Abstract
Autoantigen-specific immunological tolerance represents a central objective for prevention of type 1 diabetes (T1D). Previous studies demonstrated mucosal antigen administration results in expansion of Foxp3+ and LAP+ regulatory T cells (Tregs), suggesting oral delivery of self-antigens might represent an effective means for modulating autoimmune disease. Early preclinical experiments using the non-obese diabetic (NOD) mouse model reported mucosal administration of T1D-related autoantigens [proinsulin or glutamic acid decarboxylase 65 (GAD)] delayed T1D onset, but published data are conflicting regarding dose, treatment duration, requirement for combinatorial agents, and extent of efficacy. Recently, dogma was challenged in a report demonstrating oral insulin does not prevent T1D in NOD mice, possibly due to antigen digestion prior to mucosal immune exposure. We used transplastomic plants expressing proinsulin and GAD to protect the autoantigens from degradation in an oral vaccine and tested the optimal combination, dose, and treatment duration for the prevention of T1D in NOD mice. Our data suggest oral autoantigen therapy alone does not effectively influence disease incidence or result in antigen-specific tolerance assessed by IL-10 measurement and Treg frequency. A more aggressive approach involving tolerogenic cytokine administration and/or lymphocyte depletion prior to oral antigen-specific immunotherapy will likely be required to impart durable therapeutic efficacy.
Similar content being viewed by others
Introduction
In type 1 diabetes (T1D), immunosuppression offered in a variety of forms provides a temporary reprieve from autoimmunity, but is unable to induce lasting immunological tolerance allowing for a reversal of disease once established1. The discovery of antigen-specific mucosal tolerance mechanisms (e.g., oral, nasal) has demonstrated utility in preventing autoimmunity2,3 and as such, precipitated a number of studies in disease-specific animal models4,5,6. Such approaches for inducing tolerance for the reversal and prevention of T1D have been actively pursued both in the non-obese diabetic (NOD) mouse model of the disease as well as in human clinical trials. Indeed, mucosal autoantigen administration has been reported to delay or prevent T1D in NOD mice7,8,9,10,11. Interestingly, these preclinical studies, while all reporting positive efficacy, are somewhat conflicting regarding the frequency and dosage of antigen administration, species of antigen used, requirement for combinatorial agents and perhaps most importantly, the extent of therapeutic benefit. In humans, nasal insulin administration provided no clinical benefit while imparting subclinical immunological effects12. Oral insulin has had limited efficacy, possibly delaying T1D onset in a subpopulation of subjects having high levels of anti-insulin autoantibodies (IAA)13,14.
A recent report by Pham et al. argued that oral insulin treatment is in fact, unable to delay or prevent T1D onset in NOD mice, possibly due to autoantigen digestion prior to reaching the mucosal immune interface, a process that limits bioavailability and subsequent tolerance induction15. To address this in the setting of T1D, transgenic plant cells expressing disease-related autoantigens16,17,18,19 (i.e., insulin, glutamic acid decarboxylase 65 (GAD)) have been used to deliver intact protein to the small intestine’s gut associated lymphoid tissue. Upon oral delivery, the plant cell wall protects the expressed protein(s) of interest from acids and enzymes in the stomach via bio-encapsulation as human digestive enzymes are incapable of breaking down glycosidic bonds in carbohydrates that make up the plant cell wall. However, when intact plant cells containing autoantigen proteins reach the small intestine, commensal microbes digest the cell wall releasing the autoantigens. When fused with suitable transmucosal carriers (e.g., cholera toxin’s non-toxic subunit B (CTB)), antigens are delivered more efficiently to the immune or circulatory system20,21,22. Transgenes can be expressed in plants via the nuclear or chloroplast genome. Nuclear transgene integration presents fundamental challenges due to low autoantigen expression levels17 and risk of transgene dissemination via pollen. As a result, transplastomic tobacco plants have been engineered to express desired proteins in the chloroplast, resulting in consistent high level production of protein due to high copy number of chloroplast genomes while at the same time, eliminating the possibility of unintentional environmental release owing to the maternal inheritance of the chloroplasts19,20,23.
Our goal in this study was to induce autoantigen-specific tolerance and prevent T1D in the NOD mouse via an oral vaccine that could potentially be translated for clinical application. We used transplastomic tobacco expressing two key T1D autoantigens, human proinsulin (hpINS) and GAD. hpINS was expressed as a fusion protein with CTB which binds the monosialotetrahexosylganglioside (GM1) receptor on intestinal epithelial cells and serves as a transmucosal carrier to increase autoantigen bioavailability19,21. CTB-hpINS ─ GM1 binding has been previously characterized in vitro19, and CTB-mediated delivery has been validated in vivo using tobacco leaves expressing CTB fused to green fluorescent protein21. Oral treatment with plant cells expressing CTB-hpINS tobacco was shown to reduce insulitis in NOD mice at 15 weeks of age, but animals were not followed for disease prevention19. In similar experiments, oral delivery of plant cells expressing coagulation factors 8 and 9 (FVIII and FIX, respectively) prevented anaphylaxis in murine models of hemophilia by inducing antigen-specific tolerance to those proteins24,25. Extensive studies on the mechanism of tolerance induction using bioencapsulated CTB-FIX or CTB-FVIII suggest a complex, IL-10 dependent mechanism, ultimately resulting in the induction of CD4+CD25+Foxp3+ Tregs and LAP+CD4+CD25−Foxp3− Tregs that actively suppress anti-FIX or FVIII antibody formation25,26. Indeed, both CD4+LAP+ and CD8+LAP+ cells have been shown to confer tolerance and suppress autoimmune disease27,28. Moreover even at low doses (1.5 μg), oral administration of the plant-made CTB-acid alpha-glucosidase (GAA) fusion protein prior to GAA injection significantly suppressed GAA-specific immunoglobulin formation in a mouse model of Pompe disease29.
In order to improve upon past attempts to prevent T1D via mucosal tolerance and to address unanswered questions regarding dosing and the potential synergistic benefits of combination therapy, we conducted two phases of experimentation. The purpose of the first phase of study was dose optimization. Two transplastomic plants expressing T1D-related autoantigens were each administered at three doses: 25 μg (low), 250 μg (medium), and 500 μg (high) of CTB-hpINS or GAD, once per week for 10 weeks to female NOD mice beginning at 5 weeks of age. Oral treatments were given alone and in combination with 100 μg all-trans retinoic acid (ATRA), which was expected to facilitate mucosal tolerance induction due to its role in TGF-β-mediated induction of Foxp3 expression in T cells30,31,32. In the second phase, we sought to test the optimal dose of each plant material, as determined in phase 1, combine the therapies, and elucidate their potential mechanisms in preventing the disease. The overall aim of this work was to optimize a combination oral therapy to induce autoantigen-specific immunological tolerance and prevent T1D in NOD mice using plants expressing two key autoantigens; however, oral treatments of varying dose, duration, and combination were unable to significantly prevent T1D onset in NOD mice.
Methods
Creation of Transplastomic Plants Expressing CTB-hpINS and GAD, and Confirmation of Homoplasmic Lines
Transplastomic tobacco plant leaves expressing CTB-hpINS were created as published19. Human GAD65 cDNA was purchased (American Type Culture Collection, Manassas, VA), and the amplified PCR product was cloned into the chloroplast transformation vector, pLD-utr. Plants were transformed using biolistic particle delivery system, and specific integration of the GAD65 expression cassette was investigated as previously described33. Total genomic DNA was digested with restriction enzyme, ApaI, and the blotted membrane was probed with radioisotope-labeled flanking region fragment for Southern blot analysis.
Western Blot Analysis and Quantification of GAD by ELISA
Protein extraction, western blot, densitometric analysis of CTB-hpINS expression, and ELISA for GAD expression were performed as described previously19,34. Briefly, to extract total soluble proteins, leaves were ground in liquid nitrogen to fine powders; then 100 mg was resuspended in 300 μL extraction buffer [100 mM NaCl, 10 mM EDTA, 200 mM Tris-Cl pH 8.0, 0.1% (v/v) Triton X-100, 400 mM sucrose, 2 mM PMSF, and proteinase inhibitor cocktail] with vigorous vortexing (~30 seconds) followed by sonication using a Misonix Sonicator 3000 (twice pulse on for 5 seconds and pulse off for 10 seconds). The soluble fraction was obtained by centrifuging the homogenate at 13,000 g at 4 °C for 10 minutes. For 10% SDS PAGE, 15 μg soluble proteins were combined with 1X Laemmli sample buffer included with 100 mM DTT and 2% SDS, and boiled for 3 minutes. To detect GAD65 expressed in tobacco leaves by western blot, we applied goat anti-human GAD65 polyclonal primary antibody (Abcam, Cambridge, MA) diluted 1 in 1,500 and donkey anti-goat IgG-HRP detection antibody (Santa Cruz Biotechnology, Dallas, TX) diluted 1 in 3,000 in 3% non-fat dry milk in PBST (0.1% tween 20) solution. Glutathione S-transferase (GST)-tagged GAD2 (molecular weight = 91.8 kDa) was included as a positive control, and untransformed (UT) WT tobacco extracts were included as for a negative control.
NOD Mice
4 week-old female NOD/ShiLtJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed 5 mice per cage in the Animal Care Services Biomedical Sciences Building SPF facility at the University of Florida, with food and water available ad libitum. All procedures were performed in accordance with guidelines and regulations put forth by the University of Florida Institutional Animal Care and Use Committee (UF IACUC) and according to a protocol approved by UF IACU.
Autoantigen Preparation and Delivery
Phase 1
For phase 1 studies, animals were allocated into 14 treatment groups: three doses of each autoantigen-expressing tobacco leaf material— CTB-hpINS or GAD (25 μg, 250 μg, and 500 μg autoantigen protein), and WT control— each with or without 100 μg ATRA (Sigma-Aldrich Co. LLC; St. Louis, MO) dissolved in tocopherol-stripped corn oil (Fisher Scientific; Pittsburgh, PA). Aliquots were prepared for frozen powdered plant cells: 2.5 g CTB-hpINS plant cells (5 mg CTB-hpINS/g plant cells yielded a total of 12.5 mg CTB-hpINS per aliquot) or 4 g GAD (3 mgGAD/g plant cells yielded a total of 12 mg GAD per aliquot). Frozen powdered plant cells were then, suspended in PBS (final volume of 2.5 mL CTB-hpINS or 2.4 mL GAD) for the “high” dose (500 μg autoantigen/100 μL). The suspension was homogenized for one minute on ice using an Omni GLH (Omni International; Kennesaw, GA), and serial dilutions were performed for the “medium” 250 μg and “low” 25 μg doses. ATRA was dissolved in tocopherol-stripped corn oil at 2 mg/mL. Immediately prior to oral delivery, 300 μL corn oil (with or without ATRA) was emulsified with 600 μL homogenized plant cells between two sterile 3 mL syringes using an emulsification needle. 150 μL of the emulsion was delivered to each mouse via oral gavage. Beginning at 5 weeks of age, the tobacco-oil emulsion was delivered via oral gavage once per week for ten weeks. Mice were followed until T1D onset (treatment failure) or 32 weeks of age.
Phase 2
For phase 2 efforts, animals were allocated into 5 treatment groups: 1) Untreated control; 2) WT control tobacco; 3) 500 μg GAD; 4) 250 μg CTB-hpINS; and 5) 500 μg GAD + 250 μg CTB-hpINS. WT and CTB-hpINS plant materials were provided in frozen form (same batch as phase 1) while GAD tobacco materials were in lyophilized form. Plant materials were suspended in PBS and homogenized similar to the methods described above to yield the desired autoantigen doses. The homogenate was not emulsified with oil as ATRA was excluded. 150 μL homogenate was delivered via oral gavage once per week with the combination therapy group receiving two gavages per week (one containing CTB-hpINS and one containing GAD). Weekly oral treatment began at 5 weeks of age and continued until animals reached one of the defined endpoints: cross-sectional time points − 6, 10, and 14 weeks of age (n = 3 per group) or longitudinal time points - T1D onset or 32 weeks of age (n = 9–11 per group).
Progression to Diabetes
Beginning at 10 weeks of age, blood glucose was measured once per week via tail prick using an AlphaTRAK2 (Abbott Animal Health; Abbott Park, IL) glucose meter. Animals with blood glucose values (BGVs) ≥ 250 mg/dL were rescreened the following day. Diabetes was defined as two consecutive BGVs ≥ 250 mg/dL within 24 hours.
Necropsy and Processing of Tissues
At the defined endpoints, mice were sacrificed and blood, spleen, pancreas, and when possible, mesenteric and pancreatic lymph nodes (MLN and PLN, respectively) harvested. Serum was stored at −20 °C. Spleens, MLN, and PLN were freshly processed to single cell suspensions and red blood cells lysed as previously described35. Pancreata were fixed in 10% neutral buffered formalin at room temperature overnight, transferred to PBS, and stored at 4 °C.
Flow Cytometry
106 spleen, MLN, and PLN cells were stained as described35 for flow cytometric analysis [CD8b-FITC (clone eBioH35–17.2), CD4-PE Cyanine7 (clone GK1.5), CD25-APC (clone PC61.5), Foxp3-PE (clone NRRF-30), and in phase 2, LIVE/DEAD® Fixable Near-IR Dead Cell Stain Kit (Life Technologies; Grand Island, NY), LAP-PerCP eFluor710 (clone TW7-16B4)] using a BD LSRFortessa (Franklin Lakes, NJ) and FCS Express 4 Flow Research Edition software (De Novo Software; Glendale, CA). Lymphocytes were gated on forward and side scatter. Dead lymphocytes staining strongly positive for LIVE/DEAD® Fixable Near-IR were excluded. Antibodies were purchased from eBioscience (San Diego, CA).
ELISPOT
Phase 2
Splenocytes were analyzed for IFN-γ and IL-10 production in response to antigen-specific stimulation via ELISPOT kits (BD; Franklin Lakes, NJ) according to manufacturer’s instructions. Briefly, cells were plated in triplicate at 3 × 105 cells/well with the following stimulatory conditions: 1) unstimulated; 2) recombinant human insulin (1 μg/ml) (Sigma-Aldrich Co. LLC; St. Louis, MO); 3) recombinant human GAD (1 μg/ml) (KRONUS Inc.; Star, ID); 4) recombinant CTB (1 μg/ml) (Sigma-Aldrich Co.); and 5) soluble anti-CD3 (5 μg/ml) + anti-CD28 (2.5 μg/ml) (eBioscience). Cells were incubated at 37 °C and 5% CO2 for 65 hours. Cytokine spots were developed (6 minutes for IFN-γ and 15 minutes for IL-10) using 3-amino-9-ethylcarbazole (AEC) substrate (BD) according to manufacturer’s instructions. Spots were enumerated using a Bioreader® 4000 Pro-X and the Bioreader® software generation 8 (BIOSYS USA LLC; Miami, FL). Spots per well were summed, averaged across triplicates, and reported as a stimulation index relative to unstimulated cells.
Luminex
Sera collected at 4 and 16 weeks of age were diluted 1:12,500 and analyzed for total IgA, IgM, IgG1, IgG2a, and IgG2b titers via Milliplex® MAP Mouse Immunoglobulin Magnetic Bead Panel─Isotyping Multiplex Assay kit according to manufacturer’s filter plate procedure instructions (EMD Millipore; Billerica, MA). Plates were run on a Luminex® 200 Multiplexing Instrument with xPONENT® software version 3.1 (Luminex; Austin, TX) and analyzed using Milliplex® Analyst software version 5.1.0 (EMD Millipore; Billerica, MA).
ELISA
Sera collected at 4, 8, and 12 weeks of age were analyzed for insulin- and CTB-specific IgG, IgA, and/or IgM titers via ELISA. All antibodies were purchased from Abcam® except anti-mouse IgA detection antibody which came from ELISA Ready-Set-Go!® kit (eBioscience, Inc.; San Diego, CA). High binding clear 96-well microplates (Greiner Bio-One; Monroe, NC) were coated with 50 μL/well recombinant human insulin (Sigma-Aldrich Co.) or CTB (Sigma-Aldrich Co.) at 5 μg/mL in 0.1 M NaHCO3 (pH 9) overnight (O/N) at 4 °C and then, blocked with 200 μL/well 10% FBS O/N at 4 °C. For IgG, anti-insulin and anti-CTB standard curves were generated using mouse anti-insulin IgG clone IN-05 and mouse monoclonal anti-CTB IgG diluted in 10% FBS. Sera were diluted 1:30 in 10% FBS and added at 50 μL/well to duplicate wells. Plates were incubated O/N at 4 °C and washed five times with PBS containing 0.05% Tween 20. HRP-conjugated detection antibodies - polyclonal goat anti-mouse IgG-Fc, anti-mouse IgA detection antibody, and rat anti-mouse IgM-mu chain clone SB73a - were diluted in 10% FBS (1:40,000 IgG, 1:500 IgA, and 1:3,000 IgM), added at 50 μL/well, and incubated for 1 hour at RT. Plates were washed as described above. 50 μL/well TMB (eBioscience, Inc.; San Diego, CA) was added and developed at RT in the dark for 15 minutes. The reaction was stopped with 50 μL/well 1 N HCl (Fisher Scientific). Optical density was read immediately at 450 nm on a SpectraMax M5 microplate reader with SoftMax® Pro 4.8 Data Acquisition and Analysis Software (Molecular Devices, LLC.; Sunnyvale, CA).
Histology
Fixed, paraffin-embedded pancreata were stained via IHC at the University of Florida Molecular Pathology Core lab and evaluated for immune-phenotyping of the insulitic lesion. One section/pancreas was stained for CD3 (peroxidase-DAB) and B220 (alkaline phosphatase-Fast Red) and counterstained with hematoxylin as previously described36. Stained slides were scanned using an Aperio ScanScope CS and Spectrum Plus version 11 at 20 × magnification (Aperio Technologies; Vista, CA). Images were annotated using the Aperio ScanScope viewing program to exclude exocrine tissue. An area quantification macro was optimized, and analysis was completed using for the CytoNuclear IHC quantification software (Indica Laboratories; Albuquerque, NM) to enumerate islet area occupied by CD3+ T cells and B220+ B cells. Pancreata were flattened prior to processing, allowing for examination of the entire tissue, with an average number of islets examined per section = 31.84 ± 1.805 (Mean ± standard error of the mean (SEM)).
Statistical Methods
Data analyses were conducted using GraphPad Prism software v5.01 (GraphPad Software, Inc.; La Jolla, CA) for one-way ANOVA, two-way ANOVA, unpaired student’s t-test, chi-square test, and Kaplan-Meier life table analysis. Statistical significance was defined as P < 0.05. Error bars represent the mean ± SEM.
Results
Creation of Transplastomic Plants Expressing CTB-hpINS and GAD
Transplastomic tobacco plants expressing CTB-hpINS were created as published previously19. Plants expressing human GAD were created to test the potential for synergistic benefit in combining two autoantigens to induce oral tolerance and prevent TID in NOD mice. Antigen fusion to CTB has been shown to lower the dose required for oral tolerance induction17,37. However, attempts to express CTB-GAD in plant chloroplasts were unsuccessful. Therefore, this study was performed only with GAD expressed in chloroplasts and not CTB-GAD. The human GAD (hGAD65) gene was inserted into the chloroplast transformation vector – pLDutr. The constructed transformation vector was designed to allow for the specific integration of hGAD65 expression cassette into the intergenic space between isoleucyl-tRNA synthetase (trnI) and alanyl-tRNA synthetase (trnA) of the wild type chloroplast genome by double homologous recombination (Fig. 1A). The hGAD65 gene expression in chloroplasts was driven by the light regulated psbA promoter and 5′ untranslated region (UTR) to increase expression, and the expressed transcripts were stabilized by the psbA 3′ UTR (Fig. 1A). Transplastomic plants were subject to Southern blot assay to evaluate homoplasmy (transformation of all chloroplast genomes). Three independent lines showed transformed fragments of 7.3 kb while the untransformed wild type control fragment migrated to 4.0 kb (Fig. 1B) when probed with the flanking sequence (Fig. 1A). Absence of the native untransformed chloroplast genome 4.0 kb fragment in transplastomic lines (Fig. 1B) confirmed homoplasmy.
(A) Schematic diagram of chloroplast vectors containing the hGAD65 gene constructed into the chloroplast transformation vector. (Prrn = chloroplast rRNA operon promoter; aadA = aminoglycoside 3′-adenylytransferase gene; PpsbA = psbA gene promoter; hGAD65 = coding sequence of human GAD; TpsbA = 3′ UTR of psbA gene; trnI = isoleucyl-tRNA; trnA = alanyl-tRNA). (B) Southern blot analysis of ApaI digested total DNA from untransformed control (UT) and GAD transplastomic tobacco lines (#1, #2, and #3) probed with the radioisotope-labeled flanking region fragment as shown in (A). (C) Western blot probed with GAD antibody; 15 μg of total leaf soluble protein was loaded in each lane; UT: untransformed plant; young, mature, and old transplastomic leaves were harvested at 6 PM. GST-GAD: 100 ng, positive control. ELISA quantitation using goat polyclonal anti-GAD65 (diluted 1:1500), is shown as a percentage of the total soluble proteins (TSP). Data expressed as mean ± SD of three independent experiments.
To evaluate GAD expression, leaves of different ages were harvested at 6 pm for maximum yield, and total soluble proteins were extracted and analyzed via western blot. In addition to polypeptides at approximately 65 kDa (correct size), >140 kDa and 40 kDa polypeptides cross-reacting with the GAD antibody were also observed (Fig. 1C), probably representing a cleaved product and post-translational modification of GAD65 within the chloroplasts including phosphorylation, acetylation38, and palmitoylation39,40. Additions of these small molecules via post-translational modification appeared to cause a small increase in molecular weight at the 65 kDa band (Fig. 1C), supporting the notion that lipidation of GAD65 could cause hydrophobic aggregation during the boiling step of sample preparation, resulting in the higher molecular weight bands41. However, the cleaved product was not observed in native gels (data not shown), indicating that this may be the artifact of the SDS gel. Primary antibody specificity was confirmed using GST-tagged GAD2 positive control and WT tobacco extracts as a negative control. GAD expression levels were quantified via ELISA. Expression varied between 12.9% and 24.4% of total soluble protein (TSP) and was highest in mature leaves (Fig. 1D). Therefore, the mature leaves harvested at 6 pm were used for animal studies. The average expression levels (mg/g plant powder) were 5.42 mg/g CTB-hpINS (frozen) and 3.33 mg/g GAD (frozen) or 15.3 mg/g GAD (lyophilized).
Preclinical Effects of Oral Therapies on Diabetes Progression in NOD mice
Oral Therapy with Plants Expressing CTB-hpINS or GAD appeared to delay T1D Onset in NOD Mice but this effect was not statistically significant
In the first round of experiments (phase 1), NOD mice received weekly oral gavages for 10 weeks beginning at 5 weeks of age. Gavages containing three doses of GAD, CTB-hpINS or WT control were administered alone as well as in combination with ATRA (Fig. 2A,B). Six weeks after completion of oral feeding (at 20 weeks of age), 57% of NOD mice fed with untransformed leaves were diabetes free, whereas 100% of mice fed with the highest dose of GAD (n = 7, Fig. 2C) and 87% of mice fed with the medium dose of CTB-hpINS (n = 15, Fig. 2D) were diabetes free. Thus, there was a trend (P = 0.07) toward reduced frequency of early diabetes onset (prior to 20 weeks of age) in both of these groups compared to animals treated with WT tobacco (Fig. S1). These results are similar to previous studies using the same CTB-hpINS plant materials19 even though the amounts of antigen fed in this study are approximately 1.8, 17.9 and 35.7 fold higher for the low, medium, and high doses, respectively. However at 32 weeks of age, there was no significant difference in T1D incidence (Fig. 2). Addition of ATRA did not have any beneficial effect (Fig. 2B).
T1D progression was not significantly different in animals receiving autoantigen-expressing tobacco versus control (WT) tobacco (A) without or (B) with ATRA (P = 0.71 and P = 0.47, respectively). (C,D) Early T1D onset was defined as occurring prior to 20 weeks of age, indicated by a dashed line. There was a trend toward delayed T1D onset in mice that received oral treatment with (C) 500 μg GAD (GAD High) and (D) 250 μg CTB-hpINS (Proinsulin Medium; gray lines) versus WT (black line) control tobacco, P = 0.58 and P = 0.16, respectively, (Gehan-breslow-wilcoxon test).
Combination Oral Therapy with CTB-hpINS plus GAD. Did Not Result in Synergism to Prevent T1D Onset in NOD Mice
Since monotherapy appeared to delay early diabetes onset, we sought to determine whether combination therapy would be synergistic for sustained tolerance induction and disease prevention. We also hypothesized that the tolerogenic effect of phase 1 therapy might be lost upon treatment cessation and therefore, continued oral therapy throughout the study’s entirety. We chose the two most effective therapies (i.e., the medium dose of CTB-hpINS and the highest dose of GAD) for use in combination for phase 2 efforts. Beyond this, since the phase 1 experiments demonstrated no therapeutic benefit of combination with ATRA, this was not included during phase 2.
There was a 22% difference in the proportion of diabetic mice between those autoantigen-treated and WT-treated animals (Fig. 3A); however, the study was not sufficiently powered to detect a difference less than 50%, an effectiveness in line with previous oral antigen studies in NOD mice. Thus, T1D incidence and progression did not differ significantly between NOD mice that received oral autoantigen therapy (either alone or in combination). Compared to tobacco-treated animals (including WT), untreated mice had a more dramatic onset of diabetes with at least one of the two consecutive diagnostic BGVs exceeding 400 mg/dL in the majority of those animals (Figs 3B, S2A–E). The CTB-hpINS fed group showed the lowest rate of severity, with 11% (n = 1/9) mice showing >400 mg/dL diagnostic BGV (Fig. 3B). In the previously reported study19, none of the untreated mice showed >400 mg/dL BGV at any point, though those animals were sacrificed prior to expected time of peak T1D incidence.
(A) Continuous oral treatment with tobacco leaves expressing 500 μg GAD (gray, short dash), 250 μg CTB-hpINS (black, short dash), or the combination of both (gray, solid line), were compared to untreated (black, solid line) or WT control tobacco-treated animals (black, long dash), P = 0.24 (Log-rank test). (B) Animals experiencing catastrophic failure (black), non-catastrophic failure (gray), or no failure (those animals that survived to 32 weeks of age, white) are represented P = 0.08 (Chi-square test). T1D incidence did not differ between Phase 1 (dashed lines, treatment duration: 10 weeks) and Phase 2 (solid lines, treatment duration: ongoing through study endpoint) for (C) CTB-hpINS, (D) GAD, and (E) WT tobacco-treated animals P = 0.97, P = 0.96, and P = 0.32, respectively (Log-rank test). Number of animals (n) per group is indicated in the figure.
Results were highly reproducible between phase 1 and phase 2: 87% in phase 1 (n = 13/15) and 67% in phase 2 (n = 6/9) of animals fed 250 ug CTB-hpINS remained diabetes free at 20 weeks of age with total T1D incidence of 60% (phase 1) and 56% (phase 2) by 32 weeks (Fig. 3C). Similarly, 100% (n = 7) in phase 1 and 89% (n = 8/9) in phase 2 of NOD mice that received 500 ug GAD alone remained T1D free to 20 weeks (Fig. 3B). T1D progression was also comparable between phases 1 and 2 for animals receiving WT control tobacco (Fig. 3E). Continued feeding of either CTB-hpINS or GAD did not sustain the possible beneficial effects observed up to 20 weeks suggesting the need to explore other key factors involved in long-term maintenance of tolerance.
Immunological Effects of Oral Therapies in NOD mice
Prolonged Oral Autoantigen Therapy Increased Tregs in the Spleen but Not Disease-Associated Draining Lymph Nodes
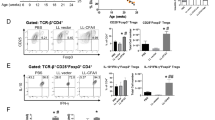
In phase 1, there were no observed changes in CD4+CD25+Foxp3+ Treg frequency in the spleens of mice treated with tobacco expressing 500 μg GAD, 250 μg CTB-hpINS, or WT tobacco (Fig. 4A). The inclusion of ATRA was similarly not associated with an increase in Treg frequency in the spleen (Fig. 4B). In phase 2, there were no differences in CD4+CD25+Foxp3+ Treg frequency within the spleen at 6, 10, and 14 weeks of age (Fig. 4C–E). However, splenic Treg frequencies were increased in mice treated with CTB-hpINS as well as CTB-hpINS plus GAD, relative to untreated animals at longitudinal endpoints (Fig. 4F). This effect was particularly evident among treated mice that did not progress to hyperglycemia (Fig. 4G). LAP+ T cells were not detectable in the spleen for any time point or treatment group. There were no changes in the frequency of CD4+CD25+Foxp3+ Tregs or LAP+ T cells in the PLN or MLN in any animals – progressors or not (Fig. 5 and S3).
(A) Phase 1 treatment of NOD mice with tobacco expressing 500 μg GAD (n = 7) or 250 μg CTB-hpINS (n = 14) without ATRA did not affect Treg frequency in the spleen compared to treatment with WT (n = 14), P = 0.73. (B) Treatment with ATRA + WT tobacco (n = 14) did not affect Treg frequency compared to WT tobacco-treated mice (n = 14), P = 0.62 (ANOVA).At (C) 6, (D) 10, and (E) 14 weeks of age as well as (F,G) at T1D onset or 32 weeks of age, fresh splenocytes were stained for CD4, CD8, CD25, LAP, and Foxp3 for flow cytometric analysis. Live lymphocytes were analyzed for the percent of CD4+CD25+Foxp3+ Tregs. (C–E) There was no difference in Treg frequency at cross-sectional time points, P = ns (all). (F) At study endpoint (T1D onset or 32 weeks of age), mice treated with CTB-hpINS tobacco, alone or in combination with GAD tobacco, demonstrated significantly greater Treg frequencies compared to untreated NOD mice, P < 0.05. (G) Among animals that received combination therapy, Treg frequency was significantly higher in those that were euglycemic at 32 weeks of age compared to diabetic animals, P < 0.001 (ANOVA). No Tx indicates untreated control NOD mice.
At T1D onset or 32 weeks of age, (A–D) PLN and (E-H) MLN cells were stained for CD4, CD8, CD25, LAP, and Foxp3 for flow cytometric analysis. Live lymphocytes gated on CD4 were analyzed for the percent of CD25+Foxp3+ Tregs. There was no difference in Treg frequency in the (A) PLN or (E) MLN, P = 0.29 and P = 0.12, respectively. There was no significant difference in the percent of live lymphocytes that were (B,F) CD8+LAP+ T cells, P = 0.997 and P = 0.32, respectively; (C,G) CD4+LAP+ T cells, P = 0.58 and P = 0.23, respectively; and (D,H) CD4+CD25+Foxp3+LAP+ Tregs, P = 0.89 and P = 0.06, respectively (ANOVA). No Tx indicates untreated control NOD mice.
Oral Autoantigen Therapy Did Not Induce Antigen-Specific Tolerance
Oral autoantigen therapy failed to induce antigen-specific tolerance as measured by ELISPOT for splenocyte IL-10 and IFN-γ production in response to insulin, GAD, or CTB stimulation (Fig. S4). While IFN-γ production was reduced in response to insulin stimulation in CTB-hpINS-treated animals at 14 weeks of age (Fig. S4I), there was no increase in IL-10 production (Fig. S4K). However, these responses were not sustained at longitudinal endpoints (T1D onset or 32 weeks of age) (Fig. S4M,O). In fact, at these time points, splenocytes from animals treated with CTB-hpINS demonstrated reduced IL-10 production in response to GAD stimulation compared to splenocytes from untreated controls, which may indicate an unexpected off-target side-effect and failure to induce bystander suppression (Fig. S4P).
Oral Therapy Induced Differential Effects on Humoral Immunity
In phase 1, total serum IgM, IgG1, IgG2b, or IgG3 levels did not differ between treatment groups prior to the initiation of oral treatment (Fig. S5A–D). At 16 weeks of age, total IgG1, IgG3, and IgA did not differ across treatment groups (Fig. S5E–G) or between progressors and non-progressors to T1D (Fig. S5H–J), but total serum IgM was elevated in the 250 μg CTB-hpINS-treated animals (Fig. 6A) that progressed to diabetes (Fig. 6B). However, the effect was not antigen-specific (for insulin or CTB) (Fig. S5K–L). Similarly, total IgG2b was increased in mice that received CTB-hpINS compared to WT-treated animals at 16 weeks (Fig. 6C). Because IgG2b class switching is induced by TGF-β, it has been used as a marker for mucosal tolerance42,43,44, but IgG2b levels did not associate with disease onset among CTB-hpINS-treated animals (Fig. 6D).
Phase 1: At 16 weeks of age, total serum (A) IgM and (C) IgG2b were increased in animals treated with tobacco expressing 250 μg CTB-hpINS compared to WT-treated animals, P < 0.01 and P < 0.01, respectively. (B) High IgM was unique to animals that would progress to T1D, P < 0.0001. (D) Total serum IgG2b was higher in euglycemic animals that received 250 μg CTB-hpINS compared to diabetic controls but not compared to treated animals that would become diabetic, P < 0.05 (ANOVA). (E) Anti-insulin IgA, as measured by OD fold change relative to 4 weeks of age, was significantly different between WT and CTB-hpINS-treated animals at 12 weeks of age, P < 0.05 (unpaired student’s t-test). (F) Anti-CTB IgA as measured by absolute OD was significantly increased in animals treated with tobacco expressing 250 μg CTB-hpINS compared to WT, P < 0.001 (ANOVA). Among animals treated with plant leaves expressing CTB-hpINS, (G) anti-insulin and (H) anti-CTB IgA levels did not differ between animals that would remain euglycemic (filled shapes) or become diabetic (open shapes) during the course of the study, P < 0.0001 and P < 0.001, respectively (ANOVA). (I) Anti-insulin IgG titers did not differ significantly over time in animals treated with WT tobacco, P = 0.08. (J) Animals treated with 250 μg CTB-hpINS displayed increased anti-insulin IgG at 8 and 12 weeks, relative to 4 weeks of age, P < 0.001 (ANOVA), but there was no difference in directly comparing WT- and CTB-hpINS treated animals at 12 weeks of age, P = 0.99 (unpaired student’s t-test). (K) Anti-insulin IgG titers did not differ between animals that would become diabetic or remain euglycemic during the study, P < 0.001 (ANOVA). No Tx indicates untreated control NOD mice.
Anti-insulin and -CTB IgA levels were significantly increased at 12 weeks of age in mice treated with CTB-hpINS compared to WT tobacco (Fig. 6E,F). IgA class switching has been shown to occur in the presence of TGF-β45,46. Although this might suggest a tolerogenic effect, in those animals treated with CTB-hpINS tobacco, neither anti-insulin IgA (Fig. 6G) nor anti-CTB IgA (Fig. 6H) positivity predicted T1D onset. Anti-insulin IgG was also significantly elevated in CTB-hpINS-treated animals at 8 and 12 weeks of age (relative to 4 weeks) while titers did not increase significantly in WT-treated animals over time (Fig. 6I,J). However, titers were not higher in CTB-hpINS-treated animals compared directly against WT-treated mice at 12 weeks of age. Among CTB-hpINS-treated animals, insulin-specific IgG levels were not associated with eventual progression to T1D (Fig. 6K). During phase 2, however, the oral delivery of CTB-hpINS tobacco did not induce an insulin- or CTB-specific IgG or IgA response (Fig. 7). Serum collected from mice treated with CTB-hpINS during phase 1 was included as a positive control (Fig. 7C).
Phase 2: Serum (A) anti-insulin IgG, (B) anti-CTB IgG, (C,D) anti-insulin IgA, and (E,F) anti-CTB IgA were measured at 4, 8, and 12 weeks of age via ELISA. (A) Anti-insulin IgG was elevated in untreated and WT tobacco treated animals at 12 weeks of age relative to 4 weeks of age, P < 0.0001. (B) Anti-CTB IgG did not differ significantly across treatment groups over time, P = 0.37. (C) Serum anti-insulin IgA levels (as measured by absolute OD) were significantly higher in phase 1 CTB-hpINS treated mice (positive control) compared to all time points and treatment groups except for 12 week-old GAD tobacco treated animals, P < 0.0001, but (D) at 12 weeks of age, there was no difference between treatment groups as measured by OD fold change, P = 0.43. (E) At 12 weeks of age, anti-CTB IgA was elevated in mice that received combination therapy as measured by absolute OD, P < 0.01, but (F) the difference was not significant when measured by OD fold change at 12 weeks of age, P = 0.07 (ANOVA).
Oral Antigen Therapy Did Not Modulate the Immune Phenotype of the Insulitic Lesion
During phase 2, pancreata harvested at 6, 10, and 14 weeks of age were analyzed for CD3+ and B220+ cells within the islets. As expected, the percentage of islet area infiltrated by CD3+ and B220+ cells appeared to increase over time; however, for each time point, there were no significant differences between treatment groups (Fig. 8) suggesting that therapy did not reduce or delay T or B cell-mediated insulitis.
(A) Pancreas sections were stained for B220 (red) and CD3 (brown) via IHC. Annotations were drawn to include only islet area for analysis. (B) The percentage of islet area infiltrated by B220+ (red) and CD3+ (green) was quantified using an optimized algorithm. At (C,F) 6, (D,G) 10, and (E,H) 14 weeks of age there was no difference between treatment groups regarding (C–E) B220+ and (F–H) CD3+ islet area, P = ns, all (ANOVA). N = 2–3 mice per group per time point.
Discussion/Conclusions
In this two-phase study, we sought to improve upon previous attempts to induce autoantigen-specific oral tolerance for the prevention of T1D by utilizing an innovative method for the mucosal delivery of two T1D-related autoantigens in a combination therapy. Transplastomic tobacco plants expressing GAD and CTB-hpINS were tested alone and in combination to optimize an oral tolerogenic vaccine for the prevention of T1D using the leading animal model of spontaneous autoimmune diabetes, the NOD mouse. We hypothesized that oral therapy would not only result in synergism but also, overcome limitations associated with oral therapy - namely, autoantigen digestion - affording efficient autoantigen delivery for improved efficacy. Furthermore, we expected that co-administration of the tolerogenic adjuvant, ATRA, would promote tolerance induction to elicit the desired immunological effect in NOD mice, a strain that reportedly exhibits impaired mucosal tolerance mechanisms relative to non-diabetes-prone strains47,48.
In this study dosage is referred to the amount autoantigen gavaged in NOD mice, but dose delivered to circulation or the immune system was not quantifiable. The observed suboptimal outcome with monotherapies (phase 1) left room for us to test for synergism in combination treatment and suggests that a short-course of treatment may not be sufficient for lasting tolerance induction, which is in agreement with previous reports using plant cells expressing FVIII and FIX to induce oral tolerance24,25. Expressed another way, we hypothesized that the tolerogenic effect of therapy was lost upon the cessation of treatment in phase 1 and that continued oral therapy would result in extended protection from T1D onset. Thus, the medium dose of CTB-hpINS and the highest dose of GAD were selected for a combination therapy during phase 2. Unfortunately, these protocol modifications did not result in improvements in terms of disease prevention. Kaplan-Meier survival curves for each tobacco treatment were comparable between phases 1 and 2, suggesting that initial stopping of therapy did not precipitate T1D onset and that ongoing treatment in phase 2 did not improve efficacy. Oral autoantigen therapy alone may thus, not be successful in preventing T1D, regardless of treatment duration. Moving forward, it may be beneficial to consider a combination of two individual treatments that demonstrate some, though limited success, as shown by Bressen et al.49.
With respect to other observations of potential importance, oral tobacco therapy (including WT) resulted in reduced weight gain in young mice compared to untreated animals. Hence, it is possible that the metabolic stimulus of nicotine or reduced food consumption may have been attributable to gavage of large quantities of plant cells; nonetheless, the metabolic effects of treatment may have influenced disease kinetics. Oral therapy with plant cells expressing CTB-hpINS or GAD appeared to delay T1D onset in both phase 1 and phase 2 studies in NOD mice, but this effect was not statistically significant or sustained with prolonged treatment. Beyond this, while our cohorts were not undersized by current standards, without greater statistical power it cannot be definitively determined whether small differences (22% in phase 2) in T1D incidence were attributable to autoantigen therapy or unforeseen effects of oral tobacco treatment. This argues that the pressure to use minimal animal numbers may not be suitable when evaluating combination therapies for disease prevention in NOD mice where colony- and batch-specific effects may influence T1D kinetics.
We anticipated oral therapy would induce antigen specific tolerance and potentially, infectious tolerance. Instead, splenocytes from CTB-hpINS treated animals showed reduced production of IL-10 production under GAD stimulation compared to untreated controls signifying a potential off-target effect of the therapy that none-the-less, failed to induce bystander suppression capable of preventing disease. In most previous studies using antigens bioencapsulated in plant cells for the induction of oral tolerance, IL-10 and/or Treg induction was routinely observed3,22,24,25,26,29. Thus, our findings may be related to the known T1D-related defects in Treg function50 and indicate a need for a more aggressive approach to tolerogenic therapy. Alternatively, this could be the result of insufficient delivery of GAD to the mucosal immune system since this protein could not be expressed in tobacco as a fusion protein with CTB. Oral delivery of plant leaves expressing autoantigens also failed to modulate the frequency of CD4+CD25+Foxp3+ Tregs or LAP+ T cells in MLN and PLN. This said, future investigations should include flow cytometric analysis of cytokine expression and Th signature transcription factors among insulin-tetramer+ T cells in each of these tissues to more conclusively determine the antigen-specific effects of oral therapy.
Interestingly in phase 1, oral CTB-hpINS treatment resulted in increased levels of insulin- and CTB-specific IgA and IgG, but neither was associated with eventual progression to T1D. Oral tolerance has been shown to reduce antigen-specific IgA and IgG in response to oral challenge51, hence our findings may indicate therapy failure which aligns with the observed therapeutic outcome. Additionally, it is possible that CTB- and/or insulin- specific IgA could reduce bioavailability of the fusion protein at the mucosal surface, thereby limiting therapeutic effect over time. Thus, further investigation is needed to discern the significance of these humoral immune responses observed during phase 1. Serum collected at T1D onset or 32 weeks of age did not contain elevated anti-insulin or -CTB IgG and IgA, and mice from phase 2 efforts did not seroconvert at any point during the course of treatment. This discordance is certainly intriguing as the only difference in vaccine formulation was the exclusion of tocopherol-stripped corn oil during the second phase of the study. Indeed, this is most likely due to adjuvant effect of corn oil as shown in a recent study in which plant oil compounds increased polio antigen antibodies (IgA, IgG1)52. It is possible that the oil, which is largely composed of linoleic acid, may have directly exerted unforeseen immunological effects53,54,55,56 or alternatively, might have affected antigen or immune cell trafficking in the lymph57,58.
Together, our data suggest that weekly oral autoantigen therapy, alone, is not sufficient to prevent spontaneous T1D in the NOD mouse, and that the delivery of two autoantigens in combination does not result in synergism, possibly because GAD was not fused with CTB. This result was quite surprising given comparison to previous accounts of oral tolerance induction in NOD mice8,9,10,16,17,18,19,59. However, we do find it corroborative of somewhat less-discussed notions that mucosal tolerance mechanisms are likely confounded in NOD mice and autoimmune-prone individuals15,47,48,60,61, even in instances of nasal immunization with foreign antigen, such as hen egg lysozyme or ovalbumin47,48. In that regard, inclusion of some form of “positive control” would have clearly benefitted these studies and if identified, should be included in future efforts. This said, previous studies using transplastomically expressed antigens for oral tolerance have demonstrated robust efficacy in preventing the development of anaphylactic response to peripherally administered antigen24,25,26 suggesting that the inability to prevent autoimmunity and T1D may stem from the disease and its animal model (e.g., self antigen vs. foreign, autoimmune NOD vs. immunologically “normal” mice) rather than the delivery vehicle.
A critical evaluation of the early literature would reveal that in studies involving multiple NOD colonies, T1D incidence in NOD mice was incredibly low even among control animals8,10. For example, “prevention” in one study saw a disease frequency of approximately 48 percent in control female NOD mice, versus 30 percent of oral insulin treated mice, at one year. Beyond this, other studies yielded inconsistent outcomes regarding the most effective form of antigen, combinatorial agents, and treatment regimen10,16,17,18,59,62. In fact, a recent effort by Pham et al. demonstrated that oral therapy with unprotected insulin protein does not prevent T1D in NOD mice suggesting a need for autoantigen encapsulation15. Here we encapsulated two autoantigens (hpINS and GAD) using a plant-based expression system yet were unable to induce oral tolerance or significantly prevent disease.
However, in no way does this unequivocally discount antigen-specific immunotherapy as a means for intervention but rather, calls for further treatment optimization. For example, low dose anti-CD3 plus oral inoculation with Lactococcus lactis expressing IL-10 and either GAD or hpINS has demonstrated remarkable efficacy toward the reversal of new-onset T1D in NOD mice35,63. Plant-based autoantigen delivery provides the benefits of more accurate dosing and reduced complications associated with maintaining live bacterial cultures for clinical translation. Therapeutic proteins are expressed at exceptionally high levels, and lyophilized plant cells can be stored for several months or years without a decrease in their functionality, thereby eliminating prohibitively expensive processes currently required for the production of protein drugs including fermentation, purification, cold storage/transportation and sterile delivery64,65. Thus, we anticipate that a similar approach using potent immune-depleting agents (e.g., Thymoglobulin, anti-CD3) prior to oral treatment and tolerizing agents (e.g., GCSF, IL-10) in combination with the transplastomic plant cells will build upon these findings and potentially provide a therapy more suitable for clinical use.
Additional Information
How to cite this article: Posgai, A. L. et al. Plant-based vaccines for oral delivery of type 1 diabetes-related autoantigens: Evaluating oral tolerance mechanisms and disease prevention in NOD mice. Sci. Rep. 7, 42372; doi: 10.1038/srep42372 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Staeva, T. P., Chatenoud, L., Insel, R. & Atkinson, M. A. Recent lessons learned from prevention and recent-onset type 1 diabetes immunotherapy trials. Diabetes 62, 9–17, doi: 10.2337/db12-0562 (2013).
Faria, A. M. & Weiner, H. L. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol 13, 143–157, doi: 10.1080/17402520600876804 (2006).
Wang, X. et al. Mechanism of oral tolerance induction to therapeutic proteins. Adv Drug Deliv Rev 65, 759–773, doi: 10.1016/j.addr.2012.10.013 (2013).
Kasarello, K., Kwiatkowska-Patzer, B., Lipkowski, A. W., Bardowski, J. K. & Szczepankowska, A. K. Oral Administration of Lactococcus lactis Expressing Synthetic Genes of Myelin Antigens in Decreasing Experimental Autoimmune Encephalomyelitis in Rats. Med Sci Monit 21, 1587–1597, doi: 10.12659/msm.892764 (2015).
Huber, A., Diedrichs-Mohring, M. & Wildner, G. Spontaneously relapsing-remitting experimental autoimmune uveitis in rats allows successful therapeutic oral tolerance induction in ongoing disease. Mol Immunol 63, 215–226, doi: 10.1016/j.molimm.2014.07.009 (2015).
Yoshinari, O. et al. Water-soluble undenatured type II collagen ameliorates collagen-induced arthritis in mice. J Med Food 16, 1039–1045, doi: 10.1089/jmf.2013.2911 (2013).
Ploix, C. et al. Oral administration of cholera toxin B-insulin conjugates protects NOD mice from autoimmune diabetes by inducing CD4+ regulatory T-cells. Diabetes 48, 2150–2156 (1999).
Zhang, Z. J., Davidson, L., Eisenbarth, G. & Weiner, H. L. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci USA 88, 10252–10256 (1991).
Bergerot, I., Fabien, N., Maguer, V. & Thivolet, C. Oral administration of human insulin to NOD mice generates CD4+ T cells that suppress adoptive transfer of diabetes. J Autoimmun 7, 655–663, doi: 10.1006/jaut.1994.1050 (1994).
Ramiya, V. K., Shang, X. Z., Wasserfall, C. H. & Maclaren, N. K. Effect of oral and intravenous insulin and glutamic acid decarboxylase in NOD mice. Autoimmunity 26, 139–151 (1997).
Ma, Y. et al. Oral administration of recombinant Lactococcus lactis expressing HSP65 and tandemly repeated P277 reduces the incidence of type I diabetes in non-obese diabetic mice. PLoS One 9, e105701, doi: 10.1371/journal.pone.0105701 (2014).
Fourlanos, S. et al. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes 60, 1237–1245, doi: 10.2337/db10-1360 (2011).
Orban, T. et al. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 32, 2269–2274, doi: 10.2337/dc09-0934 (2009).
Vehik, K. et al. Long-term outcome of individuals treated with oral insulin: diabetes prevention trial-type 1 (DPT-1) oral insulin trial. Diabetes Care 34, 1585–1590, doi: 10.2337/dc11-0523 (2011).
Pham, M. N. et al. Oral insulin (human, murine, or porcine) does not prevent diabetes in the non-obese diabetic mouse. Clin Immunol 164, 28–33, doi: 10.1016/j.clim.2016.01.013 (2016).
Ma, S. W. et al. Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance. Nat Med 3, 793–796 (1997).
Arakawa, T. et al. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol 16, 934–938, doi: 10.1038/nbt1098-934 (1998).
Ma, S. et al. Induction of oral tolerance to prevent diabetes with transgenic plants requires glutamic acid decarboxylase (GAD) and IL-4. Proc Natl Acad Sci USA 101, 5680–5685, doi: 10.1073/pnas.03074201010307420101 (2004).
Ruhlman, T., Ahangari, R., Devine, A., Samsam, M. & Daniell, H. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts–oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol J 5, 495–510, doi: 10.1111/j.1467-7652.2007.00259.x (2007).
Davoodi-Semiromi, A., Samson, N. & Daniell, H. The green vaccine: A global strategy to combat infectious and autoimmune diseases. Hum Vaccin 5, 488–493, doi: 8247 (2009).
Limaye, A., Koya, V., Samsam, M. & Daniell, H. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J 20, 959–961, doi: 10.1096/fj.05-5134fje (2006).
Jin, S. & Daniell, H. Engineered chloroplast genome just got smarter. Trends in Plant Science, in press (2015).
Ruhlman, T., Verma, D., Samson, N. & Daniell, H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol 152, 2088–2104, doi: 10.1104/pp.109.152017 (2010).
Verma, D. et al. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci USA 107, 7101–7106, doi: 10.1073/pnas.0912181107 (2010).
Sherman, A. et al. Suppression of inhibitor formation against factor VIII in hemophilia A mice by oral delivery of antigens bioencapsulated in plant cells. Blood, doi: 10.1182/blood-2013-10-528737 (2014).
Wang, X. et al. Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells. Blood 125, 2418–2427, doi: 10.1182/blood-2014-08-597070 (2015).
Chen, M. L., Yan, B. S., Bando, Y., Kuchroo, V. K. & Weiner, H. L. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol 180, 7327–7337, doi: 180/11/7327 (2008).
Chen, M. L., Yan, B. S., Kozoriz, D. & Weiner, H. L. Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms. Eur J Immunol 39, 3423–3435, doi: 10.1002/eji.200939441 (2009).
Su, J. et al. Oral delivery of Acid Alpha Glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant Biotechnol J, doi: 10.1111/pbi.12413 (2015).
Coombes, J. L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204, 1757–1764, doi: 10.1084/jem.20070590 (2007).
Sun, C. M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204, 1775–1785, doi: 10.1084/jem.20070602 (2007).
Mucida, D. et al. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity 30, 471–472, author reply 472–473, doi: 10.1016/j.immuni.2009.03.008 (2009).
Verma, D., Samson, N. P., Koya, V. & Daniell, H. A protocol for expression of foreign genes in chloroplasts. Nat Protoc 3, 739–758, doi: 10.1038/nprot.2007.522 (2008).
Shenoy, V. et al. Oral delivery of Angiotensin-converting enzyme 2 and Angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension 64, 1248–1259, doi: 10.1161/HYPERTENSIONAHA.114.03871 (2014).
Robert, S. et al. Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes 63, 2876–2887, doi: 10.2337/db13-1236 (2014).
Tewari, N. et al. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: an immunohistochemical study of tissue microarrays. BMC Cancer 13, 436, doi: 10.1186/1471-2407-13-436 (2013).
Bergerot, I. et al. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci USA 94, 4610–4614 (1997).
Lehtimaki, N., Koskela, M. M. & Mulo, P. Posttranslational Modifications of Chloroplast Proteins: An Emerging Field. Plant Physiol 168, 768–775, doi: 10.1104/pp.15.00117 (2015).
Webster, D. E. & Thomas, M. C. Post-translational modification of plant-made foreign proteins; glycosylation and beyond. Biotechnol Adv 30, 410–418, doi: 10.1016/j.biotechadv.2011.07.015 (2012).
Glenz, K. et al. Production of a recombinant bacterial lipoprotein in higher plant chloroplasts. Nat Biotechnol 24, 76–77, doi: 10.1038/nbt1170 (2006).
Sagne, C., Isambert, M. F., Henry, J. P. & Gasnier, B. SDS-resistant aggregation of membrane proteins: application to the purification of the vesicular monoamine transporter. Biochem J 316 (Pt 3), 825–831 (1996).
van Helvoort, J. M. et al. Preferential expression of IgG2b in nose draining cervical lymph nodes and its putative role in mucosal tolerance induction. Allergy 59, 1211–1218, doi: 10.1111/j.1398-9995.2004.00510.x (2004).
García, B. et al. Differential effects of transforming growth factor-β1 on IgA vs. IgG2b production by lipopolysaccharide-stimulated lymph node B cells: a comparative study with spleen B cells. Eur J Immunol 26, 2364–2370 (1996).
McIntyre, T. M. et al. Transforming growth factor beta 1 selectivity stimulates immunoglobulin G2b secretion by lipopolysaccharide-activated murine B cells. J Exp Med 177, 1031–1037 (1993).
Sonoda, E. et al. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med 170, 1415–1420 (1989).
Coffman, R. L., Lebman, D. A. & Shrader, B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med 170, 1039–1044 (1989).
Presa, M. et al. Cholera toxin subunit B peptide fusion proteins reveal impaired oral tolerance induction in diabetes-prone but not in diabetes-resistant mice. Eur J Immunol 43, 2969–2979, doi: 10.1002/eji.201343633 (2013).
Quinn, A., Melo, M., Ethell, D. & Sercarz, E. E. Relative resistance to nasally induced tolerance in non-obese diabetic mice but not other I-A(g7)-expressing mouse strains. Int Immunol 13, 1321–1333 (2001).
Bresson, D. et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest 116, 1371–1381, doi: 10.1172/JCI27191 (2006).
Lindley, S. et al. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes 54, 92–99, doi: 54/1/92 [pii] (2005).
Kato, H., Fujihashi, K., Kato, R., Yuki, Y. & McGhee, J. R. Oral tolerance revisited: prior oral tolerization abrogates cholera toxin-induced mucosal IgA responses. J Immunol 166, 3114–3121 (2001).
Chan, H. T. et al. Cold Chain and Virus Free chloroplast-made Booster Vaccine to Confer Immunity Against Different Polio Virus Serotypes. Plant Biotechnol J, doi: 10.1111/pbi.12575 (2016).
Luongo, D., Bergamo, P. & Rossi, M. Effects of conjugated linoleic acid on growth and cytokine expression in Jurkat T cells. Immunol Lett 90, 195–201 (2003).
Yaqoob, P. Fatty acids as gatekeepers of immune cell regulation. Trends Immunol 24, 639–645 (2003).
Pariza, M. W., Park, Y. & Cook, M. E. Mechanisms of action of conjugated linoleic acid: evidence and speculation. Proc Soc Exp Biol Med 223, 8–13 (2000).
Masuda, K., Horie, K., Suzuki, R., Yoshikawa, T. & Hirano, K. Oral-antigen delivery via a water-in-oil emulsion system modulates the balance of the Th1/Th2 type response in oral tolerance. Pharm Res 20, 130–134 (2003).
de Veer, M. et al. Cell recruitment and antigen trafficking in afferent lymph after injection of antigen and poly(I:C) containing liposomes, in aqueous or oil-based formulations. Vaccine 31, 1012–1018, doi: 10.1016/j.vaccine.2012.12.049 (2013).
Makidon, P. E. et al. Nanoemulsion mucosal adjuvant uniquely activates cytokine production by nasal ciliated epithelium and induces dendritic cell trafficking. Eur J Immunol 42, 2073–2086, doi: 10.1002/eji.201142346 (2012).
Homann, D., Dyrberg, T., Petersen, J., Oldstone, M. B. & von Herrath, M. G. Insulin in oral immune “tolerance”: a one-amino acid change in the B chain makes the difference. J Immunol 163, 1833–1838 (1999).
Westerholm-Ormio, M., Vaarala, O., Pihkala, P., Ilonen, J. & Savilahti, E. Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes 52, 2287–2295 (2003).
Savilahti, E. et al. Jejuna of patients with insulin-dependent diabetes mellitus (IDDM) show signs of immune activation. Clin Exp Immunol 116, 70–77 (1999).
Kobayashi, M. et al. Altered B: 9-23 insulin, when administered intranasally with cholera toxin adjuvant, suppresses the expression of insulin autoantibodies and prevents diabetes. J Immunol 179, 2082–2088 (2007).
Takiishi, T. et al. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest 122, 1717–1725, doi: 10.1172/JCI60530 (2012).
Kwon, K. C., Verma, D., Singh, N. D., Herzog, R. & Daniell, H. Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Deliv Rev 65, 782–799, doi: 10.1016/j.addr.2012.10.005 (2013).
Kwon, K. C. & Daniell, H. Oral Delivery of Protein Drugs Bioencapsulated in Plant Cells. Mol Ther 24, 1342–1350, doi: 10.1038/mt.2016.115 (2016).
Acknowledgements
This work was supported by the JDRF (17-2011-286, 25-2013-268, 17-2012-3, and 25-2012-516) and NIH NIAID (AI42288), NHLBI (HL 107904 and HL109442). Publication of this article was funded in part by the University of Florida Open Access Publishing Fund.
Author information
Authors and Affiliations
Contributions
A.P. conceived of the study, wrote the manuscript and researched data. C.W. conceived of the study, contributed to discussion, and reviewed/edited the manuscript. D.S. contributed to discussion and reviewed/edited the manuscript. H.D. conceived of the study, contributed to discussion and reviewed/edited the manuscript. K.K. reviewed/edited the manuscript and researched data. M.A.A. conceived of the study, contributed to discussion and reviewed/edited the manuscript. M.A.A. is the guarantor of this work and, as such, takes responsibility for the integrity of the contents herein.
Corresponding author
Ethics declarations
Competing interests
Conflicts of interest exist in that applications for intellectual property have been filed for certain technologies described in this application.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Posgai, A., Wasserfall, C., Kwon, KC. et al. Plant-based vaccines for oral delivery of type 1 diabetes-related autoantigens: Evaluating oral tolerance mechanisms and disease prevention in NOD mice. Sci Rep 7, 42372 (2017). https://doi.org/10.1038/srep42372
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42372