Abstract
Background
Women with polycystic ovary syndrome (PCOS) exhibit a low fertility by chronic hyperandrogenemia. Different evidence have shown that androgens could regulate the endoplasmic reticulum (ER) homeostasis and glucose metabolism. However, it is unclear whether androgens can exert these effects on human endometrial stromal cells. Our goal was to study the protein content of GRP78 (an ER homeostasis marker) in endometria from women with PCOS and healthy women and to assess the GRP78 protein levels and its relationship with glucose uptake on a human endometrial stromal cell line stimulated with testosterone.
Methods
Immunohistochemistry assays for GRP78 were performed on endometrial samples obtained from women with PCOS (n = 8) and control women subjected to hysterectomy (n = 8). Western blot analysis for GRP78 and glucose uptake was assessed in a telomerase-immortalized human endometrial stromal cell line (T-HESC) exposed to testosterone for 24 or 48 hours and challenged to an insulin short-term stimulation. Tukey test was performed for human samples comparison. Student t test or ANOVA–Bonferroni test was carried out according to the in vitro experiment. P < .05 was considered as significant.
Results
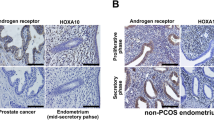
GRP78 stromal immunostaining was reduced in PCOS endometria compared to controls (P < .05). The T-HESC shows a testosterone-dependent downregulation of GRP78 protein content (P < .05), concomitant with half-reduction in glucose uptake compared to controls (P < .05). Moreover, enhanced small interfering RNA against GRP78 messenger RNA leads to a decrease in glucose uptake (P < .05). Such effects were reverted by hydroxyflutamide, an inhibitor of androgen receptor.
Conclusion
These results suggest that hyperandrogenemic PCOS environment could compromise the endometrial homeostasis confirmed by the decrease in glucose uptake induced by testosterone and exhibited by stromal cells.
Similar content being viewed by others
References
Giudice LC. Endometrium in PCOS: implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab. 2006;20(2):235–244.
Frolova AI, Moley KH. Glucose transporters in the uterus: an analysis of tissue distribution and proposed physiological roles. Reproduction. 2011;142(2):211–220.
Frolova AI, Moley KH. Quantitative analysis of glucose transporter mRNAs in endometrial stromal cells reveals critical role of GLUT1 in uterine receptivity. Endocrinology. 2011;152(5): 2123–2128.
Carvajal R, Rosas C, Kohan K, et al. Metformin augments the levels of molecules that regulate the expression of the insulin-dependent glucose transporter GLUT4 in the endometria of hyperinsulinemic PCOS patients. Hum Reprod. 2013;28(8): 2235–2244.
Kohan K, Carvajal R, Gabler F, Vantman D, Romero C, Vega M. Role of the transcriptional factors FOXO1 and PPARG on gene expression of SLC2A4 in endometrial tissue from women with polycystic ovary syndrome. Reproduction. 2010;140(1):123–131.
Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030.
Rivero R, Garin CA, Ormazabal P, et al. Protein expression of PKCZ (Protein Kinase C Zeta), Munc18c, and Syntaxin-4 in the insulin pathway in endometria of patients with polycystic ovary syndrome (PCOS). Reprod Biol Endocrinol. 2012;10:17.
Rosas C, Gabler F, Vantman D, Romero C, Vega M. Levels of Rabs and WAVE family proteins associated with translocation of GLUT4 to the cell surface in endometria from hyperinsulinemic PCOS women. Hum Reprod. 2010;25(11):2870–2877.
Fornes R, Ormazabal P, Rosas C, et al. Changes in the expression of insulin signaling pathway molecules in endometria from polycystic ovary syndrome women with or without hyperinsulinemia. Mol Med. 2010;16(3–4):129–136.
Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol. 2007;192(3):585–594.
Hojlund K, Glintborg D, Andersen NR, et al. Impaired insulin-stimulated phosphorylation of Akt and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes. 2008;57(2):357–366.
Nakatsuka A, Wada J, Iseda I, et al. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/ MTJ-1 complex. Diabetes. 2012;61(11):2823–2832.
Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58(3):693–700.
Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004; 306(5695):457–461.
Segawa T, Nau ME, Xu LL, et al. Androgen-induced expression of endoplasmic reticulum (ER) stress response genes in prostate cancer cells. Oncogene. 2002;21(57):8749–8758.
Ma C, Yoshioka M, Boivin A, et al. Atlas of dihydrotestosterone actions on the transcriptome of prostate in vivo. Prostate. 2009; 69(3):293–316.
Zhu G, Lee AS. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol. 2015;230(7): 1413–1420.
Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122(2):262–263.
Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91(11):4237–4245.
Garcia V, Orostica L, Poblete C, et al. Endometria from obese PCOS women with hyperinsulinemia exhibit altered adiponectin signaling. Horm Metab Res. 2015;47(12):901–909.
Krikun G, Mor G, Alvero A, et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145(5):2291–2296.
Plaza-Parrochia F, Bacallao K, Poblete C, et al. The role of androst-5-ene-3beta,17beta-diol (androstenediol) in cell proliferation in endometrium of women with polycystic ovary syndrome. Steroids. 2014;89:11–19.
Ormazabal P, Romero C, Quest AF, Vega M. Testosterone modulates the expression of molecules linked to insulin action and glucose uptake in endometrial cells. Horm Metab Res. 2013; 45(9):640–645.
Vera JC, Reyes AM, Velasquez FV, et al. Direct inhibition of the hexose transporter GLUT1 by tyrosine kinase inhibitors. Biochemistry. 2001;40(3):777–790.
Perez A, Ojeda P, Ojeda L, et al. Hexose transporter GLUT1 harbors several distinct regulatory binding sites for flavones and tyrphostins. Biochemistry. 2011;50(41):8834–8845.
Bacallao K, Plaza-Parrochia F, Cerda A, et al. Levels of regulatory proteins associated with cell proliferation in endometria from untreated patients having polycystic ovarian syndrome with and without endometrial hyperplasia. Reprod Sci. 2016;23(2): 211–218.
DeFronzo RA, Matsuda M. Reduced time points to calculate the composite index. Diabetes Care. 2010;33(7):e93.
McAuley KA, Mann JI, Chase JG, Lotz TF, Shaw GM. Point: HOMA—satisfactory for the time being: HOMA: the best bet for the simple determination of insulin sensitivity, until something better comes along. Diabetes Care. 2007;30(9):2411–2413.
Mozzanega B, Mioni R, Granzotto M, et al. Obesity reduces the expression of GLUT4 in the endometrium of normoinsulinemic women affected by the polycystic ovary syndrome. Ann N Y Acad Sci. 2004;1034:364–374.
Biswas N, Friese RS, Gayen JR, Bandyopadhyay G, Mahata SK, O’Connor DT. Discovery of a novel target for the dysglycemic chromogranin A fragment pancreastatin: interaction with the chaperone GRP78 to influence metabolism. PLoS One. 2014;9(1): e84132.
Yamagishi N, Ueda T, Mori A, Saito Y, Hatayama T. Decreased expression of endoplasmic reticulum chaperone GRP78 in liver of diabetic mice. Biochem Biophys Res Commun. 2012;417(1): 364–370.
Perrini S, Natalicchio A, Laviola L, et al. Dehydroepiandrosterone stimulates glucose uptake in human and murine adipocytes by inducing GLUT1 and GLUT4 translocation to the plasma membrane. Diabetes. 2004;53(1):41–52.
Sato K, Iemitsu M, Aizawa K, Ajisaka R. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294(5): E961–E968.
Sato K, Iemitsu M, Aizawa K, Ajisaka R. DHEA improves impaired activation of Akt and PKC zeta/lambda-GLUT4 pathway in skeletal muscle and improves hyperglycaemia in streptozotocin-induced diabetes rats. Acta Physiol (Oxf). 2009; 197(3):217–225.
Zhang L, Liao Q. Effects of testosterone and metformin on glucose metabolism in endometrium. Fertil Steril. 2010;93(7): 2295–2298.
Allemand MC, Irving BA, Asmann YW, et al. Effect of testosterone on insulin stimulated IRS1 Ser phosphorylation in primary rat myotubes—a potential model for PCOS-related insulin resistance. PLoS One. 2009;4(1):e4274.
Chen X, Li X, Huang HY, Li X, Lin JF. Effects of testosterone on insulin receptor substrate-1 and glucose transporter 4 expression in cells sensitive to insulin [in Chinese]. Zhonghua Yi Xue Za Zhi. 2006;86(21):1474–1477.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosas, C., Oróstica, L., Poblete, C. et al. Hyperandrogenism Decreases GRP78 Protein Level and Glucose Uptake in Human Endometrial Stromal Cells. Reprod. Sci. 23, 761–770 (2016). https://doi.org/10.1177/1933719115618283
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719115618283