Abstract
Japanese encephalitis (JE), the most important cause of epidemic encephalitis worldwide, is confined to Asia, but its geographical area is spreading. West Nile virus, and other closely related flaviviruses, cause similar disease elsewhere. Recent cryoelectron microscopic studies have characterized the flavivirus envelope protein as a new class of viral fusion protein (class II), and examined its arrangement on the virion surface. Changes in the envelope protein’s hinge region, or its putative receptor-binding domain, are associated with changes in neurovirulence in animal models of JE. Clinically, JE causes a wide range of presentations, including a poliolike flaccid paralysis. Seizures and raised intracranial pressure are associated with a poor outcome, and may be potentially treatable. A safe efficacious formalin-inactivated vaccine against JE has been available for many years, but is too expensive for use in most Asian countries. A newer live attenuated vaccine has been used in China, but its use elsewhere has been restricted by regulatory concerns. A chimeric vaccine in which JE structural proteins are inserted into the 17D yellow fever vaccine backbone is one of several vaccines in development. There are no established antiviral treatments against JE. Interferon alpha was the most promising drug in small open trials, but a recent double-blind placebo controlled trial showed that it did not affect the outcome in children with JE.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Anderson JF, Rahal JJ (2002). Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg Infect Dis 8: 107–108.
Arroyo J, Guirakhoo F, Fenner S, Zhang ZX, Monath TP, Chambers TJ (2001). Molecular basis for attenuation of neurovirulence of a yellow fever Virus/Japanese encephalitis virus chimera vaccine (ChimeriVax-JE). J Virol 75: 934–942.
Asnis DS, Conetta R, Teixeira AA, Waldman G, Sampson BA (2000). The West Nile virus outbreak of 1999 in New York: the Flushing Hospital experience. Clin Infect Dis 30: 413–418.
Burke DS, Morrill JC (1987). Levels of interferon in the plasma and cerebrospinal fluid of patients with acute Japanese encephalitis. J Infect Dis 155: 797–799.
Cecilia D, Gould EA (1991). Nucleotide changes respsonsible for loss of neuroinvasiveness in Japanese encephalitis virus neutralisation-resistant mice. Virology 181: 70–77.
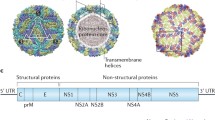
Chambers TJ, Hahn CS, Galler R, Rice CM (1990). Flavivirus genome organisation, expression and replication. Annu Rev Microbiol 44: 649–688.
Chen CJ, Raung SL, Kuo MD, Wang YM (2002). Suppression of Japanese encephalitis virus infection by non-steroidal anti-inflammatory drugs. J Gen Virol 83: 1897–1905.
Chen WR, Ricco-Hesse R, Tesh RB (1992). A new genotype of Japanese virus from Indonesia. Am J Trop Med Hyg 47: 61–69.
Chen WR, Tesh RB, Ricco-Hesse R (1990). Genetic variation of Japanese encephalitis virus in nature. J Gen Virol 71: 2915–2922.
Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM (1997). Dengue virus infectivity depends on envelope protein binding to target cell heparan sulphate. Nat Med 3: 866–871.
Chiou SS, Chen WJ (2001). Mutations in the NS3 gene and 3′-NCR of Japanese encephalitis virus isolated from an unconventional ecosystem and implications for natural attenuation of the virus. Virology 289: 129–136.
Despres P, Frenkiel MP, Ceccaldi PE, Duarte Dos Santos C, Deubel V (1998). Apoptosis in the mouse central nervous system in response to infection with mouse-neurovirulent dengue viruses. J Virol 72: 823–829.
Ferlenghi I, Clarke M, Ruttan T, Allison SL, Schalich J, Heinz FX, Harrison SC, Rey FA, Fuller SD (2001). Molecular organization of arecombinant subviral particle from tick-borne encephalitis virus. Mol Cell 7: 593–602.
Guirakhoo F, Pugachev K, Arroyo J, Miller C, Zhang ZX, Weltzin R, Georgakopoulos K, Catalan J, Ocran S, Draper K, Monath TP (2002). Viremia and immunogenicity in nonhuman primates of a tetravalent yellow fever-dengue chimeric vaccine: genetic reconstructions, dose adjustment, and antibody responses against wild-type dengue virus isolates. Virology 298: 146–159.
Harinasuta C, Nimmanitya S, Titsyakorn U (1985). The effect of interferon alpha on two cases of Japanese encephalitis in Thailand. Southeast Asian J Trop Med Pub Health 16: 332–336.
Harinasuta C, Wasi C, Vithanomsat S (1984). The effect of interferon on Japanese encephalitis virus in vitro. Southeast Asian J Trop Med Pub Health 15: 564–568.
Hasegawa H, Yoshida M, Shiosaka T, Fujita S, Kobayashi Y (1992). Mutations in the envelope protein of Japanese encephalitis virus affect entry into cultured cells and virulence in mice. Virology 191: 158–165.
Heinz FX, Allison SL (2001). The machinery for flavivirus fusion with host cell membranes. Curr Opin Microbiol 4: 450–455.
Hennessy S, Zhengle L, Tsai TF, Strom BL, Chao-Min W, Hui-Lian L, Tai-Xiang W, Hong-Ji Y, Qi-Mau L, Karabatsos N, Bilker WB, Halstead SB (1996). Effectiveness of live-attenuated Japanese encephalitis vaccine (SA14-14-2): a case control study. Lancet 347: 1583–1586.
Holzmann H, Heinz FX, Mandl C, Guirakhoo F, Kunz C (1990). A single amino acid substitution in envelope protein of tick borne encephalitis virus leads to attenuation in the mouse model. J Gen Virol 64: 5156–5159.
Huang CH, Wong C (1963). Relation of the peripheral multiplication of Japanese B encephalitis virus to the pathogenesis of the infection in mice. Acta Virol 7: 322–330.
Jan JT, Chen BH, Ma SH, Liu CI, Tsai HP, Wu HC, Jiang SY, Yang KD, Shaio MF (2000). Potential dengue virustriggered apoptotic pathway in human neuroblastoma cells: arachidonic acid, superoxide anion, and NF-kappaB are sequentially involved. J Virol 74: 8680–8691.
Jan LR, Chen KL, Lu CF, Wu YC, Horng CB (1996). Complete nucleotide sequence of the genome of Japanese encephalitis virus ling strain: the presence of a 25-nucleotide deletion in the 3′-nontranslated region. Am J Trop Med Hyg 55: 603–609.
Johnson RT, Burke DS, Elwell M, Leake CJ, A N, Hoke CH, Lorsomrudee W (1985). Japanese encephalitis: immunocytochemical studies of viral antigen and inflammatory cells in fatal cases. Ann Neurol 18: 567–573.
Johnston LJ, Halliday GM, King NJ (2000). Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J Invest Dermatol 114: 560–568.
Kaiser R (1995). Tick-borne encephalitis in southern Germany. Lancet 345: 463.
Kalita J, Misra UK (2000). Markedly severe dystonia in Japanese encephalitis. Mov Disord 15: 1168–1172.
Kanesa-thasan N, Smucny JJ, Hoke CH, Marks DH, Konishi E, Kurane I, Tang DB, Vaughn DW, Mason PW, Shope RE (2000). Safety and immunogenicity of NYVAC-JEV and ALVAC-JEV attenuated recombinant Japanese encephalitis virus—poxvirus vaccines in vaccinia-nonimmune and vaccinia-immune humans. Vaccine 19: 483–491.
Konishi E, Mason PW, Innis BI, Ennis FA (1995). Japanese encephalitis virus-specific proliferative responses of human peripheral blood T lymphocytes. Am J Trop Med Hyg 53: 278–283.
Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH (2002). Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108: 717–725.
Kumar S, Misra UK, Kalita J, Sawani V, Gupta RK, Gujral R (1997). MRI in Japanese encephalitis. Am J Med Sci 39: 180–184.
Kumar R, Mathur A, Kumar A, Sharma S, Chakraborty S, Chaturvedi UC (1990). Clinical features and prognostic indicators of Japanese encephalitis in children in Lucknow (India). Indian J Med Res 91: 321–327.
Lee E, Lobigs M (2000). Substitutions at the putative receptor-binding site of an encephalitic flavivirus alter virulence and host cell tropism and reveal a role for glycosaminoglycans in entry. J Virol 74: 8867–8875.
Leis AA, Stokic DS, Polk JL, Dostrow V, Winkelmann M (2002). A Poliomyelitis-like syndrome from West Nile virus infection. N Engl J Med 347: 1279–1280.
Lescar J, Roussel A, Wien MW, Navaza J, Fuller SD, Wengler G, Rey FA (2001). The Fusion glycoprotein shell of Semliki Forest virus: an icosahedral assembly primed for fusogenic activation at endosomal pH. Cell 105: 137–148.
Leyssen P, De Clercq E, Neyts J (2000). Perspectives for the treatment of infections with Flaviviridae. Clin Microbiol Rev 13: 67–82.
Liao CL, Lin YL, Wang JJ, Huang YL, Yeh CT, Ma SH, Chen LK (1997). Effect of enforced expression of human bcl-2 on Japanese encephalitis virus induced apoptosis in cultured cells. J Virol 71: 5963–5971.
Liao CL, Lin YL, Wu BC, Tsao CH, Wang MC, Liu CI, Huang YL, Chen JH, Wang JP, Chen LK (2001). Salicylates inhibit flavivirus replication independently of blocking nuclear factor kappa B activation. J Virol 75: 7828–7839.
Lin CF, Lei HY, Shiau AL, Liu HS, Yeh TM, Chen SH, Liu CC, Chiu SC, Lin YS (2002). Endothelial cell apoptosis induced by antibodies against dengue virus nonstructural protein 1 via production of nitric oxide. J Immunol 169: 657–664.
Ma X, Yu YX, Wang SG (1993). Observations on safety and serological efficacy from a large scale field trial of Japanese encephalitis vaccine. Chin J Biol 6: 188–191.
McMinn PC, Dalgarno L, Weir RC (1996). A comparison of the spread of Murray Valley encephalitis viruses of high or low neuroinvasiveness in the tissues of Swiss mice after peripheral inoculation. Virology 220: 414–423.
Misra UK, Kalita J (1997). Movement disorders in Japanese encephalitis. J Neurol 244: 299–303.
Misra UK, Kalita J (2001). Seizures in Japanese encephalitis. J Neurol Sci 190: 57–60.
Miyake M (1964). The pathology of Japanese encephalitis. WHO Bull 30: 153–160.
Monath TP (2002). Japanese encephalitis vaccines: current vaccines and future prospects. Curr Top Microbiol Immunol 267: 105–138.
Monath TP, Arroyo J, Levenbook I, Zhang ZX, Catalan J, Draper K, Guirakhoo F (2002a). Single mutation in the flavivirus envelope protein hinge region increases neurovirulence for mice and monkeys but decreases viscerotropism for monkeys: relevance to development and safety testing of live, attenuated vaccines. J Virol 76: 1932–1943.
Monath TP, Levenbook I, Soike K, Zhang ZX, Ratterree M, Draper K, Barrett AD, Nichols R, Weltzin R, Arroyo J, Guirakhoo F (2000). Chimeric yellow fever virus 17D-Japanese encephalitis virus vaccine: dose-response effectiveness and extended safety testing in rhesus monkeys. J Virol 74: 1742–1751.
Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, Marchesani R, Knauber M, Wells KH, Arroyo J, Guirakhoo F (2002b). Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine 20: 1004–1018.
Murgod UA, Muthane UB, Ravi V, Radhesh S, Desai A (2001). Persistent movement disorders following Japanese encephalitis. Neurology 57: 2313–2315.
Ni H, Barrett ADT (1996). Molecular differences between wild-type Japanese encephalitis virus strains of high and low mouse neuroinvasiveness. J Gen Virol 77: 1449–1455.
Ni H, Burns NJ, Chang GJ, Zhang MJ, Wills MR, Trent DW, Sanders PG, Barrett AD (1994). Comparison of nucleotide and deduced amino acid sequence of the 5′ non-coding region and structural protein genes of the wild-type Japanese encephalitis virus strain SA14 and its attenuated vaccine derivatives. J Gen Virol 75: 1505–1510.
Ni H, Chang GJ, Xie H, Trent DW, Barrett AD (1995). Molecular basis of attenuation of neurovirulence of wild-type Japanese encephalitis virus strain SA14. J Gen Virol 76 (Pt 2): 409–413.
Parquet MC, Kumatori A, Hasebe F, Mathenge EG, Morita K (2002). St. Louis encephalitis virus induced pathology in cultured cells. Arch Virol 147: 1105–1119.
Pyke AT, Williams DT, Nisbet DJ, van den Hurk AF, Taylor CT, Johansen CA, Macdonald J, Hall RA, Simmons RJ, Mason RJ, Lee JM, Ritchie SA, Smith GA, Mackenzie JS (2001). The appearance of a second genotype of Japanese encephalitis virus in the Australasian region. Am J Trop MedHyg 65: 747–753.
Ramakrishna C, Desai A, Shankar SK, Chandramuki A, Ravi V (1999). Oral immunisation of mice with live Japanese encephalitis virus induces a protective immune response. Vaccine 17: 3102–3108.
Raung SL, Kuo MD, Wang YM, Chen CJ (2001). Role of reactive oxygen intermediates in Japanese encephalitis virus infection in murine neuroblastoma cells. Neurosci Lett 315: 9–12.
Rey FA, Heinz FX, Mandl C, Kunz C, Harrison C (1995). The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature 375: 291–298.
Roehrig JT, Hunt AR, Johnson AJ, Hawkes RA (1989). Synthetic peptides derived from the deduced amino acid sequence of the E-glycoprotein of Murray Valley encephalitis virus elicit antiviral antibody. Virology 171: 49–60.
Shlim DR, Solomon T (2002). Japanese encephalitis vaccine for travelers: exploring the limits of risk. Clin Infect Dis 35: 183–188.
Solomon T (2000). Japanese encephalitis. In: Neurobase. Gilman S, Goldstein GW, Waxman SG, (eds). San Diego: Medlink Publishing. Available on CD-ROM.
Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT (2000a). Japanese encephalitis. J Neurol Neurosurg Psychiatry 68: 405–415.
Solomon T, Dung NM, Kneen R, Thao LT, Gainsborough M, Nisalak A, Day NP, Kirkham FJ, Vaughn DW, Smith S, White NJ (2002 a). Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain 125: 1084–1093.
Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LTT, Raengsakulrach B, Day NPJ, Farrar J, Myint KSA, Nisalak A, White NJ (2000b). Neurological manifestations of dengue infection. Lancet 355: 1053–1059.
Solomon T, Dung NM, Wills B, Kneen R, Gainsborough M, Diet TV, Thuy TTN, Loan HT, Khanh VC, Vaughn DW, White NJ, Farrar JJ (2002b). A double-blind placebocontrolled trial of interferon alpha in Japanese encephalitis. Lancet 361: 821–826.
Solomon T, Kneen R, Dung NM, Khanh VC, Thuy TTN, Ha DQ, Day NPJ, Nisalak A, Vaughn DW, White NJ (1998). Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet 351: 1094–1097.
Solomon T, Vaughn DW (2002). Clinical features and pathophysiology of Japanese encephalitis and West Nile virus infections. In: Current topics in microbiology and immunology: Japanese encephalitis and West Nile virus infections. Mackenzie JS, Barrett AD, Deubel V (eds). Berlin: Springer-Verlag, pp 171–194.
Stadler K, Allison SL, Schalich J, Heinz FX (1997). Proteolytic activation of tick-borne encephalitis virus by furin. J Virol 71: 8475–8481.
Su CM, Liao CL, Lee YL, Lin YL (2001). Highly sulfated forms of heparin sulfate are involved in japanese encephalitis virus infection. Virology 286: 206–215.
Tsai TF (2000). New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine 18 (Suppl 2): 1–25.
Uchil PD, Satchidanandam V (2001). Phylogenetic analysis of Japanese encephalitis virus: envelope gene based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am J Trop Med Hyg 65: 242–251.
Vaughn DW, Hoke CH (1992). The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol Rev 14: 197–221.
Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, Louder MK, Filgueira L, Marovich MA, Wong HK, Blauvelt A, Murphy GS, Robb ML, Innes BL, Birx DL, Hayes CG, Frankel SS (2000). Human skin Langerhans cells are targets of dengue virus infection. Nat Med 6: 816–820.
Xiao SY, Zhang H, Guzman H, Tesh RB (2001). Experimental yellow fever virus infection in the Golden hamster (Mesocricetus auratus). II. Pathology. J Infect Dis 183: 1437–1444.
Xin YY, Ming ZG, Peng GY, Jian A, Min LH (1988). Safety of a live-attenuated Japanese encephalitis virus vaccine (SA14-14-2) for children. Am J Trop Med Hyg 39: 214–217.
Author information
Authors and Affiliations
Corresponding author
Additional information
Some of the work described in this review was funded by the Wellcome Trust of Great Britain.
Rights and permissions
About this article
Cite this article
Solomon, T. Recent advances in Japanese encephalitis. Journal of NeuroVirology 9, 274–283 (2003). https://doi.org/10.1080/13550280390194037
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/13550280390194037