized as a crucial epigenetic hallmark for delineating super enhancer (SE) landscapes across promoter regions and genome-wide remodeling of H3K27ac distribution in response to increasing UV exposure was observed. Notably, an enrichment of genes linked to UV-elicited SEs was detected within cancer-related and DNA damage response pathways [141]. UV damage leads to rapid and significant decondensation of heterochromatin, primarily mediated by DNA damage binding protein 2 (DDB2), which facilitates the displacement of linker histones from chromatin. This instability of heterochromatin structure is closely associated with cancer development and could be partly repaired by histone methyltransferase SETDB1 that is specifically recruited to the UV-damaged heterochromatic regions and maintains the H3K9me3 modification on newly deposited H3 histones, helping to protect the cell from the effects of genomic instability [153].
Noncoding RNAs modification
In addition, the carcinogenic effects of UV radiation are mediated by epigenetic modifications that extend beyond the DNA to encompass RNA as well. Upon UVA radiation, melanocytes emit extracellular vesicles laden with diverse cellular components notably miR-21 mediating the signal transduction in the keratinocytes and leading to their increased proliferation and heightened anti-apoptotic mechanisms, which links to the development of skin cancer [154].UVB irradiation could reduce the expression of miR-137 that could inhibit the tumorigenic phenotype in human epidermal melanocyte cell and increase the expression of miR-29a-3p and miR-183-5p that could downregulate the phosphatase and tensin homolog (PTEN) involved in the metastatic progression of cSCC [155,156,157]. In the latest research findings, the m6A methylation modification mediated by the methyltransferase METTL14 can regulate the maturation process of miRNAs under UVB exposure, thereby controlling the photo-aging of skin fibroblasts [158]. Further, UV radiation triggers a miRNA signature in primary SCC cells that mirrors aspects of the metastatic phenotype, implying a role for UV-induced epigenetic modifications in both cancer initiation and subsequent metastatic spread [157].
As mentioned above, epigenetic modifications can induce the upregulation of chemokine genes, thereby facilitating mast cell migration to induce immunosuppression [159, 160]. However, epigenetic changes are reversible and can be reversed. Phytochemicals such as epigallocatechin gallate (EGCG) have shown potential in preventing the malignant conversion of UV-induced papilloma into carcinoma or reactivating tumor suppressor genes that have been epigenetically silenced by reversing UV-induced epigenetic modifications for its function in suppressing DNA methyltransferases (DNMTs) [161,162,163,164,165]. These findings suggest that epigenetic alterations could potentially serve as a viable target in strategies aimed at preventing UV-mediated carcinogenesis.
The role of UV in cancer treatment
While previous research has primarily focused on the intricate mechanisms underpinning UV-induced carcinogenesis and progression, UV light remains a paradoxical force (Fig. 2). This dual nature is particularly reflected in contemporary research where nanomaterials are extensively explored and UV-light is often harnessed as an activating energy source to eradicate cancer cells. Below, we delve into some of the prevalent scenarios where UV light finds widespread application in cancer therapy today (Fig. 4).
Summary of UV in cancer treatment. UV light can be generated by X-rays and near-infrared light through the excitation of scintillating nanoparticles (SCNPs) and upconversion nanoparticles (UCNPs), respectively, thereby circumventing their poor penetration drawbacks. In addition to its inherent ability to induce apoptosis in tumor cells, UV light can also be combined with nanomaterials to achieve various therapeutic effects in cancer treatment. These effects include synergistic actions with anti-cancer agents, radiosensitization, excitation of photosensitizers in photodynamic therapy, controlled drug release, induction of ferroptosis, and activation of novel particles or molecules
UV alone
UV alone can induce apoptosis and inhibit growth of a variety of tumor cells through different pathways depending on wave length, dose and cell lines with UVC irradiation at the highest frequency [166]. This effect has been observed in vitro in various tumor cell lines including human osteosarcoma cells, fibrosarcoma cells, glioblastoma cells, pancreatic cancer cells and colon cancer cells with EGFR signaling pathway being of great significance [166,167,168,169,170,171,172]. One possible mechanism is that UV damages DNA and induces cell cycle arrest followed by apoptosis [167]. Moreover, UVC radiation can affect the growth and spread of tumors by regulating cell-to-cell interactions in the tumor microenvironment. For instance, exposure to UVC radiation stimulates the secretion of many cytokines including TNF-α and IFN-γ of bone marrow-derived mesenchymal stromal cells (BM-MSCs), which collectively exert an inhibitory effect on the growth capacity of colorectal cancer (CRC) cells and induce their apoptosis [173]. The use of UVC in vivo experiments in cancer therapy has also been explored. UVC exposure not only suppressed cancer cells growth in nude mice with minimal residual cancer (MRC) but also appeared to be devoid of any significant side effects, which means the potential use of UVC following surgical resection in patients with MRC. Moreover, UVC exhibits greater efficacy in inhibiting cancer cell proliferation in 2D monolayer cultures, whereas UVB is more effective in reducing cancer growth in a skin-flap mouse model implanted with tumor cells. Additionally, in an experimental brain metastasis model using Lewis lung carcinoma (LLC), UVC irradiation was delivered through a craniotomy open window successfully and induced apoptosis in cancer cells. This UVC irradiation was particularly effective against LLC and significantly prolonged the survival of mice with brain metastasis. In clinical trials, near-ultraviolet light (NUV) has been used for nerve visualization in surgery and shown beneficial effects within the surgical field [174]. Clinically, similar to the carcinogenesis mechanism discussed above, UV alone plays a role mainly by inducing T cell apoptosis and regulating immune and inflammatory responses as a kind of physiotherapy for cutaneous T-cell lymphomas (CTCL), which is a relatively superficial skin tumor. The results obtained emphasize the capacity of UV light to combat cancer, specifically in the eradication of superficial tumors, thus introducing a prospective strategy in cancer management [166, 175, 176].
Synergistic with anti-cancer agents
Further, UVC combined with anti-cancer agents may produce synergistic effects. Kawaguchi et al. reported that small dosages of UVC and cisplatin together have a synergistic against cancer impact by suppressing receptor tyrosine kinases (RTK), offering a unique therapy option for attempts to treat colorectal cancer [177]. Chang et al. has undertaken an extensive exploration in this field. Their team reported that the combined treatment of anti-oral cancer natural products with UVC radiation has enhanced the inhibition of cells proliferation compared to individual treatments alone and promoted apoptosis of oral cancer cells by enhancing ROS generation, cell cycle disturbance, mitochondrial dysfunction and DNA damage [178,179,180,181,182,183,184]. The relevant contents are summarized in Table 1. In general, UVC has a certain clinical translational application prospect in combined therapy [185].
Radiosensitization
Many tumors exhibit radiotherapy resistance due to their hypoxic characteristics so enhancing radiotherapy sensitivity has become an urgent problem [188, 189]. Unlike X-ray, UVC photons can damage genetic material without much reliance on oxygen, thereby enhancing radiotherapy sensitivity in tumors with hypoxic characteristics [190]. Interestingly, scintillating nanoparticles (SCNPs) namely low-conversion nanoparticles with the ability to absorb X-rays and convert them into lower energetic UVC photons have been explored in radiation therapy. Due to the substantially higher X-ray absorption cross-sections of inner-shell electrons relative to those of outer-shell electrons, elements with high atomic numbers, particularly transition metals and heavy metals which possess a significant number of inner-shell electrons, are promising candidates for the development of nanoscintillators. Lutetium phosphate doped lanthanide elements such as Tb3+ or Ce3+, for example LuPO4:Pr3+, are the very ideal material with high values of atomic number, emission intensity and appropriate emission spectrum [191]. Müller et al. has reported that combining LuPO4:Pr3+ with the ability to convert X-rays into UVC radiation due to the inter-configurational 4f15d1-4f transition in Pr3+ with traditional X-ray treatment could result in a significant viability loss in hypoxic tumor areas compared to radiation alone and a significant formation of UV-specific DNA damage namely CPDs can be detected. This novel material can utilize UV radiation to reduce the ability of tumor cells to resist radiation [192]. At the same time, it helps to reduce the radiation dose while maintaining the same tumor control rate from the perspective of radiotherapy [191, 193].
Excit photosensitizers in PDT
Harnessing the relatively high energy of UV light, researchers have explored its applications in combination with materials to activate photosensitizers to generate ROS in PDT that triggers downstream effects upon most occasions. PDT possesses the characteristics of non-invasiveness, excellent tumor selectivity, easy operation and few side effects. The three primary elements of PDT are photosensitizer (PS), light, and tissue oxygen [194]. When the photosensitizers are ingested by tumor cells with high metabolic activity, ROS will be produced and cause tumor cell apoptosis, necrosis or autophagy after irradiation with an appropriate wavelength light source [195, 196]. Although UV wavelengths match the absorption wavelength of many photosensitizers, it simultaneously poses several challenges for UV to excite materials directly for therapy as mentioned previously for prolonged UV exposure potentially causing DNA damage, immunosuppression, inflammation, photoaging and other harmful effects and finally leading to photocarcinogenesis. Moreover, the limited penetration depth of UV light restricts its reach to tissues at depths of approximately 100 μm [197]. Thus avoiding UV light's unsatisfactory penetration capacity while making use of its excitation property is a pressing concern. Intriguingly, scintillating nanoparticles and upconversion nanoparticles (UCNPs), which are typically activated by X-rays and near-infrared light (NIR) respectively, serve precisely this function. These two different forms of PDT excitation are called X-PDT and NIR-PDT respectively. These materials both avoid the low penetration of UV and take advantage of its excitation power in a transformational manner. The former has been described in the previous section. The latter has the ability to convert two or more low-energy pump photons from the near-infrared spectrum into a higher-energy UV photon with a shorter wavelength if it has been doped with appropriate type and amount of ions like Ce3+, which could better match the absorption band of the selected photosensitizers [198, 199]. In order to understand the conversion generation process of UV photons, it is also necessary to understand the structure of UCNPs. UCNPs typically incorporate dopant ions such as the activator like Tm3+ and sensitizers like Ce3+or Yb3+ within a host lattice commonly NaYF4 or NaGdF4, which are integral to the UCNPs' functionality. The activator ions are primarily responsible for the upconversion whereas sensitizers enhance the efficiency of this process. Upon NIR irradiation, sensitizers facilitate energy transfer to the activators, which promotes their excitation to a higher energy state and enables the emission of upconversion luminescence. This synergistic interaction between sensitizers and activators is essential for the realization of efficient upconversion luminescence in UCNPs [200]. Upon UV light, compatible photosensitizers notably TiO2 are energized, triggering the promotion of electrons from their valence band to the conduction band, resulting in the formation of highly reactive electron-hole (e-/h+) pairs [201, 202]. These electron-hole pairs can subsequently engage in reduction or oxidation reactions with chemical species present on the TiO2 surface and lead to the production of various cytotoxic ROS such as superoxide anions, which exhibit potential for cancer cell eradication in PDT [203,204,205,206]. In parallel, Cu-Cy nanoparticles exhibited a pronounced generation of ROS predominantly in the form of singlet oxygen (1O2) offering compelling substantiation for their promising role as potent ROS generators capable of eliminating cancer cell [207, 208]. To sum up, the application of SCNPs and UCNPs has indirectly made it possible for UV to be used in PDT for treatment of deep cancers. Some typical X-PDT applications together with UV sensitization are summarized in Table 2.
Controlled drug release
Selective and efficacious drug delivery systems significantly augment the efficacy of cancer treatment strategies. However, the traditional chemotherapy drugs commonly used in clinical practice are not selective to target tissues and organs so that treatment may require large amounts of drugs with many side effects. A selective method of delivering medication to certain diseased tissues makes use of drug carrier systems, which are often based on materials on the nanoscale [216]. Nanocarriers are taken in by diseased tissues through passive or active targeting strategies in which process achieving a controlled release of therapeutic drugs at precise sites and times is imperative for maximizing the therapeutic impact while minimizing side effects [217]. Synthetic stimuli-responsive biomaterials can change its physical and chemical properties under internal and external stimuli to achieve controlled drug release. Among various stimulation types, light stimulation has attracted considerable interest due to its noninvasiveness and precise temporal and spatial control [218, 219]. UV light stands as the preeminent light source in photo-controlled drug delivery systems attributed to its ample energy that elicits structural transformations in a wide array of light-responsive materials, which mainly includes three types namely cleavage, isomerization, cross-linking/decross-linking reactions, and then therapeutic drugs are released [22, 217, 220]. Regarding the field of precision medicine, central to promising UV-controlled drug release lies the utilization of UV-responsive photoremovable protecting groups (PPGs) such as nitrobenzyl (NB), coumarin (CM), azobenzene (AZO), spiropyran (SP) derivatives [22, 218, 221, 222]. These moieties serve as bridges between the therapeutic payload and the carrier, ensuring that the drug remains inactive during circulation. The application of UV light to PPGs within the tumor microenvironment elicits sophisticated structural transformations, achieving the precisely timed and spatially controlled release of drug. Coumarin and cinnamates are capable of undergoing photo-induced cross-linking reactions through [2π + 2π]-cycloaddition when exposed to UV light while they undergo decross-linking reactions when irradiated with light of a shorter wavelength [223]. Similarly, the hydrophobic moiety SP can isomerize into the hydrophilic conformation of merocyanine (MC) by UV to break intramolecular carbon–oxygen bonds [224].
This property of UV light has been harnessed to facilitate its application in targeted cancer therapy. The application of UV photo-control technology in antibody-drug conjugates (ADC) achieves the harmonious unification of targeting effects and highly spatiotemporal controllability in drug release. Li et al. reported a novel ADC in which UV has been used to cleave o-nitrobenzyl structure in its linker. Upon exposure to UV light, the ADC swiftly liberated cytotoxins, effectively exerting profound cytotoxicity against drug-resistant tumor cells. More than that, the UV-activated ADC exhibited a targeting efficacy equivalent to the native antibody, coupled with enhanced stability and photoresponsive properties [23]. According to Wang et al., coumarin-anchored low generation dendrimers were assembled. The coumarin substitutes were cross-linked with one another at 365 nm UV irradiation while the cross-linked assemblies broke down at 254 nm UV irradiation. This nanoparticle loaded 5-fluorouracil and a therapeutic gene encoding tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) also demonstrated complementary anti-cancer efficacy under UV exposure [225].
Induce ferroptosis
The exploitation of ferroptosis as a means to suppress tumor growth has garnered significant attention, surfacing as a burgeoning therapeutic approach for the management of treatment-resistant cancers [226,227,228,229]. It has been reported that UVB induces keratinocytes to accumulate oxygenated phospholipids specific to ferroptosis. Additionally, UVB causes a significant decrease in intracellular glutathione (GSH) levels and the expression of glutathione peroxidase 4 (GPX4), which fuels photoaging and contributes to keratinocyte death through ferroptosis [20, 230,231,232]. Interestingly, this process may be regulated by circular RNA and autophagy [233, 234]. Concurrently, the utilization of liproxstatin-1, an inhibitor of ferroptosis, has been effective in minimizing UV-induced cutaneous damage [235].
Correspondingly, glioblastoma and breast cancer development could be inhibited by this property of UV radiation. When UV radiation from NIR excitation of UCNPs causes oxidative stress, GSH synthesis is consumed and inhibited, reducing the antioxidant capacity of the cells and encouraging lipid peroxidation events [236,237,238,239]. Besides, UV light prompts the reduction of intracellular Fe3+ to Fe2+, a catalyst for the Fenton reaction, which generates hydroxyl radicals (OH•) from H2O2 substrates and subsequently attacks the lipids of the cell membrane and initiates lipid peroxidation. In addition, the UV light from UCNPs can also directly photolyze H2O2 to produce •OH, increasing the generation of ROS and further promoting ferroptosis [240] (Fig. 5). Several animal experiments have further confirmed this result, showing that UV radiation emitted by the material induces ferroptosis and significantly inhibiting tumor growth as well as extending the survival time of animals [237,238,239,240].
Summary of UV induced ferroptosis. During the PDT process, intracellular UV radiation can trigger ferroptosis. UV light emanating from excited SCNPs and UCNPs can reduce Fe3+ to Fe2+ within the cell, and then by Fenton reactions or directly catalyzing H2O2 into ·OH radicals, which lead to lipid peroxidation and finally ferroptosis. Additionally, the production of Fe2+ is accompanied by the depletion of GSH and a reduction in the activity of GPX4, which can further promote lipid peroxidation, ultimately leading to ferroptosis in cancer cells as well
Stimulate novel particles or molecules
UV light could act as a high-energy stimulator to stimulate specific particles and molecules to eliminate cancer cells. Metal nanoparticles composed of multiple precious metals, which can selectively target cancer cells, exhibit a characteristic to be activated by ionizing radiation to generate high-energy photons that produce charged particles and free radicals in tissues and thus damage cancer cells. Nevertheless, conventional X-ray radiotherapy often causes irreparable damage to healthy cells along with cancer cells due to its high photon energy. UV light could be designed as a relatively safer approach by activating the nanoparticles within cancer cells without causing irreparable biological damage to normal tissues. When metal nanoparticles were irradiated with UV light in the wavelength range of 100-180 nm, the kinetic energy of the photoelectrons exceeded the minimum energy of 4.52 eV required for H-bond dissociation. Furthermore, irradiation with UV radiation in the range of 100-150 nm could achieve even higher energy levels of 6-7 eV that is sufficient to disrupt DNA double-strands and destroy cancer cell organelles. Notably, just one or two nanoparticles delivered to cancer cells are sufficient to generate numerous photoelectrons [241, 242]. Moreover, infiltrating the cancer cell membrane to facilitate targeted drug delivery and elicit anti-tumor responses remains a pivotal objective in cancer therapeutics. In 2017, García-López et al. used molecular machines (or molecular nanomachines) to drill through the lipid bilayers of cellular membranes and kill tumor cells by activating molecular motors with UV light to generate nanomechanical action. The UV light activated the smaller rotor part of the molecule, which together with the larger stator part formed this novel molecule. Additionally, this molecule can target tumor cells through specific cell surface recognition sites [24, 26, 27, 243].
However, it is necessary to conduct further animal-based studies to corroborate these results and ensure their efficacy and safety profile for future human applications. But in general, it holds promise for further development and application of UV radiation.
Preclinical and clinical application and their limitations of UV radiation
UV light is a double-edged sword for cancer. UV light can cause skin cancer, while it can also synthesis some beneficial substances such as Vitamin D3 and can be used as an adjuvant therapy of treating tumor. In the early days, researchers often considered UV light a risk factor. Now UV light could also be used for phototherapy and play an auxiliary role in many aspects of tumor treatment.
For directly, UV therapy is a clinical treatment method used for cutaneous T-cell lymphomas (CTCL), especially for Mycosis Fungoides (MF) and Sézary Syndrome (SS) [244,245,246]. The majority of patients can experience remission from MF with phototherapy using UVA light in conjunction with 8-methoxypsoralen (PUVA) and narrow-band UVB light (NB-UVB). Extracorporeal photochemotherapy (ECP) works by exposing peripheral blood to 8-methoxypsoralen and UVA, and then re-infusing it back into the body to exert its therapeutic effect, which is also a kind of phototherapy method.
PUVA is primarily utilized for patients with deeper pigmentation and for higher-level phases [247, 248]. It is often combined with systemic active drugs and the most commonly used are interferon-alpha (IFN-α) and retinoids (isotretinoin). On a different note, NB-UVB therapy is primarily used to treat early MF and can be taken alone with a decreased risk [249]. However, for patients who are resistant to NB-UVB and have thick plaques, PUVA may be still essential [245]. However, there are few CTCL cases, and long-term reliable follow-up data are key to further determining the UV treatment methods. Simultaneously, it is essential to recognize that the restricted penetration of UV may be a key determinant in its restricted application, suggesting its suitability primarily for treating tumors confined to the body's surface. Considering the widely proven carcinogenic properties of UV, the evaluation of the safety of direct use of UV in the treatment of skin diseases and the risk of new skin cancers is very valuable. According to the retrospective risk assessment study and Meta-analysis of the use of UV for other diseases, which included a larger number of clinical patients, it was found that UV phototherapy did not increase the risk of skin cancer [250,251,252]. However, due to the limitations of the type of direct application of UV to tumor therapy, there have not been large-scale studies on the risks and safety of this type of phototherapy for skin tumors. Larger prospective studies and ongoing risk assessment in tumor will help further evaluate its safety. To enhance therapeutic benefits, more precise adjustments to therapy periods, dosage management, duration, and continuation would be needed.
For indirectly, UV radiation produced by excitation during PDT is used in the treatment areas of tumors mentioned above that, which type of tumor can be much deeper than direct-applied approaches. It must be considered that current research on generating UV for tumor treatment by exciting materials is still mainly focused on in vitro cell experiments and small animal models, and its potential for clinical translation, long-term safety, and effectiveness in larger animals or humans has not yet been verified [236]. Moreover, although small animal experiments have shown certain biocompatibility, the safety and potential side effects of using UV through these nanoparticles in the body over the long term still require further research [240]. Although several researches have evaluated the characteristics of in vivo metabolic kinetics, material clearance, tissue accumulation, and potential toxicity of materials used to induce UV, the boundary between the impact of different doses of nanoparticles on tumor growth through UV generation and the possibility of tissue damage also needs further demonstration [236, 238, 240].
As it is essential to consider that these nanoparticles did not cause significant damage to the normal tissues of mice, above all studies demonstrated acceptable biocompatibility and safety in cell or small animal models [236,237,238,239,240]. The fact that the excitation light source NIR is administered solely in the tumor region and that the nanoparticles generally have passive tumor targeting due to their enhanced permeability and retention effect suggests that there may not be as much of an impact on the surrounding normal tissues. Additionally, researchers have discovered that there is little to no heat produced when the material is excited by a laser [237, 240]. Although UV light is generally damaging to biological tissues, the UV light is generated internally within the nanoparticles from NIR light OR X-ray and this localized UV light production reduces the potential damage to surrounding normal tissues and avoid secondary carcinogenesis under the conditions as far as possible [239, 240].
Meanwhile, in order to take advantage of some of the characteristics of UV, PDT is often necessary. However, the significant risks and limitations associated with the therapeutic use of PDT have been appeared, most of which is mainly in the basic research stage. The existing clinical practice is to induce intracellular production of UV through PDT for the treatment of precancerous lesions and tumors. However, the clinical application of PDT is limited by light intensity, photoinitiators and tissue oxygen content [253]. For example, precancerous lesions and tumors of the oral mucosa are most often found on the tongue, palate, and buccal region. These areas are usually not effective for direct light sources thus diminishing the efficacy of PDT. Therefore, multiple phototherapy sessions are often required to minimize the risk of recurrence. In this process, PDT generates large amounts of ROS when it catalyzes the production of intracellular UV. Meanwhile, ROS induced by PDT may be toxic to normal cells, which may cause damage to normal tissue [254]. Photoinitiators also are the key factor for PDT effect to antitumor therapy. Traditional photoinitiators have the defects of poor water solubility and relatively poor initiation effect. Based on this, the researchers developed a series of novel photoinitiators such as UCNPs or SCNPs. However, as mentioned above, the degradation properties and biocompatibility of these nanomaterials need further consideration. Higher concentrations of nanomaterials can be significantly toxic to normal cells. What's more, PDT is dependent on the oxygen content of the tissue and requires oxygen as a supply to participate in ROS formation. However, the tumor microenvironment exhibits a state of hypoxia, which may be a limiting factor for PDT application. To solve these problems, researches developed to reconstruct the oxygen supplyment in the tumor microenvironment are needed.
UV radiation is most commonly used to trigger photocatalytic reactions, but the application of UV light is limited by low penetration depth. Apart from these, the followings should be unresolved in the future: Is it feasible to disrupt the traditional ROS pathway to prevent further exacerbation of hypoxia within the tumor microenvironment? Can adequate ROS be synthesized within the organism's reductive setting and directed towards DNA in tumor cells? How to accurately explain the process of photocatalytic therapy in vivo, and can the effects of photocatalytic materials be intuitively seen in vivo by means of in situ characterization? How can the toxicity of photocatalytic materials and the consumption of drugs due to metabolism or immunity be controlled?
Conclusion and perspectives
Materials science has revolutionized the narrative of UV light, shifting from a historical perspective of harm to a modern horizon of therapeutic promise in cancer. Among the spectrum of UV light, UVC and UVB stand out as the most promising candidate for directly cancer treatment applications whereas UVA often serves as a stimulating factor for cancer treatment in conjunction with other materials. As new materials continue to surface, the exploration of UV light's utility in cancer therapy is set to expand, unveiling new avenues for research and cancer therapy. Nonetheless, we still lack innovative research models connecting basic research and clinical application, developing translational medicine and promoting the transformation of achievements. Theoretically, these following unresolved issues seem worthy of further exploration:
Since UV can be used directly or indirectly in cancer treatment, more studies of the dose-response relationship between UV and tumor development may help clarify the boundary between prospect and risk. It is also worth exploring whether this kind of intracellular UV radiation has different effects on tumor development than that exposed to extracellular under other similar conditions. Most malignant tumors are located deep in the body rather than on the surface. Although NIR light can improve the treatment depth, it cannot accurately locate the tumor. Advancing the application of UV light in tumor therapies necessitates further exploration on overcoming the constraints on light penetration, achieving selective targeting of tumors with photosensitizers and optimizing photo-response efficiency, which demands precision from both the UV and material perspectives. Optimizing the performance of UV radiation also necessitates the delicate manipulation of its temporal and spatial characteristics. Precise and accurate control of its application to diseased areas while protecting surrounding healthy tissue is also one of the directions and challenges for future research. Exploration is needed on the precise delivery of nanomaterials and the wavelength as well as dose effects of UV light for the dual effect of UV on tumor.
It is likely to start with the precise control of the light source. Digitalization and computer-controlled technology may assist to tackle these problems by automating the setting of UV light exposure time and irradiation dosage. It utilizes an integrated camera system and software algorithms to identify the contours of lesions, accurately projecting therapeutic light onto the affected areas. Through programming, it can systematically vary the light intensity to improve the efficacy of phototherapy [255, 256]. In addition, the local implantable ionic wireless light delivery device can also achieve precise control of the light source, but the disadvantage is that it requires surgical implantation of the device [257]. For materials that are excited to produce UV radiation, their surfaces can be modified to target the tumor microenvironment or specific antigens on the tumor cell surface to improve their delivery accuracy [258]. The effectiveness of indirect UV treatment based on materials should also consider the biocompatibility of the "all-in-one" strategy and the factors such as the loading efficiency of phototherapy agents, the sequence of stimulus response release, and the complexity of the environment where the stimulus factors are placed. On the one hand, it is necessary to find more suitable phototherapy agents. On the other hand, it is not entirely dependent on the idea of "assembly", but more necessary to go into the photo-response mechanism and develop new photo-response materials. Besides, the introduction of in vivo imaging such as fluorescence in phototherapy or multi-modality medical imaging including MRI, CT, PET can also determine the location and morphology of tumor lesions and monitor the distribution of therapeutic agents to produce intracellular UV radiation, so as to realize the integration of diagnosis and treatment [259, 260].
In general, UV directly or indirectly, alone or combined materials show certain application prospects in tumor therapy in the current research. However, its wide application also faces many challenges. The type of tumor that can be treated directly with UV is limited and exploration of clinical translation of the indirect application about UV in tumor treatment based on materials is still shallow. Long-term safety data of above materials in vivo remains further study. In addition, the ethical implications of using UV radiation directly or indirectly in therapeutic contexts, particularly in terms of patient safety and informed consent, should be concerned. And the practical challenges of translating these therapies from research to clinical practice, such as the development of safe delivery systems, dose optimization, and patient-specific factors, should be adequately addressed, as they are often significant barriers to the successful implementation of new therapies from bench to bedside.
Availability of data and materials
Not applicable.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024. https://doi.org/10.3322/caac.21834.
Roh E, Han Y, Reddy K, Zykova TA, Lee MH, Yao K, et al. Suppression of the solar ultraviolet-induced skin carcinogenesis by TOPK inhibitor HI-TOPK-032. Oncogene. 2020;39(21):4170–82. https://doi.org/10.1038/s41388-020-1286-4.
Sivapragasam S, Stark B, Albrecht AV, Bohm KA, Mao P, Emehiser RG, et al. CTCF binding modulates UV damage formation to promote mutation hot spots in melanoma. Embo j. 2021;40(20):e107795. https://doi.org/10.15252/embj.2021107795.
Wittlich M, Westerhausen S, Strehl B, Versteeg H, Stöppelmann W. The GENESIS-UV study on ultraviolet radiation exposure levels in 250 occupations to foster epidemiological and legislative efforts to combat nonmelanoma skin cancer. Br J Dermatol. 2023;188(3):350–60. https://doi.org/10.1093/bjd/ljac093.
Bai Y, Zhao Y, Li Y, Xu J, Fu X, Gao X, et al. UV-shielding alginate films crosslinked with Fe(3+) containing EDTA. Carbohydr Polym. 2020;239:115480. https://doi.org/10.1016/j.carbpol.2019.115480.
Deuschle V, Brusco I, Piana M, Faccin H, de Carvalho LM, Oliveira SM, et al. Persea americana Mill. crude extract exhibits antinociceptive effect on UVB radiation-induced skin injury in mice. Inflammopharmacology. 2019;27(2):323–38. https://doi.org/10.1007/s10787-018-0441-9.
Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br J Dermatol. 2007;157(5):874–87. https://doi.org/10.1111/j.1365-2133.2007.08108.x.
Young AR, Morgan KA, Harrison GI, Lawrence KP, Petersen B, Wulf HC, et al. A revised action spectrum for vitamin D synthesis by suberythemal UV radiation exposure in humans in vivo. Proc Natl Acad Sci U S A. 2021;118(40). https://doi.org/10.1073/pnas.2015867118.
Kim H-S, Kim H-J, Hong Y-D, Son ED, Cho S-Y. β-endorphin suppresses ultraviolet B irradiation-induced epidermal barrier damage by regulating inflammation-dependent mTORC1 signaling. Sci Rep. 2023;13(1):22357. https://doi.org/10.1038/s41598-023-49886-5.
Roh E, Lee MH, Zykova TA, Zhu F, Nadas J, Kim HG, et al. Targeting PRPK and TOPK for skin cancer prevention and therapy. Oncogene. 2018;37(42):5633–47. https://doi.org/10.1038/s41388-018-0350-9.
Rahman H, Kumar D, Liu T, Okwundu N, Lum D, Florell SR, et al. Aspirin Protects Melanocytes and Keratinocytes against UVB-Induced DNA Damage In Vivo. J Invest Dermatol. 2021;141(1):132–41.e3. https://doi.org/10.1016/j.jid.2020.06.003.
Rahman H, Liu T, Askaryar S, Grossman D. Aspirin Protects against UVB-Induced DNA Damage through Activation of AMP Kinase. J Invest Dermatol. 2023;143(1):154–62.e3. https://doi.org/10.1016/j.jid.2022.07.011.
Atigadda VR, Kashyap MP, Yang Z, Chattopadhyay D, Melo N, Sinha R, et al. Conformationally defined rexinoids for the prevention of inflammation and nonmelanoma skin cancers. J Med Chem. 2022;65(21):14409–23. https://doi.org/10.1021/acs.jmedchem.2c00735.
Marsico AR, Eaglstein WH, Weinstein GD. Ultraviolet light and tar in the Goeckerman treatment of psoriasis. Arch Dermatol. 1976;112(9):1249–50.
Hou Z, Zhang Y, Deng K, Chen Y, Li X, Deng X, et al. UV-emitting upconversion-based TiO2 photosensitizing nanoplatform: near-infrared light mediated in vivo photodynamic therapy via mitochondria-involved apoptosis pathway. ACS Nano. 2015;9(3):2584–99. https://doi.org/10.1021/nn506107c.
Liu C, Zhang Y, Liu M, Chen Z, Lin Y, Li W, et al. A NIR-controlled cage mimicking system for hydrophobic drug mediated cancer therapy. Biomaterials. 2017;139:151–62. https://doi.org/10.1016/j.biomaterials.2017.06.008.
Wang S, Bi A, Zeng W, Cheng Z. Upconversion nanocomposites for photo-based cancer theranostics. J Mater Chem B. 2016;4(32):5331–48. https://doi.org/10.1039/c6tb00709k.
Chu H, Zhao J, Mi Y, Di Z, Li L. NIR-light-mediated spatially selective triggering of anti-tumor immunity via upconversion nanoparticle-based immunodevices. Nat Commun. 2019;10(1):2839. https://doi.org/10.1038/s41467-019-10847-0.
Dong S, Xu J, Jia T, Xu M, Zhong C, Yang G, et al. Upconversion-mediated ZnFe(2)O(4) nanoplatform for NIR-enhanced chemodynamic and photodynamic therapy. Chem Sci. 2019;10(15):4259–71. https://doi.org/10.1039/c9sc00387h.
Vats K, Kruglov O, Mizes A, Samovich SN, Amoscato AA, Tyurin VA, et al. Keratinocyte death by ferroptosis initiates skin inflammation after UVB exposure. Redox Biol. 2021;47:102143. https://doi.org/10.1016/j.redox.2021.102143.
Al-Nemrawi N, Hameedat F, Al-Husein B, Nimrawi S. Photolytic Controlled Release Formulation of Methotrexate Loaded in Chitosan/TiO(2) Nanoparticles for Breast Cancer. Pharmaceuticals (Basel). 2022;15(2). https://doi.org/10.3390/ph15020149.
Zhao W, Zhao Y, Wang Q, Liu T, Sun J, Zhang R. Remote light-responsive nanocarriers for controlled drug delivery: advances and perspectives. Small. 2019;15(45):e1903060. https://doi.org/10.1002/smll.201903060.
Li J, Xiao D, Xie F, Li W, Zhao L, Sun W, et al. Novel antibody-drug conjugate with UV-controlled cleavage mechanism for cytotoxin release. Bioorg Chem. 2021;111:104475. https://doi.org/10.1016/j.bioorg.2020.104475.
Gunasekera RS, Galbadage T, Ayala-Orozco C, Liu D, García-López V, Troutman BE, et al. Molecular Nanomachines Can Destroy Tissue or Kill Multicellular Eukaryotes. ACS Appl Mater Interfaces. 2020;12(12):13657–70. https://doi.org/10.1021/acsami.9b22595.
Ayala Orozco C, Liu D, Li Y, Alemany LB, Pal R, Krishnan S, et al. Visible-light-activated molecular nanomachines kill pancreatic cancer cells. ACS Appl Mater Interfaces. 2020;12(1):410–7. https://doi.org/10.1021/acsami.9b21497.
Liu D, García-López V, Gunasekera RS, Greer Nilewski L, Alemany LB, Aliyan A, et al. Near-infrared light activates molecular nanomachines to drill into and kill cells. ACS Nano. 2019;13(6):6813–23. https://doi.org/10.1021/acsnano.9b01556.
García-López V, Chen F, Nilewski LG, Duret G, Aliyan A, Kolomeisky AB, et al. Molecular machines open cell membranes. Nature. 2017;548(7669):567–72. https://doi.org/10.1038/nature23657.
Letfullin RR, George TF. Nanotherapy of cancer by photoelectrons emitted from the surface of nanoparticles exposed to nonionizing ultraviolet radiation. Nanomedicine (Lond). 2017;12(10):1107–17. https://doi.org/10.2217/nnm-2017-0053.
Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63(1–3):8–18. https://doi.org/10.1016/s1011-1344(01)00198-1.
Augustin J, Kis A, Sorbe C, Schäfer I, Augustin M. Epidemiology of skin cancer in the German population: impact of socioeconomic and geographic factors. J Eur Acad Dermatol Venereol. 2018;32(11):1906–13. https://doi.org/10.1111/jdv.14990.
Mai ZM, Sargen MR, Curtis RE, Pfeiffer RM, Tucker MA, Cahoon EK. Ambient ultraviolet radiation and major salivary gland cancer in the United States. J Am Acad Dermatol. 2020;83(6):1775–7. https://doi.org/10.1016/j.jaad.2020.03.077.
Xiang F, Lucas R, Hales S, Neale R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978–2012: empirical relationships. JAMA Dermatol. 2014;150(10):1063–71. https://doi.org/10.1001/jamadermatol.2014.762.
VoPham T, Bertrand KA, Yuan JM, Tamimi RM, Hart JE, Laden F. Ambient ultraviolet radiation exposure and hepatocellular carcinoma incidence in the United States. Environ Health. 2017;16(1):89. https://doi.org/10.1186/s12940-017-0299-0.
Pega F, Momen NC, Streicher KN, Leon-Roux M, Neupane S, Schubauer-Berigan MK, et al. Global, regional and national burdens of non-melanoma skin cancer attributable to occupational exposure to solar ultraviolet radiation for 183 countries, 2000–2019: A systematic analysis from the WHO/ILO Joint Estimates of the Work-related Burden of Disease and Injury. Environ Int. 2023;181:108226. https://doi.org/10.1016/j.envint.2023.108226.
Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, et al. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82(3):364–80. https://doi.org/10.4065/82.3.364.
Argenziano G, Zalaudek I, Hofmann-Wellenhof R, Bakos RM, Bergman W, Blum A, et al. Total body skin examination for skin cancer screening in patients with focused symptoms. J Am Acad Dermatol. 2012;66(2):212–9. https://doi.org/10.1016/j.jaad.2010.12.039.
D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–48. https://doi.org/10.3390/ijms140612222.
Schmitt J, Seidler A, Diepgen TL, Bauer A. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164(2):291–307. https://doi.org/10.1111/j.1365-2133.2010.10118.x.
Lo JA, Fisher DE. The melanoma revolution: from UV carcinogenesis to a new era in therapeutics. Science. 2014;346(6212):945–9. https://doi.org/10.1126/science.1253735.
Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545(7653):175–80. https://doi.org/10.1038/nature22071.
Centeno PP, Pavet V, Marais R. The journey from melanocytes to melanoma. Nat Rev Cancer. 2023;23(6):372–90. https://doi.org/10.1038/s41568-023-00565-7.
Pan Y, Adler NR, Wolfe R, McLean CA, Kelly JW. Nodular melanoma is less likely than superficial spreading melanoma to be histologically associated with a naevus. Med J Aust. 2017;207(8):333–8. https://doi.org/10.5694/mja17.00232.
Memon A, Bannister P, Rogers I, Sundin J, Al-Ayadhy B, James PW, et al. Changing epidemiology and age-specific incidence of cutaneous malignant melanoma in England: An analysis of the national cancer registration data by age, gender and anatomical site, 1981–2018. Lancet Reg Health Eur. 2021;2:100024. https://doi.org/10.1016/j.lanepe.2021.100024.
Porceddu SV, Veness MJ, Guminski A. Nonmelanoma cutaneous head and neck cancer and merkel cell carcinoma: current concepts, advances, and controversies. J Clin Oncol. 2015;33(29):3338–45. https://doi.org/10.1200/jco.2014.60.7333.
Small J, Barton V, Peterson B, Alberg AJ. Keratinocyte carcinoma as a marker of a high cancer-risk phenotype. Adv Cancer Res. 2016;130:257–91. https://doi.org/10.1016/bs.acr.2016.01.003.
Krakowski AC, Hafeez F, Westheim A, Pan EY, Wilson M. Advanced basal cell carcinoma: What dermatologists need to know about diagnosis. J Am Acad Dermatol. 2022;86(6s):S1–s13. https://doi.org/10.1016/j.jaad.2022.03.023.
Schmitt J, Haufe E, Trautmann F, Schulze HJ, Elsner P, Drexler H, et al. Is ultraviolet exposure acquired at work the most important risk factor for cutaneous squamous cell carcinoma? Results of the population-based case-control study FB-181. Br J Dermatol. 2018;178(2):462–72. https://doi.org/10.1111/bjd.15906.
Green AC, Olsen CM. Cutaneous squamous cell carcinoma: an epidemiological review. Br J Dermatol. 2017;177(2):373–81. https://doi.org/10.1111/bjd.15324.
Winge MCG, Kellman LN, Guo K, Tang JY, Swetter SM, Aasi SZ, et al. Advances in cutaneous squamous cell carcinoma. Nat Rev Cancer. 2023;23(7):430–49. https://doi.org/10.1038/s41568-023-00583-5.
Viarisio D, Müller-Decker K, Accardi R, Robitaille A, Dürst M, Beer K, et al. Beta HPV38 oncoproteins act with a hit-and-run mechanism in ultraviolet radiation-induced skin carcinogenesis in mice. PLoS Pathog. 2018;14(1):e1006783. https://doi.org/10.1371/journal.ppat.1006783.
Harwood CA, Proby CM, Arron ST. Genomics of SCC: Tumor Formation, Progression, and Future Therapeutic Implications for High-Risk Cutaneous Squamous Cell Carcinoma. In: Schmults CD, editor. High-Risk Cutaneous Squamous Cell Carcinoma: A Practical Guide for Patient Management. Berlin, Heidelberg: Springer Berlin Heidelberg; 2016. p. 67–102.
Wong HY, Lee RC, Chong S, Kapadia S, Freeman M, Murigneux V, et al. Epidermal mutation accumulation in photodamaged skin is associated with skin cancer burden and can be targeted through ablative therapy. Sci Adv. 2023;9(19):eadf2384. https://doi.org/10.1126/sciadv.adf2384.
Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988;1(8589):795–7. https://doi.org/10.1016/s0140-6736(88)91658-3.
Little MP, Lee T, Kimlin MG, Kitahara CM, Zhang R, Alexander BH, et al. Lifetime Ambient UV Radiation Exposure and Risk of Basal Cell Carcinoma by Anatomic Site in a Nationwide US Cohort, 1983–2005. Cancer Epidemiol BiomarkersPrev. 2021;30(10):1932–46. https://doi.org/10.1158/1055-9965.Epi-20-1815.
Schmitt J, Haufe E, Trautmann F, Schulze HJ, Elsner P, Drexler H, et al. Occupational UV-Exposure is a Major Risk Factor for Basal Cell Carcinoma: Results of the Population-Based Case-Control Study FB-181. J Occup Environ Med. 2018;60(1):36–43. https://doi.org/10.1097/jom.0000000000001217.
Stang A, Becker JC, Nghiem P, Ferlay J. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: An international assessment. Eur J Cancer. 2018;94:47–60. https://doi.org/10.1016/j.ejca.2018.02.003.
Sergi MC, Lauricella E, Porta C, Tucci M, Cives M. An update on Merkel cell carcinoma. Biochim Biophys Acta Rev Cancer. 2023;1878(3):188880. https://doi.org/10.1016/j.bbcan.2023.188880.
Liu W, Yang R, Payne AS, Schowalter RM, Spurgeon ME, Lambert PF, et al. Identifying the Target Cells and Mechanisms of Merkel Cell Polyomavirus Infection. Cell Host Microbe. 2016;19(6):775–87. https://doi.org/10.1016/j.chom.2016.04.024.
Becker JC, Stang A, DeCaprio JA, Cerroni L, Lebbé C, Veness M, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. https://doi.org/10.1038/nrdp.2017.77.
Mogha A, Fautrel A, Mouchet N, Guo N, Corre S, Adamski H, et al. Merkel cell polyomavirus small T antigen mRNA level is increased following in vivo UV-radiation. PLoS ONE. 2010;5(7):e11423. https://doi.org/10.1371/journal.pone.0011423.
Besaratinia A, Kim SI, Bates SE, Pfeifer GP. Riboflavin activated by ultraviolet al irradiation induces oxidative DNA damage-mediated mutations inhibited by vitamin C. Proc Natl Acad Sci U S A. 2007;104(14):5953–8. https://doi.org/10.1073/pnas.0610534104.
Kim Y, He YY. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014;1(2):188–98. https://doi.org/10.1016/j.gendis.2014.08.005.
Ravanat J-L, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components. J Photochem Photobiol, B. 2001;63(1):88–102. https://doi.org/10.1016/S1011-1344(01)00206-8.
Cadet J, Berger M, Douki T, Morin B, Raoul S, Ravanat JL, et al. Effects of UV and visible radiation on DNA-final base damage. Biol Chem. 1997;378(11):1275–86.
Choi YS, Fisher DE. UV and melanoma: the TP53 link. Cell Res. 2014;24(10):1157–8. https://doi.org/10.1038/cr.2014.95.
Pfeifer GP, Besaratinia A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci. 2012;11(1):90–7. https://doi.org/10.1039/c1pp05144j.
Tommasi S, Denissenko MF, Pfeifer GP. Sunlight induces pyrimidine dimers preferentially at 5-methylcytosine bases. Cancer Res. 1997;57(21):4727–30.
Geng L, Sun Y, Zhu M, An H, Li Y, Lao Y, et al. The inhibition of PARG attenuates DNA repair in hepatocellular carcinoma. Mol Biomed. 2023;4(1):3. https://doi.org/10.1186/s43556-023-00114-6.
Ravanat JL, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components. J Photochem Photobiol B. 2001;63(1–3):88–102. https://doi.org/10.1016/s1011-1344(01)00206-8.
Kahremany S, Hofmann L, Gruzman A, Dinkova-Kostova AT, Cohen G. NRF2 in dermatological disorders: Pharmacological activation for protection against cutaneous photodamage and photodermatosis. Free Radic Biol Med. 2022;188:262–76. https://doi.org/10.1016/j.freeradbiomed.2022.06.238.
Al-Sadek T, Yusuf N. Ultraviolet radiation biological and medical implications. Curr Issues Mol Biol. 2024;46(3):1924–42. https://doi.org/10.3390/cimb46030126.
Kim JE, Roh E, Lee MH, Yu DH, Kim DJ, Lim TG, et al. Fyn is a redox sensor involved in solar ultraviolet light-induced signal transduction in skin carcinogenesis. Oncogene. 2016;35(31):4091–101. https://doi.org/10.1038/onc.2015.471.
Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal. 2004;6(3):561–70. https://doi.org/10.1089/152308604773934314.
Kamenisch Y, Ivanova I, Drexler K, Berneburg M. UVA, metabolism and melanoma: UVA makes melanoma hungry for metastasis. Exp Dermatol. 2018;27(9):941–9. https://doi.org/10.1111/exd.13561.
Kunisada M, Sakumi K, Tominaga Y, Budiyanto A, Ueda M, Ichihashi M, et al. 8-Oxoguanine formation induced by chronic UVB exposure makes Ogg1 knockout mice susceptible to skin carcinogenesis. Cancer Res. 2005;65(14):6006–10. https://doi.org/10.1158/0008-5472.Can-05-0724.
Seifermann M, Epe B. Oxidatively generated base modifications in DNA: Not only carcinogenic risk factor but also regulatory mark? Free Radic Biol Med. 2017;107:258–65. https://doi.org/10.1016/j.freeradbiomed.2016.11.018.
Hahm JY, Park J, Jang E-S, Chi SW. 8-Oxoguanine: from oxidative damage to epigenetic and epitranscriptional modification. Exp Mol Med. 2022;54(10):1626–42. https://doi.org/10.1038/s12276-022-00822-z.
Besaratinia A, Pfeifer GP. Investigating human cancer etiology by DNA lesion footprinting and mutagenicity analysis. Carcinogenesis. 2006;27(8):1526–37. https://doi.org/10.1093/carcin/bgi311.
Kappes UP, Luo D, Potter M, Schulmeister K, Rünger TM. Short- and Long-Wave UV Light (UVB and UVA) Induce Similar Mutations in Human Skin Cells. J Investig Dermatol. 2006;126(3):667–75. https://doi.org/10.1038/sj.jid.5700093.
Zhivagui M, Hoda A, Valenzuela N, Yeh YY, Dai J, He Y, et al. DNA damage and somatic mutations in mammalian cells after irradiation with a nail polish dryer. Nat Commun. 2023;14(1):276. https://doi.org/10.1038/s41467-023-35876-8.
Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991;88(22):10124–8. https://doi.org/10.1073/pnas.88.22.10124.
McAdam E, Brem R, Karran P. Oxidative Stress-Induced Protein Damage Inhibits DNA repair and determines mutation risk and therapeutic efficacy. Mol Cancer Res. 2016;14(7):612–22. https://doi.org/10.1158/1541-7786.Mcr-16-0053.
Brem R, Macpherson P, Guven M, Karran P. Oxidative stress induced by UVA photoactivation of the tryptophan UVB photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) inhibits nucleotide excision repair in human cells. Sci Rep. 2017;7(1):4310. https://doi.org/10.1038/s41598-017-04614-8.
Murray HC, Maltby VE, Smith DW, Bowden NA. Nucleotide excision repair deficiency in melanoma in response to UVA. Experimental Hematology and Oncology. 2016;5(1). https://doi.org/10.1186/s40164-016-0035-4.
Kim SH, Lee SE, Kim SJ, Fang X, Hur J, Sozen E, et al. Protective effects of an electrophilic metabolite of docosahexaenoic acid on UVB-induced oxidative cell death, dermatitis, and carcinogenesis. Redox Biol. 2023;62:102666. https://doi.org/10.1016/j.redox.2023.102666.
Kremer JL, Melo GP, Marinello PC, Bordini HP, Rossaneis AC, Sábio LR, et al. Citral prevents UVB-induced skin carcinogenesis in hairless mice. J Photochem Photobiol B. 2019;198:111565. https://doi.org/10.1016/j.jphotobiol.2019.111565.
Bailey P, Ridgway RA, Cammareri P, Treanor-Taylor M, Bailey UM, Schoenherr C, et al. Driver gene combinations dictate cutaneous squamous cell carcinoma disease continuum progression. Nat Commun. 2023;14(1):5211. https://doi.org/10.1038/s41467-023-40822-9.
Worrede A, Douglass SM, Weeraratna AT. The dark side of daylight: photoaging and the tumor microenvironment in melanoma progression. J Clin Invest. 2021;131(6). https://doi.org/10.1172/jci143763.
Fears TR, Bird CC, Guerry Dt, Sagebiel RW, Gail MH, Elder DE, et al. Average midrange ultraviolet radiation flux and time outdoors predict melanoma risk. Cancer Res. 2002;62(14):3992–6.
Arnold M, de Vries E, Whiteman DC, Jemal A, Bray F, Parkin DM, et al. Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int J Cancer. 2018;143(6):1305–14. https://doi.org/10.1002/ijc.31527.
Boniol M, Autier P, Boyle P, Gandini S. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345: e4757. https://doi.org/10.1136/bmj.e4757.
Guida S, Guida G, Goding CR. MC1R Functions, expression, and implications for targeted therapy. J Invest Dermatol. 2022;142(2):293–302.e1. https://doi.org/10.1016/j.jid.2021.06.018.
Moon H, Donahue LR, Choi E, Scumpia PO, Lowry WE, Grenier JK, et al. Melanocyte stem cell activation and translocation initiate cutaneous melanoma in response to UV exposure. Cell Stem Cell. 2017;21(5):665–78.e6. https://doi.org/10.1016/j.stem.2017.09.001.
Jakob JA, Bassett RL Jr, Ng CS, Curry JL, Joseph RW, Alvarado GC, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118(16):4014–23. https://doi.org/10.1002/cncr.26724.
Sukniam K, Manaise HK, Popp K, Popp R, Gabriel E. Role of Surgery in Metastatic Melanoma and Review of Melanoma Molecular Characteristics. Cells. 2024;13(6). https://doi.org/10.3390/cells13060465.
Teixido C, Castillo P, Martinez-Vila C, Arance A, Alos L. Molecular Markers and Targets in Melanoma. Cells. 2021;10(9). https://doi.org/10.3390/cells10092320.
Jiang W, Ananthaswamy HN, Muller HK, Kripke ML. p53 protects against skin cancer induction by UV-B radiation. Oncogene. 1999;18(29):4247–53. https://doi.org/10.1038/sj.onc.1202789.
Shain AH, Joseph NM, Yu R, Benhamida J, Liu S, Prow T, et al. Genomic and transcriptomic analysis reveals incremental disruption of key signaling pathways during melanoma evolution. Cancer Cell. 2018;34(1):45–55.e4. https://doi.org/10.1016/j.ccell.2018.06.005.
Trucco LD, Mundra PA, Hogan K, Garcia-Martinez P, Viros A, Mandal AK, et al. Ultraviolet radiation-induced DNA damage is prognostic for outcome in melanoma. Nat Med. 2019;25(2):221–4. https://doi.org/10.1038/s41591-018-0265-6.
Menzies AM, Haydu LE, Visintin L, Carlino MS, Howle JR, Thompson JF, et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res. 2012;18(12):3242–9. https://doi.org/10.1158/1078-0432.Ccr-12-0052.
Viros A, Sanchez-Laorden B, Pedersen M, Furney SJ, Rae J, Hogan K, et al. Ultraviolet radiation accelerates BRAF-driven melanomagenesis by targeting TP53. Nature. 2014;511(7510):478–82. https://doi.org/10.1038/nature13298.
Peris K, Fargnoli MC, Kaufmann R, Arenberger P, Bastholt L, Seguin NB, et al. European consensus-based interdisciplinary guideline for diagnosis and treatment of basal cell carcinoma-update 2023. Eur J Cancer. 2023;192:113254. https://doi.org/10.1016/j.ejca.2023.113254.
Lee Y, Miller HL, Russell HR, Boyd K, Curran T, McKinnon PJ. Patched2 modulates tumorigenesis in patched1 heterozygous mice. Cancer Res. 2006;66(14):6964–71. https://doi.org/10.1158/0008-5472.Can-06-0505.
Loureiro JB, Ribeiro R, Nazareth N, Ferreira T, Lopes EA, Gama A, et al. Mutant p53 reactivator SLMP53-2 hinders ultraviolet B radiation-induced skin carcinogenesis. Pharmacol Res. 2022;175:106026. https://doi.org/10.1016/j.phrs.2021.106026.
Chaiprasongsuk A, Janjetovic Z, Kim TK, Jarrett SG, D’Orazio JA, Holick MF, et al. Protective effects of novel derivatives of vitamin D(3) and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol. 2019;24:101206. https://doi.org/10.1016/j.redox.2019.101206.
Di Nardo L, Pellegrini C, Di Stefani A, Ricci F, Fossati B, Del Regno L, et al. Molecular alterations in basal cell carcinoma subtypes. Sci Rep. 2021;11(1):13206. https://doi.org/10.1038/s41598-021-92592-3.
Selvam K, Sivapragasam S, Poon GMK, Wyrick JJ. Detecting recurrent passenger mutations in melanoma by targeted UV damage sequencing. Nat Commun. 2023;14(1):2702. https://doi.org/10.1038/s41467-023-38265-3.
Griffin GK, Booth CAG, Togami K, Chung SS, Ssozi D, Verga JA, et al. Ultraviolet radiation shapes dendritic cell leukaemia transformation in the skin. Nature. 2023;618(7966):834–41. https://doi.org/10.1038/s41586-023-06156-8.
Bernard JJ, Gallo RL, Krutmann J. Photoimmunology: how ultraviolet radiation affects the immune system. Nat Rev Immunol. 2019;19(11):688–701. https://doi.org/10.1038/s41577-019-0185-9.
Frommeyer TC, Gilbert MM, Brittain GV, Wu T, Nguyen TQ, Rohan CA, et al. UVB-Induced Microvesicle Particle Release and Its Effects on the Cutaneous Microenvironment. Front Immunol. 2022;13. https://doi.org/10.3389/fimmu.2022.880850.
Damiani E, Ullrich SE. Understanding the connection between platelet-activating factor, a UV-induced lipid mediator of inflammation, immune suppression and skin cancer. Prog Lipid Res. 2016;63:14–27. https://doi.org/10.1016/j.plipres.2016.03.004.
Kuchel JM, Barnetson RS, Halliday GM. Cyclobutane pyrimidine dimer formation is a molecular trigger for solar-simulated ultraviolet radiation-induced suppression of memory immunity in humans. Photochem Photobiol Sci. 2005;4(8):577–82. https://doi.org/10.1039/b504068j.
Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195(2):171–9. https://doi.org/10.1084/jem.20011450.
Tse BCY, Ferguson AL, Koay YC, Grau GE, Don AS, Byrne SN. Exposure to solar ultraviolet radiation establishes a novel immune suppressive lipidome in skin-draining lymph nodes. Front Immunol. 2022;13:1045731. https://doi.org/10.3389/fimmu.2022.1045731.
Hart PH, Norval M. More Than Effects in Skin: Ultraviolet Radiation-Induced Changes in Immune Cells in Human Blood. Front Immunol. 2021;12. https://doi.org/10.3389/fimmu.2021.694086.
Salminen A, Kaarniranta K, Kauppinen A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm Res. 2022;71(7–8):817–31. https://doi.org/10.1007/s00011-022-01598-8.
Tse BCY, Ireland RA, Lee JY, Marsh-Wakefield F, Kok LF, Don AS, et al. Exposure to systemic immunosuppressive ultraviolet radiation alters T cell recirculation through sphingosine-1-phosphate. J Immunol. 2021;207(9):2278–87. https://doi.org/10.4049/jimmunol.2001261.
Yamazaki S, Odanaka M, Nishioka A, Kasuya S, Shime H, Hemmi H, et al. Ultraviolet B-Induced Maturation of CD11b-Type Langerin(-) Dendritic Cells Controls the Expansion of Foxp3(+) Regulatory T Cells in the Skin. J Immunol. 2018;200(1):119–29. https://doi.org/10.4049/jimmunol.1701056.
Sullivan NJ, Tober KL, Burns EM, Schick JS, Riggenbach JA, Mace TA, et al. UV Light B-Mediated Inhibition of Skin Catalase Activity Promotes Gr-1+CD11b+ Myeloid Cell Expansion. J Investig Dermatol. 2012;132(3):695–702. https://doi.org/10.1038/jid.2011.329.
Lewis JM, Monico PF, Mirza FN, Xu S, Yumeen S, Turban JL, et al. Chronic UV radiation-induced RORγt+ IL-22-producing lymphoid cells are associated with mutant KC clonal expansion. Proc Natl Acad Sci U S A. 2021;118(37). https://doi.org/10.1073/pnas.2016963118.
Manjili SH, Isbell M, Ghochaghi N, Perkinson T, Manjili MH. Multifaceted functions of chronic inflammation in regulating tumor dormancy and relapse. Semin Cancer Biol. 2022;78:17–22. https://doi.org/10.1016/j.semcancer.2021.03.023.
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. https://doi.org/10.1038/s41392-021-00658-5.
Ciążyńska M, Olejniczak-Staruch I, Sobolewska-Sztychny D, Narbutt J, Skibińska M, Lesiak A. Ultraviolet Radiation and Chronic Inflammation-Molecules and Mechanisms Involved in Skin Carcinogenesis: A Narrative Review. Life (Basel). 2021;11(4). https://doi.org/10.3390/life11040326.
Tong L, Wu S. The role of constitutive nitric-oxide synthase in ultraviolet B light-induced nuclear factor κB activity. J Biol Chem. 2014;289(38):26658–68. https://doi.org/10.1074/jbc.M114.600023.
Dawes JM, Antunes-Martins A, Perkins JR, Paterson KJ, Sisignano M, Schmid R, et al. Genome-wide transcriptional profiling of skin and dorsal root ganglia after ultraviolet-b-induced inflammation. PLoS ONE. 2014;9(4):e93338. https://doi.org/10.1371/journal.pone.0093338.
Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012;18(8):1286–90. https://doi.org/10.1038/nm.2861.
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–71. https://doi.org/10.1038/nrc3611.
Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. https://doi.org/10.1016/j.immuni.2019.06.025.
Ju T, Hernandez L, Mohsin N, Labib A, Frech F, Nouri K. Evaluation of risk in chronic cutaneous inflammatory conditions for malignant transformation. J Eur Acad Dermatol Venereol. 2023;37(2):231–42. https://doi.org/10.1111/jdv.18663.
Ke Y, Wang XJ. TGFβ Signaling in Photoaging and UV-Induced Skin Cancer. J Invest Dermatol. 2021;141(4s):1104–10. https://doi.org/10.1016/j.jid.2020.11.007.
Sinha NK, McKenney C, Yeow ZY, Li JJ, Nam KH, Yaron-Barir TM, et al. The ribotoxic stress response drives UV-mediated cell death. Cell. 2024. https://doi.org/10.1016/j.cell.2024.05.018.
Di Filippo M, Hennig P, Karakaya T, Slaufova M, Beer HD. NLRP1 in Cutaneous SCCs: An Example of the Complex Roles of Inflammasomes in Cancer Development. Int J Mol Sci. 2022;23(20). https://doi.org/10.3390/ijms232012308.
Fenini G, Karakaya T, Hennig P, Di Filippo M, Beer HD. The NLRP1 Inflammasome in Human Skin and Beyond. Int J Mol Sci. 2020;21(13). https://doi.org/10.3390/ijms21134788.
Zhong FL, Mamaï O, Sborgi L, Boussofara L, Hopkins R, Robinson K, et al. Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via inflammasome activation. Cell. 2016;167(1):187–202.e17. https://doi.org/10.1016/j.cell.2016.09.001.
Zhong QY, Lin B, Chen YT, Huang YP, Feng WP, Wu Y, et al. Gender differences in UV-induced skin inflammation, skin carcinogenesis and systemic damage. Environ Toxicol Pharmacol. 2021;81:103512. https://doi.org/10.1016/j.etap.2020.103512.
Jandova J, Snell J, Hua A, Dickinson S, Fimbres J, Wondrak GT. Topical hypochlorous acid (HOCl) blocks inflammatory gene expression and tumorigenic progression in UV-exposed SKH-1 high risk mouse skin. Redox Biol. 2021;45:102042. https://doi.org/10.1016/j.redox.2021.102042.
Singh A, Singh A, Bauer SJ, Wheeler DL, Havighurst TC, Kim K, et al. Genetic deletion of TNFα inhibits ultraviolet radiation-induced development of cutaneous squamous cell carcinomas in PKCε transgenic mice via inhibition of cell survival signals. Carcinogenesis. 2016;37(1):72–80. https://doi.org/10.1093/carcin/bgv162.
Ming T, Tao Q, Tang S, Zhao H, Yang H, Liu M, et al. Curcumin: An epigenetic regulator and its application in cancer. Biomed Pharmacother. 2022;156: 113956. https://doi.org/10.1016/j.biopha.2022.113956.
Cheng Y, He C, Wang M, Ma X, Mo F, Yang S, et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther. 2019;4:62. https://doi.org/10.1038/s41392-019-0095-0.
Parreno V, Loubiere V, Schuettengruber B, Fritsch L, Rawal CC, Erokhin M, et al. Transient loss of Polycomb components induces an epigenetic cancer fate. Nature. 2024;629(8012):688–96. https://doi.org/10.1038/s41586-024-07328-w.
Shen Y, Stanislauskas M, Li G, Zheng D, Liu L. Epigenetic and genetic dissections of UV-induced global gene dysregulation in skin cells through multi-omics analyses. Sci Rep. 2017;7:42646. https://doi.org/10.1038/srep42646.
Liu J, Liu L, He J, Xu Y, Wang Y. Multi-omic analysis of altered transcriptome and epigenetic signatures in the UV-induced DNA damage response. DNA Repair (Amst). 2021;106:103172. https://doi.org/10.1016/j.dnarep.2021.103172.
Vandiver AR, Irizarry RA, Hansen KD, Garza LA, Runarsson A, Li X, et al. Age and sun exposure-related widespread genomic blocks of hypomethylation in nonmalignant skin. Genome Biol. 2015;16(1):80. https://doi.org/10.1186/s13059-015-0644-y.
Li S, Dina Kuo HC, Wang L, Wu R, Sargsyan D, Kong AN. UVB Drives metabolic rewiring and epigenetic reprograming and protection by sulforaphane in human skin keratinocytes. Chem Res Toxicol. 2022;35(7):1220–33. https://doi.org/10.1021/acs.chemrestox.1c00432.
Shen Y, Stanislauskas M, Li G, Zheng D, Liu L. Epigenetic and genetic dissections of UV-induced global gene dysregulation in skin cells through multi-omics analyses. Sci Rep. 2017;7(1):42646. https://doi.org/10.1038/srep42646.
Yadav N, Tripathi AK, Banerjee M. UVR-Induced Epigenetic Regulation and Photocarcinogenesis. In: Ray RS, Haldar C, Dwivedi A, Agarwal N, Singh J, editors. Photocarcinogenesis & Photoprotection. Singapore: Springer Singapore; 2018. p. 9–13.
Yang YL, Zhou C, Chen Q, Shen SZ, Li JD, Wang XL, et al. YAP1/Piezo1 involve in the dynamic changes of lymphatic vessels in UVR-induced photoaging progress to squamous cell carcinoma. J Transl Med. 2023;21(1):820. https://doi.org/10.1186/s12967-023-04458-z.
Wu H, Wang J, Zhao Y, Qin Y, Chen X, Zhou Y, et al. Extracellular vesicles derived from human dermal fibroblast effectively ameliorate skin photoaging via miRNA-22-5p-GDF11 axis. Chem Eng J. 2023;452:139553. https://doi.org/10.1016/j.cej.2022.139553.
Rodríguez-Paredes M, Bormann F, Raddatz G, Gutekunst J, Lucena-Porcel C, Köhler F, et al. Methylation profiling identifies two subclasses of squamous cell carcinoma related to distinct cells of origin. Nat Commun. 2018;9(1):577. https://doi.org/10.1038/s41467-018-03025-1.
Yang Y, Wu R, Sargsyan D, Yin R, Kuo HC, Yang I, et al. UVB drives different stages of epigenome alterations during progression of skin cancer. Cancer Lett. 2019;449:20–30. https://doi.org/10.1016/j.canlet.2019.02.010.
Wilson HE, Wyrick JJ. Genome-wide impact of cytosine methylation and DNA sequence context on UV-induced CPD formation. Environ Mol Mutagen. 2024;65 Suppl 1(Suppl 1):14–24. https://doi.org/10.1002/em.22569.
Kim SI, Pfeifer GP. The epigenetic DNA modification 5-carboxylcytosine promotes high levels of cyclobutane pyrimidine dimer formation upon UVB irradiation. Genome Instab Dis. 2021;2(1):59–69. https://doi.org/10.1007/s42764-020-00030-x.
Fortuny A, Chansard A, Caron P, Chevallier O, Leroy O, Renaud O, et al. Imaging the response to DNA damage in heterochromatin domains reveals core principles of heterochromatin maintenance. Nat Commun. 2021;12(1):2428. https://doi.org/10.1038/s41467-021-22575-5.
Wäster P, Eriksson I, Vainikka L, Öllinger K. Extracellular vesicles released by melanocytes after UVA irradiation promote intercellular signaling via miR21. Pigment Cell Melanoma Res. 2020;33(4):542–55. https://doi.org/10.1111/pcmr.12860.
Poenitzsch Strong AM, Setaluri V, Spiegelman VS. MicroRNA-340 as a modulator of RAS-RAF-MAPK signaling in melanoma. Arch Biochem Biophys. 2014;563:118–24. https://doi.org/10.1016/j.abb.2014.07.012.
Jian Q, An Q, Zhu D, Hui K, Liu Y, Chi S, et al. MicroRNA 340 is involved in UVB-induced dendrite formation through the regulation of RhoA expression in melanocytes. Mol Cell Biol. 2014;34(18):3407–20. https://doi.org/10.1128/mcb.00106-14.
Chen IP, Bender M, Spassova I, Henning S, Kubat L, Fan K, et al. UV-type specific alteration of miRNA expression and its association with tumor progression and metastasis in SCC cell lines. J Cancer Res Clin Oncol. 2020;146(12):3215–31. https://doi.org/10.1007/s00432-020-03358-9.
Chen L, Hu Y, Zhang M, Liu L, Ma J, Xu Z, et al. METTL14 affects UVB-induced human dermal fibroblasts photoaging via miR-100-3p biogenesis in an m(6)A-dependent manner. Aging Cell. 2024;23(5):e14123. https://doi.org/10.1111/acel.14123.
Damiani E, Puebla-Osorio N, Gorbea E, Ullrich SE. Platelet-activating factor induces epigenetic modifications in human mast cells. J Investig Dermatol. 2015;135(12):3034–40. https://doi.org/10.1038/jid.2015.336.
Damiani E, Puebla-Osorio N, Lege BM, Liu J, Neelapu SS, Ullrich SE. Platelet activating factor-induced expression of p21 is correlated with histone acetylation. Sci Rep. 2017;7. https://doi.org/10.1038/srep41959.
Mittal A, Piyathilake C, Hara Y, Katiyar SK. Exceptionally high protection of photocarcinogenesis by topical application of (–)-epigallocatechin-3-gallate in hydrophilic cream in SKH-1 hairless mouse model: relationship to inhibition of UVB-induced global DNA hypomethylation. Neoplasia. 2003;5(6):555–65. https://doi.org/10.1016/s1476-5586(03)80039-8.
Nandakumar V, Vaid M, Katiyar SK. (-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis. 2011;32(4):537–44. https://doi.org/10.1093/carcin/bgq285.
Khan A, Khan A, Khan MA, Malik Z, Massey S, Parveen R, et al. Phytocompounds targeting epigenetic modulations: an assessment in cancer. Front Pharmacol. 2023;14:1273993. https://doi.org/10.3389/fphar.2023.1273993.
Katiyar SK. Epigenetic Regulation by Dietary Phytochemicals in Photocarcinogenesis. Curr Pharmacol Rep. 2015;1(1):52–9. https://doi.org/10.1007/s40495-015-0021-2.
Agarwal A, Kansal V, Farooqi H, Prasad R, Singh VK. Epigallocatechin Gallate (EGCG), an Active Phenolic Compound of Green Tea, Inhibits Tumor Growth of Head and Neck Cancer Cells by Targeting DNA Hypermethylation. Biomedicines. 2023;11(3). https://doi.org/10.3390/biomedicines11030789.
Kimura H, Lee C, Hayashi K, Yamauchi K, Yamamoto N, Tsuchiya H, et al. UV light killing efficacy of fluorescent protein-expressing cancer cells in vitro and in vivo. J Cell Biochem. 2010;110(6):1439–46. https://doi.org/10.1002/jcb.22693.
Hiyama H, Reeves SA. Role for cyclin D1 in UVC-induced and p53-mediated apoptosis. Cell Death Differ. 1999;6(6):565–9. https://doi.org/10.1038/sj.cdd.4400524.
Yamauchi T, Adachi S, Yasuda I, Nakashima M, Kawaguchi J, Nishii Y, et al. UVC radiation induces downregulation of EGF receptor via phosphorylation at serine 1046/1047 in human pancreatic cancer cells. Radiat Res. 2011;176(5):565–74. https://doi.org/10.1667/rr2445.1.
Yamauchi T, Adachi S, Yasuda I, Nakashima M, Kawaguchi J, Yoshioka T, et al. Ultra-violet irradiation induces apoptosis via mitochondrial pathway in pancreatic cancer cells. Int J Oncol. 2011;39(6):1375–80. https://doi.org/10.3892/ijo.2011.1188.
Adachi S, Yasuda I, Nakashima M, Yamauchi T, Kawaguchi J, Shimizu M, et al. Ultraviolet irradiation can induce evasion of colon cancer cells from stimulation of epidermal growth factor. J Biol Chem. 2011;286(29):26178–87. https://doi.org/10.1074/jbc.M111.240630.
Correia M, Thiagarajan V, Coutinho I, Gajula GP, Petersen SB, Neves-Petersen MT. Modulating the structure of EGFR with UV light: new possibilities in cancer therapy. PLoS ONE. 2014;9(11):e111617. https://doi.org/10.1371/journal.pone.0111617.
Olsen BB, Neves-Petersen MT, Klitgaard S, Issinger OG, Petersen SB. UV light blocks EGFR signalling in human cancer cell lines. Int J Oncol. 2007;30(1):181–5. https://doi.org/10.3892/ijo.30.1.181.
Feng H, Zhao JK, Schiergens TS, Wang PX, Ou BC, Al-Sayegh R, et al. Bone marrow-derived mesenchymal stromal cells promote colorectal cancer cell death under low-dose irradiation. Br J Cancer. 2018;118(3):353–65. https://doi.org/10.1038/bjc.2017.415.
Dip F, Rosenthal D, Socolovsky M, Falco J, De la Fuente M, White KP, et al. Nerve autofluorescence under near-ultraviolet light: cutting-edge technology for intra-operative neural tissue visualization in 17 patients. Surg Endosc. 2022;36(6):4079–89. https://doi.org/10.1007/s00464-021-08729-y.
Momiyama M, Suetsugu A, Kimura H, Kishimoto H, Aki R, Yamada A, et al. Imaging the efficacy of UVC irradiation on superficial brain tumors and metastasis in live mice at the subcellular level. J Cell Biochem. 2013;114(2):428–34. https://doi.org/10.1002/jcb.24381.
Uehara F, Miwa S, Tome Y, Hiroshima Y, Yano S, Yamamoto M, et al. Comparison of UVB and UVC effects on the DNA damage-response protein 53BP1 in human pancreatic cancer. J Cell Biochem. 2014;115(10):1724–8. https://doi.org/10.1002/jcb.24837.
Kawaguchi J, Adachi S, Yasuda I, Yamauchi T, Nakashima M, Ohno T, et al. Cisplatin and ultra-violet-C synergistically down-regulate receptor tyrosine kinases in human colorectal cancer cells. Mol Cancer. 2012;11:45. https://doi.org/10.1186/1476-4598-11-45.
Chang HW, Tang JY, Yen CY, Chang HS, Huang HW, Chung YA, et al. Synergistic anti-oral cancer effects of UVC and methanolic extracts of Cryptocarya concinna roots via apoptosis, oxidative stress and DNA damage. Int J Radiat Biol. 2016;92(5):263–72. https://doi.org/10.3109/09553002.2016.1145753.
Wang SC, Wang YY, Lin LC, Chang MY, Yuan SF, Tang JY, et al. Combined Treatment of Sulfonyl Chromen-4-Ones (CHW09) and Ultraviolet-C (UVC) Enhances Proliferation Inhibition, Apoptosis, Oxidative Stress, and DNA Damage against Oral Cancer Cells. Int J Mol Sci. 2020;21(17). https://doi.org/10.3390/ijms21176443.
Huang HW, Chung YA, Chang HS, Tang JY, Chen IS, Chang HW. Antiproliferative effects of methanolic extracts of Cryptocarya concinna Hance roots on oral cancer Ca9-22 and CAL 27 cell lines involving apoptosis, ROS induction, and mitochondrial depolarization. ScientificWorldJournal. 2014;2014:180462. https://doi.org/10.1155/2014/180462.
Wang SC, Chang HS, Tang JY, Farooqi AA, Kuo YT, Hsuuw YD, et al. Combined Treatment with Cryptocaryone and Ultraviolet C Promotes Antiproliferation and Apoptosis of Oral Cancer Cells. Int J Mol Sci. 2022;23(6). https://doi.org/10.3390/ijms23062981.
Peng SY, Lin LC, Yang ZW, Chang FR, Cheng YB, Tang JY, et al. Combined Treatment with Low Cytotoxic Ethyl Acetate Nepenthes Extract and Ultraviolet-C Improves Antiproliferation to Oral Cancer Cells via Oxidative Stress. Antioxidants (Basel). 2020;9(9). https://doi.org/10.3390/antiox9090876.
Chuang YT, Shiau JP, Yen CY, Hou MF, Jeng JH, Tang JY, et al. Fucoidan/UVC Combined Treatment Exerts Preferential Antiproliferation in Oral Cancer Cells but Not Normal Cells. Antioxidants (Basel). 2022;11(9). https://doi.org/10.3390/antiox11091797.
Peng SY, Yen CY, Lan TH, Jeng JH, Tang JY, Chang HW. Combined Treatment (Ultraviolet-C/Physapruin A) Enhances Antiproliferation and Oxidative-Stress-Associated Mechanism in Oral Cancer Cells. Antioxidants (Basel). 2022;11(11). https://doi.org/10.3390/antiox11112227.
Pronin S, Koh CH, Hughes M. Cytotoxicity of ultraviolet-C radiation on a heterogeneous population of human glioblastoma multiforme cells: Meta-analysis. Photodiagnosis Photodyn Ther. 2018;24:158–63. https://doi.org/10.1016/j.pdpdt.2018.10.003.
Jiang Y, Ge XY, Liu SM, Zheng L, Huang MW, Shi Y, et al. Nimotuzumab suppresses epithelial-mesenchymal transition and enhances apoptosis in low-dose UV-C treated salivary adenoid cystic carcinoma cell lines in vitro. Anticancer Drugs. 2014;25(9):1052–60. https://doi.org/10.1097/cad.0000000000000139.
Zare T, Fardid R, Naderi S. Synergetic Effect of Silver Nanoparticles and UVC Irradiation on H2AX Gene Expression in TK6 Cells. Cell J. 2019;21(2):204–9. https://doi.org/10.22074/cellj.2019.5898.
Beckers C, Pruschy M, Vetrugno I. Tumor hypoxia and radiotherapy: A major driver of resistance even for novel radiotherapy modalities. Semin Cancer Biol. 2024;98:19–30. https://doi.org/10.1016/j.semcancer.2023.11.006.
de Perrot M, Wu L, Wu M, Cho BCJ. Radiotherapy for the treatment of malignant pleural mesothelioma. Lancet Oncol. 2017;18(9):e532–42. https://doi.org/10.1016/s1470-2045(17)30459-x.
Müller M, Wang Y, Squillante MR, Held KD, Anderson RR, Purschke M. UV scintillating particles as radiosensitizer enhance cell killing after X-ray excitation. Radiother Oncol. 2018;129(3):589–94. https://doi.org/10.1016/j.radonc.2018.06.016.
Squillante MR, Jüstel T, Anderson RR, Brecher C, Chartier D, Christian JF, et al. Fabrication and characterization of UV-emitting nanoparticles as novel radiation sensitizers targeting hypoxic tumor cells. Opt Mater. 2018;80:197–202. https://doi.org/10.1016/j.optmat.2018.04.033.
Müller M, Espinoza S, Jüstel T, Held KD, Anderson RR, Purschke M. UVC-Emitting LuPO4:Pr3+ Nanoparticles Decrease Radiation Resistance of Hypoxic Cancer Cells. Radiat Res. 2019;193(1):82–7. https://doi.org/10.1667/RR15491.1.
Tran TA, Kappelhoff J, Jüstel T, Anderson RR, Purschke M. UV emitting nanoparticles enhance the effect of ionizing radiation in 3D lung cancer spheroids. Int J Radiat Biol. 2022;98(9):1484–94. https://doi.org/10.1080/09553002.2022.2027541.
Chilakamarthi U, Giribabu L. Photodynamic therapy: past, present and future. Chem Rec. 2017;17(8):775–802. https://doi.org/10.1002/tcr.201600121.
Kim HR, Luo Y, Li G, Kessel D. Enhanced apoptotic response to photodynamic therapy after bcl-2 transfection. Cancer Res. 1999;59(14):3429–32.
Zhao W, Wang L, Zhang M, Liu Z, Wu C, Pan X, et al. Photodynamic therapy for cancer: mechanisms, photosensitizers, nanocarriers, and clinical studies. MedComm. 2024;5(7):e603. https://doi.org/10.1002/mco2.603.
Vankayala R, Sagadevan A, Vijayaraghavan P, Kuo C-L, Hwang KC. Metal Nanoparticles Sensitize the Formation of Singlet Oxygen. Angew Chem Int Ed. 2011;50(45):10640–4. https://doi.org/10.1002/anie.201105236.
Wen S, Zhou J, Zheng K, Bednarkiewicz A, Liu X, Jin D. Advances in highly doped upconversion nanoparticles. Nat Commun. 2018;9(1):2415. https://doi.org/10.1038/s41467-018-04813-5.
Lin Y, Yao Y, Zhang W, Fang Q, Zhang L, Zhang Y, et al. Applications of upconversion nanoparticles in cellular optogenetics. Acta Biomater. 2021;135:1–12. https://doi.org/10.1016/j.actbio.2021.08.035.
Sun B, Teo JY, Wu J, Zhang Y. Light Conversion Nanomaterials for Wireless Phototherapy. Acc Chem Res. 2023;56(10):1143–55. https://doi.org/10.1021/acs.accounts.2c00699.
Fujishima A, Honda K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature. 1972;238(5358):37–8. https://doi.org/10.1038/238037a0.
Zhang Y, Ren K, Zhang X, Chao Z, Yang Y, Ye D, et al. Photo-tearable tape close-wrapped upconversion nanocapsules for near-infrared modulated efficient siRNA delivery and therapy. Biomaterials. 2018;163:55–66. https://doi.org/10.1016/j.biomaterials.2018.02.019.
Rozhkova EA, Ulasov I, Lai B, Dimitrijevic NM, Lesniak MS, Rajh T. A high-performance nanobio photocatalyst for targeted brain cancer therapy. Nano Lett. 2009;9(9):3337–42. https://doi.org/10.1021/nl901610f.
Wang T, Jiang H, Wan L, Zhao Q, Jiang T, Wang B, et al. Potential application of functional porous TiO2 nanoparticles in light-controlled drug release and targeted drug delivery. Acta Biomater. 2015;13:354–63. https://doi.org/10.1016/j.actbio.2014.11.010.
Yadav HM, Thorat ND, Yallapu MM, Tofail SAM, Kim JS. Functional TiO(2) nanocoral architecture for light-activated cancer chemotherapy. J Mater Chem B. 2017;5(7):1461–70. https://doi.org/10.1039/c6tb02324j.
Idris NM, Lucky SS, Li Z, Huang K, Zhang Y. Photoactivation of core–shell titania coated upconversion nanoparticles and their effect on cell death. J Mater Chem B. 2014;2(40):7017–26. https://doi.org/10.1039/C4TB01169D.
Pandey NK, Chudal L, Phan J, Lin L, Johnson O, Xing M, et al. A facile method for the synthesis of copper-cysteamine nanoparticles and study of ROS production for cancer treatment. J Mater Chem B. 2019;7(42):6630–42. https://doi.org/10.1039/c9tb01566c.
Huang X, Wan F, Ma L, Phan JB, Lim RX, Li C, et al. Investigation of copper-cysteamine nanoparticles as a new photosensitizer for anti-hepatocellular carcinoma. Cancer Biol Ther. 2019;20(6):812–25. https://doi.org/10.1080/15384047.2018.1564568.
Zhang C, Zhao K, Bu W, Ni D, Liu Y, Feng J, et al. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew Chem Int Ed. 2015;54(6):1770–4. https://doi.org/10.1002/anie.201408472.
Wang H, Lv B, Tang Z, Zhang M, Ge W, Liu Y, et al. Scintillator-based nanohybrids with sacrificial electron prodrug for enhanced x-ray-induced photodynamic therapy. Nano Lett. 2018;18(9):5768–74. https://doi.org/10.1021/acs.nanolett.8b02409.
Du Z, Wang X, Zhang X, Gu Z, Fu X, Gan S, et al. X-Ray-triggered carbon monoxide and manganese dioxide generation based on scintillating nanoparticles for cascade cancer radiosensitization. Angew Chem Int Ed Engl. 2023;62(23):e202302525. https://doi.org/10.1002/anie.202302525.
Du Z, Zhang X, Guo Z, Xie J, Dong X, Zhu S, et al. X-Ray-Controlled Generation of Peroxynitrite Based on Nanosized LiLuF(4):Ce(3+) Scintillators and their Applications for Radiosensitization. Adv Mater. 2018;30(43):e1804046. https://doi.org/10.1002/adma.201804046.
Sengar P, Juárez P, Verdugo-Meza A, Arellano DL, Jain A, Chauhan K, et al. Development of a functionalized UV-emitting nanocomposite for the treatment of cancer using indirect photodynamic therapy. J Nanobiotechnology. 2018;16(1):19. https://doi.org/10.1186/s12951-018-0344-3.
Bulin AL, Broekgaarden M, Chaput F, Baisamy V, Garrevoet J, Busser B, et al. Radiation Dose-Enhancement Is a Potent Radiotherapeutic Effect of Rare-Earth Composite Nanoscintillators in Preclinical Models of Glioblastoma. Adv Sci. 2020;7(20). https://doi.org/10.1002/advs.202001675.
Müller M, Rahmanzadeh R, Tran T, Kappelhoff J, Akam EA, Caravan P, et al. Particle Size of X-ray Pumped UVC-Emitting Nanoparticles Defines Intracellular Localization and Biological Activity Against Cancer Cells. Part Part Syst Character. 2020;37(10). https://doi.org/10.1002/ppsc.202000201.
Liu J, Zhang R, Xu ZP. Nanoparticle-based nanomedicines to promote cancer immunotherapy: recent advances and future directions. Small. 2019;15(32):e1900262. https://doi.org/10.1002/smll.201900262.
Fomina N, Sankaranarayanan J, Almutairi A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv Drug Deliv Rev. 2012;64(11):1005–20. https://doi.org/10.1016/j.addr.2012.02.006.
Shamsipur M, Ghavidast A, Pashabadi A. Phototriggered structures: Latest advances in biomedical applications. Acta Pharm Sin B. 2023;13(7):2844–76. https://doi.org/10.1016/j.apsb.2023.04.005.
Thang DC, Wang Z, Lu X, Xing B. Precise cell behaviors manipulation through light-responsive nano-regulators: recent advance and perspective. Theranostics. 2019;9(11):3308–40. https://doi.org/10.7150/thno.33888.
Karisma VW, Wu W, Lei M, Liu H, Nisar MF, Lloyd MD, et al. UVA-Triggered Drug Release and Photo-Protection of Skin. Front Cell Dev Biol. 2021;9:598717. https://doi.org/10.3389/fcell.2021.598717.
Xiao P, Zhang J, Zhao J, Stenzel MH. Light-induced release of molecules from polymers. Prog Polym Sci. 2017;74:1–33. https://doi.org/10.1016/j.progpolymsci.2017.06.002.
Chen C, Zhao J, Gao M, Meng X, Fan A, Wang Z, et al. Photo-triggered micelles: simultaneous activation and release of microtubule inhibitors for on-demand chemotherapy. Biomater Sci. 2018;6(3):511–8. https://doi.org/10.1039/c7bm01053b.
Engel S, Jeschenko PM, van Dongen M, Rose JC, Schäfer D, Bruns M, et al. Photo-cross-linked and pH-switchable soft polymer nanocapsules from polyglycidyl ethers. Macromolecules. 2024;57(2):707–18. https://doi.org/10.1021/acs.macromol.3c01698.
Ali AA, Kharbash R, Kim Y. Chemo- and biosensing applications of spiropyran and its derivatives - A review. Anal Chim Acta. 2020;1110:199–223. https://doi.org/10.1016/j.aca.2020.01.057.
Wang H, Miao W, Wang F, Cheng Y. A self-assembled coumarin-anchored dendrimer for efficient gene delivery and light-responsive drug delivery. Biomacromol. 2018;19(6):2194–201. https://doi.org/10.1021/acs.biomac.8b00246.
Roh JL, Kim EH, Jang HJ, Park JY, Shin D. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett. 2016;381(1):96–103. https://doi.org/10.1016/j.canlet.2016.07.035.
Chen L, Lin W, Zhang H, Geng S, Le Z, Wan F, et al. TRIB3 promotes malignancy of head and neck squamous cell carcinoma via inhibiting ferroptosis. Cell Death Dis. 2024;15(3):178. https://doi.org/10.1038/s41419-024-06472-5.
Liang D, Minikes AM, Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. 2022;82(12):2215–27. https://doi.org/10.1016/j.molcel.2022.03.022.
Zhou Q, Meng Y, Li D, Yao L, Le J, Liu Y, et al. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct Target Ther. 2024;9(1):55. https://doi.org/10.1038/s41392-024-01769-5.
Zhang PC, Hong Y, Zong SQ, Chen L, Zhang C, Tian DZ, et al. Variation of ferroptosis-related markers in HaCaT cell photoaging models induced by UVB. Clin Cosmet Investig Dermatol. 2023;16:3147–55. https://doi.org/10.2147/ccid.S433071.
He S, Cai J, Jia T, Mao Z, Zhou L, Zhang X, et al. New sight of renal toxicity caused by UV-aged polystyrene nanoplastics: induced ferroptosis via adsorption of transferrin. Small. 2024;20(23):e2309369. https://doi.org/10.1002/smll.202309369.
Teng Y, Cui H, Xu D, Tang H, Gu Y, Tang Y, et al. Specific knockdown of the NDUFS4 gene reveals important roles of ferroptosis in UVB-induced photoaging. Inflammation. 2024. https://doi.org/10.1007/s10753-024-02057-8.
Yi P, Huang Y, Zhao X, Qin Z, Zhu D, Liu L, et al. A novel UVA-associated circUBE2I mediates ferroptosis in HaCaT cells. Photochem Photobiol. 2023. https://doi.org/10.1111/php.13885.
Xiao T, Liang J, Li M, Guo Y, Chen S, Ke Y, et al. ATG5-mediated keratinocyte ferroptosis promotes M1 polarization of macrophages to aggravate UVB-induced skin inflammation. J Photochem Photobiol B. 2024;257:112948. https://doi.org/10.1016/j.jphotobiol.2024.112948.
Feng Z, Qin Y, Huo F, Jian Z, Li X, Geng J, et al. NMN recruits GSH to enhance GPX4-mediated ferroptosis defense in UV irradiation induced skin injury. Biochim Biophys Acta Mol Basis Dis. 2022;1868(1):166287. https://doi.org/10.1016/j.bbadis.2021.166287.
Bao W, Liu X, Lv Y, Lu GH, Li F, Zhang F, et al. Nanolongan with multiple on-demand conversions for ferroptosis-apoptosis combined anticancer therapy. ACS Nano. 2019;13(1):260–73. https://doi.org/10.1021/acsnano.8b05602.
Zhu J, Dai P, Liu F, Li Y, Qin Y, Yang Q, et al. Upconverting nanocarriers enable triggered microtubule inhibition and concurrent ferroptosis induction for selective treatment of triple-negative breast cancer. Nano Lett. 2020;20(9):6235–45. https://doi.org/10.1021/acs.nanolett.0c00502.
Nguyen NT, Kim J, Le XT, Lee WT, Lee ES, Oh KT, et al. Amplified fenton-based oxidative stress utilizing ultraviolet upconversion luminescence-fueled nanoreactors for apoptosis-strengthened ferroptosis anticancer therapy. ACS Nano. 2023;17(1):382–401. https://doi.org/10.1021/acsnano.2c08706.
Xue K, Yang R, An Y, Ding Y, Li S, Miao F, et al. NIR-promoted ferrous ion regeneration enhances ferroptosis for glioblastoma treatment. J Control Release. 2024;368:595–606. https://doi.org/10.1016/j.jconrel.2024.01.004.
Zhang K, Wang J, Peng L, Zhang Y, Zhang J, Zhao W, et al. UCNPs-based nanoreactors with ultraviolet radiation-induced effect for enhanced ferroptosis therapy of tumor. J Colloid Interface Sci. 2023;651:567–78. https://doi.org/10.1016/j.jcis.2023.07.183.
Letfullin RR, George TF. Nanotherapy of cancer by photoelectrons emitted from the surface of nanoparticles exposed to nonionizing ultraviolet radiation. Nanomedicine. 2017;12(10):1107–17. https://doi.org/10.2217/nnm-2017-0053.
Rezaee M, Hunting DJ, Sanche L. Correlation between energy deposition and molecular damage from Auger electrons: A case study of ultra-low energy (5–18 eV) electron interactions with DNA. Med Phys. 2014;41(7):072502. https://doi.org/10.1118/1.4881329.
Patil S, Raj AT. Commentary: molecular machines open cell membranes. Front Oncol. 2017;7:277. https://doi.org/10.3389/fonc.2017.00277.
Yang H, Xun Y, Ke C, Tateishi K, You H. Extranodal lymphoma: pathogenesis, diagnosis and treatment. Mol Biomed. 2023;4(1):29. https://doi.org/10.1186/s43556-023-00141-3.
Trautinger F. Phototherapy of cutaneous T-cell lymphomas. Photochem Photobiol Sci. 2018;17(12):1904–12. https://doi.org/10.1039/c8pp00170g.
Dummer R, Vermeer MH, Scarisbrick JJ, Kim YH, Stonesifer C, Tensen CP, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 2021;7(1):61. https://doi.org/10.1038/s41572-021-00296-9.
Brumfiel CM, Patel MH, Puri P, Besch-Stokes J, Lester S, Rule WG, et al. How to sequence therapies in mycosis fungoides. Curr Treat Options Oncol. 2021;22(11):101. https://doi.org/10.1007/s11864-021-00899-0.
Carter J, Zug KA. Phototherapy for cutaneous T-cell lymphoma: online survey and literature review. J Am Acad Dermatol. 2009;60(1):39–50. https://doi.org/10.1016/j.jaad.2008.08.043.
Hristov AC, Tejasvi T, Wilcox RA. Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(1):193–209. https://doi.org/10.1002/ajh.26760.
Lopes C, Trevisani VF, Melnik T. Efficacy and safety of 308-nm monochromatic excimer lamp versus other phototherapy devices for vitiligo: a systematic review with meta-analysis. Am J Clin Dermatol. 2016;17(1):23–32. https://doi.org/10.1007/s40257-015-0164-2.
Hearn RM, Kerr AC, Rahim KF, Ferguson J, Dawe RS. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. 2008;159(4):931–5. https://doi.org/10.1111/j.1365-2133.2008.08776.x.
Ko MJ, Tsai WC, Tsai PH, Hsu LY, Chien KL, Wu HY. Ultraviolet B phototherapy does not increase the risk of skin cancer among patients with atopic dermatitis: a population-based retrospective cohort study. J Am Acad Dermatol. 2023;89(3):496–503. https://doi.org/10.1016/j.jaad.2023.05.037.
Su Y, Lu K, Huang Y, Zhang J, Sun X, Peng J, et al. Targeting Warburg effect to rescue the suffocated photodynamic therapy: A cancer-specific solution. Biomaterials. 2023;294: 122017. https://doi.org/10.1016/j.biomaterials.2023.122017.
Chen Z, Wu Y, Yao Z, Su J, Wang Z, Xia H, et al. 2D Copper(II) metalated metal-organic framework nanocomplexes for dual-enhanced photodynamic therapy and amplified antitumor immunity. ACS Appl Mater Interfaces. 2022;14(39):44199–210. https://doi.org/10.1021/acsami.2c12990.
Wang M, Dai Z. Advances in equipment for tumor photodynamic therapy. Chin Sci Bull. 2017;62(15):1591–601. https://doi.org/10.1360/N972017-00150.
Werfel T, Holiangu F, Niemann KH, Schmerling O, Lüllau F, Zedler A, et al. Digital ultraviolet therapy: a novel therapeutic approach for the targeted treatment of psoriasis vulgaris. Br J Dermatol. 2015;172(3):746–53. https://doi.org/10.1111/bjd.13464.
Jeong SH, Lee MG, Kim CC, Park J, Baek Y, Park BI, et al. An implantable ionic therapeutic platform for photodynamic therapy with wireless capacitive power transfer. Mater Horiz. 2023;10(6):2215–25. https://doi.org/10.1039/d2mh01548j.
Pan Y, Luan X, Gao Y, Zeng F, Wang X, Zhou D, et al. In-tumor biosynthetic construction of upconversion nanomachines for precise near-infrared phototherapy. ACS Nano. 2023;17(5):4515–25. https://doi.org/10.1021/acsnano.2c10453.
Ding Q, Mei L, Liu Y, Zhou S, Chen L, Liang Y, et al. Mitochondria-targeted fluorophores for in vivo NIR-II imaging-guided PDT/PTT. Chem Commun (Camb). 2023;59(52):8127–30. https://doi.org/10.1039/d3cc02380j.
Vermandel M, Quidet M, Vignion-Dewalle AS, Leroy HA, Leroux B, Mordon S, et al. Comparison of different treatment schemes in 5-ALA interstitial photodynamic therapy for high-grade glioma in a preclinical model: An MRI study. Photodiagnosis Photodyn Ther. 2019;25:166–76. https://doi.org/10.1016/j.pdpdt.2018.12.003.
Acknowledgements
Several elements in Figures 2, 3 and 4 were modified from Servier Medical Art(http://smart.servier.com/), licensed under a Creative Common Attribution 4.0 Generic License. (https://creativecommons.org/licenses/by/4.0/) The authors acknowledge the use of Microsoft PowerPoint in the creation of the figures presented in this review.
We thank Dr. Yi-hang Hao and Xu Zhang for their kind supports in production of schematic diagram and manuscript integrity check and giving precious opinions.
Funding
This work was supported by National Natural Science Foundation of China grants (Nos. 82173326, 82473058 and 82073000), Interdisciplinary innovation project of West China College of Stomatology Sichuan University (No. RD-03–202004) and Clinical Research Program, West China Hospital of Stomatology Sichuan University (LCYJ-MS-202308).
Author information
Authors and Affiliations
Contributions
Manuscript designing and outlining: Ya-ling Tang and Xin-hua Liang; Manuscript writing: Zhen-wei Yu and Hua-yang Fan; Figure and table creating: Zhen-wei Yu and Hua-yang Fan; Clinical consulting and revision: Min Zheng. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
This manuscript has not been published elsewhere before.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, Zw., Zheng, M., Fan, Hy. et al. Ultraviolet (UV) radiation: a double-edged sword in cancer development and therapy. Mol Biomed 5, 49 (2024). https://doi.org/10.1186/s43556-024-00209-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43556-024-00209-8