Abstract
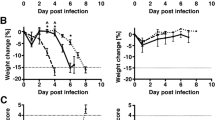
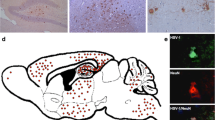
Type I interferon (IFN) is critical for resistance of mice to infection with vesicular stomatitis virus (VSV). Wild type (wt) VSV infection did not induce type I IFN production in vitro or in the central nervous system (CNS) of mice; however IFN-β was detected in lungs, spleen, and serum within 24 h. The M protein mutant VSV, T1026R1 (also referred to as M51R), induced type I IFN production in vitro and in the CNS, with poor expression in spleens. In addition, VSV T1026R1 was not pathogenic to mice after intranasal infection, illustrating the importance of IFN in controlling VSV replication in the CNS. Experiments with chemical sympathectomy, sRAGE, and neutralizing antibody to VSV were performed to investigate the mechanism(s) utilized for induction of peripheral IFN; neither sRAGE infusion nor chemical sympathectomy had an effect on peripheral IFN production. In contrast, administration of neutralizing antibody (Ab) readily blocked the response. Infectious VSV was transiently present in lungs and spleens at 24 h post infection. The results are consistent with VSV traffic from the olfactory neuroepithelium to peripheral lymphoid organs hematogenously or via lymphatic circulation. These results suggest that VSV replicates to high titers in the brains of mice because of the lack of IFN production in the CNS after intranasal VSV infection. In contrast, replication of VSV in peripheral organs is controlled by the production of large amounts of IFN.
Similar content being viewed by others
References
Ahmed M, McKenzie MO, Puckett S, Hojnacki M, Poliquin L, Lyles DS (2003). Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J Virol 77: 4646–4657.
Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM (2005). Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol 64: 706–715.
Bailey M, Engler H, Hunzeker J, Sheridan JF (2003). The hypothalamic-pituitary-adrenal axis and viral infection. Viral Immunol 16: 141–157.
Bailey SL, Schreiner B, McMahon EJ, Miller SD (2007). CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ TH-17 cells in relapsing EAE. Nat Immunol 8: 172–180.
Barchet W, Cella M, Odermatt B, Asselin-Paturel C, Colonna M, Kalinke U (2002). Virus-induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J Exp Med 195: 507–516.
Barna M, Komatsu T, Bi Z, Reiss CS (1996a). Sex differences in susceptibility to viral infection of the central nervous system. J Neuroimmunol 67: 31–39.
Barna M, Komatsu T, Reiss CS (1996b). Activation of type III nitric oxide synthase in astrocytes following a neurotropic viral infection. Virology 223: 331–343.
Bi Z, Barna M, Komatsu T, Reiss CS (1995). Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol 69: 6466–6472.
Bi Z, Reiss CS (1995). Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol 69: 2208–2213.
Blalock JE (1989). A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev 69: 1–32.
Boehme KW, Compton T (2004). Innate sensing of viruses by toll-like receptors. J Virol 78: 7867–7873.
Breakfield XO, Neale EA, Neale JH, Jacobowitz DM (1975). Localized catecholamine storage associated with granules in murine neuroblastoma cells. Brain Res 92: 237–256.
Browning MJ, Huneycutt BS, Huang AS, Reiss CS (1991). Replication-defective viruses modulate immune responses. J Immunol 147: 2685–2691.
Campbell SJ, Perry VH, Pitossi FJ, Butchart AG, Chertoff M, Waters S, Dempster R, Anthony DC (2005). Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol 166: 1487–1497.
Chen G, Ward MF, Sama AE, Wang H (2004). Extracellular HMGB1 as a proinflammatory cytokine. J Interferon Cytokine Res 24: 329–333.
Chesler DA, Munoz-Jordan JL, Donelan N, Garcia-Sastre A, Reiss CS (2003). PKR is not required for interferon-gamma inhibition of VSV replication in neurons. Viral Immunol 16: 87–96.
Christian AY, Barna M, Bi Z, Reiss CS (1996). Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BL/6 mice. Viral Immunol 9: 195–205.
Conzelmann KK (2005). Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. J Virol 79: 5241–5248.
Crozat K, Beutler B (2004). TLR7: a new sensor of viral infection. Proc Natl Acad Sci USA 101: 6835–6836.
Dafny N, Yang PB (2005). Interferon and the central nervous system. Eur J Pharmacol 523: 1–15.
Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T (2006). Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci USA 103: 7835–7840.
Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa (2004). Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303: 1529–1531.
Durbin JE, Hackenmiller R, Simon MC, Levy DE (1996). Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84: 443–450.
Durbin RK, Mertz SE, Koromilas AE, Durbin JE (2002). PKR protection against intranasal vesicular stomatitis virus infection is mouse strain dependent. Viral Immunol 15: 41–51.
Ferran MC, Lucas-Lenard JM (1997). The vesicular stomatitis virus matrix protein inhibits transcription from the human beta interferon promoter. J Virol 71: 371–377.
Francoeur AM, Lam T, Stanners CP (1980). PIF, a highly sensitive plaque assay for the induction of interferon. Virology 105: 526–536.
Francoeur AM, Poliquin L, Stanners CP (1987). The isolation of interferon-inducing mutants of vesicular stomatitis virus with altered viral P function for the inhibition of total protein synthesis. Virology 160: 236–245.
Gaddy DF, Lyles DS (2005). Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways. J Virol 79: 4170–4179.
Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, Bahram S, Oldstone MBA, Beutler B. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology 362: 304–313.
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S (2004). Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303: 1526–1529.
Herrmann M, Ehrenreich H (2003). Brain derived proteins as markers of acute stroke: their relation to pathophysiology, outcome prediction and neuroprotective drug monitoring. Restor Neurol Neurosci 21: 177–190.
Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM (1999). RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97: 889–901.
Huneycutt BS, Plakhov IV, Shusterman Z, Bartido SM, Huang A, Reiss CS, Aoki C (1994). Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice following intranasal inoculation: an immunohistochemical analysis. Brain Res 635: 81–95.
Ireland DD, Reiss CS (2004). Expression of IL-12 receptor by neurons. Viral Immunol 17: 411–422.
Ireland DD, Reiss CS (2006). Gene expression contributing to recruitment of circulating cells in response to vesicular stomatitis virus infection of the CNS. Viral Immunol 19: 536–545.
Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B (2005). CD14 is required for MyD88-independent LPS signaling. Nat Immunol 6: 565–570.
Kawai T, Akira S (2005). Toll-like receptor downstream signaling. Arthritis Res Ther 7: 12–19.
Komatsu T, Bi Z, Reiss CS (1996). Interferon-gamma induced type I nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol 68: 101–108.
Lalla E, Lamster IB, Feit M, Huang L, Spessot A, Qu W, Kislinger T, Lu Y, Stern DM, Schmidt AM (2000). Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J Clin Invest 105: 1117–1124.
Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B (2004). Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest 113: 1641–1650.
Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA (2004). Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl Acad Sci USA 101: 5598–5603.
Madden KS (2003). Catecholamines, sympathetic innervation, and immunity. Brain Behav Immun 17(Suppl 1): S5-S10.
Malmgaard L (2005). Dendritic cells, toll-like receptors, and T-cell responses: lessons from viral infections in vivo. Viral Immunol 18: 584–594.
Marcus PI, Rodriguez LL, Sekellick MJ (1998). Interferon induction as a quasispecies marker of vesicular stomatitis virus populations. J Virol 72: 542–549.
Marie I, Durbin JE, Levy DE (1998). Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J 17: 6660–6669.
Matyszak MK, Perry VH (1997). Dendritic cells in inflammatory responses in the CNS. Adv Exp Med Biol 417: 295–299.
McEwen BS, Biron CA, Brunson KW, Bullock K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM (1997). The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev 23: 79–133.
Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M (1994). Functional role of type I and type II interferons in antiviral defense. Science 264: 1918–1921.
Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A (1992). Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem 267: 14998–15004.
Newman TA, Galea I, van RN, Perry VH (2005). Blood-derived dendritic cells in an acute brain injury. J Neuroimmunol 166: 167–172.
Petersen JM, Her LS, Varvel V, Lund E, Dahlberg JE (2000). The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol Cell Biol 20: 8590–8601.
Plakhov IV, Aoki C, Reiss CS, Huang AS (1995a). Pathogenesis of murine encephalitis limited by defective interfering particles. An immunohistochemical study. J NeuroVirol 1: 207–218.
Plakhov IV, Arlund EE, Aoki C, Reiss CS (1995b). The earliest events in vesicular stomatitis virus infection of the murine olfactory neuroepithelium and entry of the central nervous system. Virology 209: 257–262.
Publicover J, Ramsburg E, Robek M, Rose JK (2006). Rapid pathogenesis induced by a vesicular stomatitis virus matrix protein mutant: viral pathogenesis is linked to induction of tumor necrosis factor alpha. J Virol 80: 7028–7036.
Publicover J, Ramsburg E, Rose JK (2005). A single-cycle vaccine vector based on vesicular stomatitis virus can induce immune responses comparable to those generated by a replication-competent vector. J Virol 79: 13231–13238.
Ramsburg E, Publicover J, Buonocore L, Poholek A, Robek M, Palin A, Rose JK (2005). A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced t-cell responses and is highly attenuated for replication in animals. J Virol 79: 15043–15053.
Rempel JD, Murray SJ, Meisner J, Buchmeier MJ (2004). Differential regulation of innate and adaptive immune responses in viral encephalitis. Virology 318: 381–392.
Rosicarelli B, Serafini B, Sbriccoli M, Lu M, Cardone F, Pocchiari M, Aloisi F (2005). Migration of dendritic cells into the brain in a mouse model of prion disease. J Neuroimmunol 165: 114–120.
Sabin AB, Olitsky PK (1938). Influence of host factors on neuroinvasiveness of vesicular stomatitis virus: III. Effect of age and pathway of infection on the character and localization of lesions in the central nervous system. J Exp Med 67: 201–228.
Sandberg K, Eloranta ML, Campbell IL (1994). Expression of alpha/beta interferons (IFN-alpha/beta) and their relationship to IFN-alpha/beta-induced genes in lymphocytic choriomeningitis. J Virol 68: 7358–7366.
Schubert D, Humphreys S, Baroni C, Cohn M (1969). In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci USA 64: 316–323.
Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Capello E, Mancardi GL, Aloisi F (2006). Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol 65: 124–141.
Silverman MN, Pearce BD, Biron CA, Miller AH (2005). Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol 18: 41–78.
Stojdl DF, Abraham N, Knowles S, Marius R, Brasey A, Lichty BD, Brown EG, Sonenberg N, Bell JC (2000). The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J Virol 74: 9580–9585.
Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC (2003). VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4: 263–275.
Trottier M, Schlitt BP, Kung AY, Lipton HL (2004). Transition from acute to persistent Theiler’s virus infection requires active viral replication that drives proinflammatory cytokine expression and chronic demyelinating disease. J Virol 78: 12480–12488.
Trottier MDJ, Palian BM, Shoshkes Reiss C (2005). VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology 333: 215–225.
Weihe E, Nohr D, Michel S, Muller S, Zentel HJ, Fink T, Krekel J (1991). Molecular anatomy of the neuroimmune connection. Int J Neurosci 59: 1–23.
Yan SS, Wu ZY, Zhang HP, Furtado G, Chen X, Yan SF, Schmidt AM, Brown C, Stern A, LaFaille J, Chess L, Stern DM, Jiang H (2003). Suppression of experimental autoimmune encephalomyelitis by selective blockade of encephalitogenic T-cell infiltration of the central nervous system. Nat Med 9: 287–293.
Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, Picard C, von BH, Senechal B, Plancoulaine S, Al-Hajjar S, Al-Ghonaium A, Marodi L, Davidson D, Speert D, Roifman C, Garty BZ, Ozinsky A, Barrat FJ, Coffman RL, Miller RL, Li X, Lebon P, Rodriguez-Gallego C, Chapel H, Geissmann F, Jouanguy E, Casanova JL (2005). Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity 23: 465–478.
Yetter RA, Lehrer S, Ramphal R, Small PA Jr (1980). Outcome of influenza infection: effect of site of initial infection and heterotypic immunity. Infect Immun 29: 654–662.
Zhou S, Kurt-Jones EA, Fitzgerald KA, Wang JP, Cerny AM, Chan M, Finberg RW (2007). Role of MyD88 in route-dependent susceptibility to vesicular stomatitis virus infection. J Immunol 178: 5173–5181.
Zozulya AL, Reinke E, Baiu DC, Karman J, Sandor M, Fabry Z (2007). Dendritic cell transmigration through brain microvessel endothelium is regulated by MIP-1alpha chemokine and matrix metalloproteinases. J Immunol 178: 520–529.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by postdoctoral fellowship NS11073 to M.D.T. and a Research Challenge Fund award N5385 from New York University, and DC003536 and NS039746 to C.S.R.
Rights and permissions
About this article
Cite this article
Trottier, M.D., Lyles, D.S. & Reiss, C.S. Peripheral, but not central nervous system, type I interferon expression in mice in response to intranasal vesicular stomatitis virus infection. Journal of NeuroVirology 13, 433–445 (2007). https://doi.org/10.1080/13550280701460565
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/13550280701460565