Abstract
Exposure to cigarette smoking cues can trigger physiological arousal and desire to smoke. The brain substrates of smoking cue-induced craving (CIC) are beginning to be elucidated; however, it has been difficult to study this state independent of the potential contributions of pharmacological withdrawal from nicotine. Pharmacological withdrawal itself may have substantial effects on brain activation to cues, either by obscuring or enhancing it, and as CIC is not reduced by nicotine replacement strategies, its neuro-anatomical substrates may differ. Thus, characterizing CIC is critical for developing effective interventions. This study used arterial spin-labeled (ASL) perfusion fMRI, and newly developed and highly appetitive, explicit smoking stimuli, to examine neural activity to cigarette CIC in an original experimental design that strongly minimizes contributions from pharmacological withdrawal. Twenty-one smokers (12 females) completed smoking and nonsmoking cue fMRI sessions. Craving self-reports were collected before and after each session. SPM2 software was employed to analyze data. Blood flow (perfusion) in a priori-selected regions was greater during exposure to smoking stimuli compared to nonsmoking stimuli (p<0.01; corrected) in ventral striatum, amygdala, orbitofrontal cortex, hippocampus, medial thalamus, and left insula. Perfusion positively correlated with intensity of cigarette CIC in both the dorsolateral prefrontal cortex (r2=0.54) and posterior cingulate (r2=0.53). This pattern of activation that includes the ventral striatum, a critical reward substrate, and the interconnected amygdala, cingulate and OFC, is consistent with decades of animal research on the neural correlates of conditioned drug reward.
Similar content being viewed by others
INTRODUCTION
Rationale
Both nicotine and conditioned cues (reminders) maintain cigarette smoking and lead to relapse (Henningfield and Goldberg, 1983; Rose, 2006). Thus, effective smoking cessation treatments should address both factors. Nicotine replacement therapy (NRT) and bupropion are both effective at ameliorating nicotine withdrawal-induced craving, but neither block the craving elicited by learned associations formed between environmental cues and nicotine (Teneggi and Tiffany, 2002; Robinson and Berridge, 1993; Wise, 1988; Hughes et al, 1999; Hurt et al, 1997; Jorenby et al, 1999). Currently, medications that unequivocally prevent or reduce cue-induced craving (CIC) are not available. Further understanding of the underlying neurobiology of CIC would facilitate the development of effective therapies and improve the currently low (10–20% at 6 months to a year) success rates associated with current smoking interventions (Hughes et al, 1999; West et al, 2001). Thus, a major goal of this work is to examine the element of neurobiology underlying CIC distinct from that observed in CIC studies associated with nicotine withdrawal.
Craving generated by drug cues may accrue slowly over time, recruiting supplementary neural substrates as self-reported craving increases. Additionally, craving may persist for extended periods after stimulus exposure, often extinguished only by smoking. Because perfusion is quantitative and provides an absolute blood flow measure (ml of blood/100 g of tissue/minute; Detre et al, 1992), it is well-suited to our model in which stimuli are presented over several minutes. BOLD fMRI studies provide a relative measure of brain activity requiring subjects to remain in the scanner throughout both drug and nondrug stimulus presentations. As CIC and pharmacological withdrawal may involve overlapping and/or separate brain substrates, this can interfere with obtaining a clear signal. Having separate scanning sessions also, and importantly, provides an opportunity for subjects to smoke before each stimulus presentation, minimizing withdrawal further. A third benefit to separated cue sessions is that any nicotine-induced craving that may accrue during scanning can be equated across stimulus sets and subtracted out of final analyses.
Further mitigating basis for the use of perfusion imaging over the well-known event-related BOLD fMRI is that it provides a resting baseline. As mentioned, CIC is a prolonged state that accrues and is maintained over time. With the event-related on/off stimulus presentation of BOLD imaging, craving to cigarette cues is measured over each seconds-long cue presentation and may not return to baseline within the interval before noncue stimulus presentation. Thus, as craving to cues increases throughout scanning, distinguishing cue from noncue signal becomes more difficult. Second, baseline drift associated with BOLD may ‘ride on top’ of brain activation to cues, masking portions of the signal. Signal collected with perfusion fMRI has no associated baseline drift and collects cue and noncue stimulus set data separately to aid in delineating a sharper stronger CIC signal.
A Priori CNS Regions of Interest
A priori CNS regions of interest were chosen based on over 20 years of preclinical literature on drug-seeking behavior (Robinson and Berridge, 1993; Wise, 1988; Galvan et al, 2005), and the more recent advances in functional neuroimaging in smoking cue and other drug cue reactivity studies (Brody et al, 2002; Childress et al, 1999; Due et al, 2002; Wilson et al, 2005; Smolka et al, 2006; McClernon et al, 2005). We expected to see greater neural activity to smoking cues in the areas of the pedunculopontine nucleus, ventral tegmental area, and ventral striatum, the proposed pathway through which nicotine and associated cues modulate the mesolimbic dopaminergic system (Corrigall et al, 1994; Laviolette et al, 2002). Further, we hypothesized greater perfusion in the ventral striatum and the interconnected amygdala, anterior and posterior cingulate, select prefrontal regions, hippocampus, and thalamus as these regions are implicated in modulating behavioral responses to drug-related stimuli (Brody et al, 2002; Childress et al, 1999; Franklin and Druhan, 2000a, 2000b; Grant et al, 1996; Wang et al, 1999). Insulae are important in mediating autonomic responses to drugs of abuse and their predictors and thus were examined in the analysis (Bechara et al, 2000; Garavan et al, 2000). Lastly, we predicted that smoking cues would activate the fusiform gyrus, as it plays a role in attention, and has been activated in previous nicotine cue reactivity studies (David et al, 2005; Due et al, 2002; Wilson et al, 2005). We hypothesized that increases in perfusion in ventral medial prefrontal and deep limbic structures (amygdala, insula, and ventral striatum), regions important in mediating responses to drug cues, would correlate with subjective craving scores.
Arterial Spin-Labeled (ASL) Perfusion MRI
We used the novel technique of ASL perfusion MRI to characterize regional brain activation during cigarette CIC (Alsop and Detre, 1996). This noninvasive, nonradioactive technique uses radio frequency pulses to magnetically label arterial blood (water) to reflect changes in brain blood flow (Detre et al, 1992). Changes in blood flow result in changes in tissue magnetization, measured directly with MRI. Perfusion refers to the delivery of life-sustaining materials to tissue via blood flow. Theoretically, it is regionally coupled to brain metabolism to replenish constituents used to provide energy to activated neurons and associated cells. With this technique, arterial blood is labeled by pulses of electromagnetic energy in an interleaved manner, such that tagged and untagged (control) images are acquired in pairs and subtracted from one another, providing contrast that is subsequently used to estimate cerebral blood flow (CBF) (Williams et al, 1992, 2005).
SUBJECTS AND SCREENING PROCEDURES
The study, approved by the University of Pennsylvania Institutional Review Board, adhered to the Declaration of Helsinki. Smokers were compensated $100.00 for completion of both MRI sessions. Subjects (∼75%) were recruited from those presenting for treatment for nicotine dependence at the University of Pennsylvania Treatment Research Center. The remaining subjects were recruited through word of mouth. Subjects were screened, tested on study knowledge, and consented before a psychological and physical examination. Psychological tests were administered by a clinical psychologist and included administration of the MINI (Minnesota International Neuro-psychiatric Interview), a structured diagnostic tool that assesses current DSM-IV diagnosis of other psychoactive substance dependence and severe psychiatric symptoms (eg psychosis, dementia, acute suicidal or homicidal ideation, mania or depression; Sheehan et al, 1998; DSM IV, 1994). As a higher percentage of people with psychiatric illness, including drug dependence, smoke cigarettes than the general population, only those with current diagnoses of severe psychiatric illness were excluded. The physical examination included a complete medical history, medical evaluation, and urine testing to verify absence of psychoactive substance use other than nicotine. Severity of nicotine dependence was determined from a laboratory-developed Smoking History Questionnaire (SHQ) that included a modified Fagerstrom Test for Nicotine Dependence (FTND; Fagerstrom and Schneider, 1989).
Subjects with an abnormal structural MRI, history of head trauma or injury causing loss of consciousness lasting more than 3 min or associated with skull fracture or inter-cranial bleeding were excluded. For safety reasons and owing to the magnetic environment of the MRI, smokers with magnetically active prosthetics, plates, pins, permanent retainers, or bullets were also excluded.
Thirty-three smokers were scanned for this study. Data from 12 subjects were excluded: six data sets were unusable because of technical difficulties, four subjects did not meet inclusion/exclusion criteria, and two subjects slept during scanning.
Data from 21 physically healthy and mentally stable male and nonpregnant female smokers between the ages of 18 and 60 (mean: 34.4 SEM: (±2.6)) who met DSM-IV criteria for nicotine dependence (FTND: 4.8 (±0.4); indicating moderate dependence) were available for the study. Subjects smoked from 15 to 40 cigarettes per day (19.6±1.7). The sample was 57% female, 50% black, 48% white, 2% Hispanic, and averaged 13.7 (±0.4) years of education. The Edinburgh Handedness Inventory identified 76% of the subjects as right-handed (Oldfield, 1971).
DESIGN
Stimuli
Nonsmoking stimuli
After subjects were positioned in the MRI bed, but before the actual scanning session, a freshly sharpened pencil was placed in the subject's left hand. Audio-visual cues were presented via a video that portrayed individuals differing in race, age, and sex relating interesting short stories or anecdotes.
Smoking stimuli
After positioning in the MRI bed, and before scanning, subjects were given one of their own cigarettes to hold in the left hand, while a technician lit and blew out a match and placed it in an ashtray within the subject's line of vision. The audio-video clip was similar to that of the nonsmoking cue video, however, was heavily peppered with smoking, cigarette cues, and unambiguous language designed to induce appetitive desire for a cigarette (eg ‘I love a cigarette after a hard day at work. It relaxes me completely and tastes so good’). Our goal in developing these explicit stimuli was to further enhance cue responses over those elicited by the implicit stimuli used in our previous work (Droungas et al, 1995; Brody et al, 2002).
Both sets of cues were intentionally devoid of other appetitive stimuli (eg sex, food, alcohol, caffeine, gaming, etc).
fMRI Experimental Design
To reduce possible anxiety elicited by the unfamiliar MRI environment, subjects completed a virtual fMRI simulation before scanning. As withdrawal increases over time since last cigarette smoked (Shiffman, 1979), two separate MRI sessions were administered (see Figure 1 for session time line). The second session was scheduled on the same day, approximately 1 h later. Subjects smoked one of their own cigarettes ad lib before both cue sessions to maintain individual and characteristic pharmacological, physiological, and psychological states. Functional MRI of cue reactivity took place 20–25 min after smoking to permit the cardiovascular effects of smoking to dissipate. Subjects were informed of the opportunity to smoke immediately after each session because the expectation of drug availability has been shown to enhance subjective craving reports and physiological responses to smoking cues (Droungas et al, 1995; Carter and Tiffany, 2001; Dols et al, 2002; although see Wertz and Sayette, 2001).
Timeline for fMRI session. Approximate length, in minutes, of each session component is indicated on the left. Subjects smoked a cigarette before each session. Subjects reported on CWQ before and immediately following counterbalanced sets of smoking and nonsmoking stimuli. Each scanning session lasted 45 min with the ASL perfusion scan situated midway. The second session was identical however in place of a DTI scan, a high-resolution scan was acquired.
The head coil and memory pads were firmly and comfortably positioned to reduce movement. Visual stimuli were delivered by LCD projector and observed via mirrors, attached to the head coil to focus attention on a screen placed at the head of the MRI bed. Audio stimuli were transmitted through headphones. For each session, subjects remained in the scanner for approximately 45 min. ASL Perfusion functional data were acquired during the presentation of the stimulus sets. While in the scanner and before and after presentation of both stimulus sets, a nine-item Craving and Withdrawal Questionnaire (CWQ) was administered. Changes in subject-rated assessment of items were used to calculate change in mood over the course of scanning. The first item is designed to capture the subjective craving experience and two others may reflect indices of withdrawal. Four of the most relevant questions are presented in Table 1.
Imaging Parameters
MR scanning was conducted on a Siemens 3.0 Tesla Trio whole-body scanner (Siemens AG, Erlangen, Germany), using a standard transmit/receive head coil. A 3-min localizer scan was acquired before functional scanning. This scan (sagittal, axial, and coronal slices) was acquired with FOV=280 mm, TR/TE=20/5 ms, 192 × 144 matrix, and slice thickness of 5 mm, and was used for subsequent normalization and anatomical co-registration of the images. The localizer scan was followed by a 5-min resting baseline perfusion scan. These scans also served as a habituation period to the MR environment. The perfusion ASL technique was used to acquire baseline and cue scans. Interleaved images with and without labeling were acquired using a gradient echo echo-planar imaging sequence. A delay of 700 ms was inserted between the end of the labeling pulse and image acquisition to reduce transit artifact. Acquisition parameters were: FOV=22 cm, matrix=64 × 64, TR/TE=3000/17 ms, and flip angle=90°. Fourteen slices (8 mm thickness with 1.5 mm gap) were acquired from inferior to superior brain in a sequential order. Each ASL cue scan with 200 acquisitions was 10 min in length. Following acquisition of the first ASL cue scan, a T1-weighted high-resolution scan was acquired, and a diffusion tensor imaging (DTI) scan was acquired after the second cue scan (each approximately 6 min). Acquisition parameters for the three-dimensional (3D) high-resolution MPRAGE structural in the axial plane were: FOV=250 mm, TR/TE=1620/3 ms, 192 × 256 matrix, and slice thickness 1 mm. Only data from the perfusion ASL cue reactivity scans are presented here.
Imaging Data Processing and Analysis
Perfusion fMRI data were analyzed using SPM2 software (www.fil.ion.ucl.ac.uk/spm). MR image series were realigned to correct for head movements, co-registered with each subject's anatomical MRI, and smoothed in space with a 3D isotropic, 10 mm full-width at half-maximum (FWHM) Gaussian kernel. Perfusion weighted image series were generated by pairwise subtraction of the label and control images, followed by conversion to absolute CBF image series based on a single compartment ASL perfusion model (Detre et al, 1992; Williams et al, 1992).
Voxel-wise analyses of the CBF data were conducted on each subject, using a general linear model (GLM) with global time course as a nuisance covariate to reduce the effect of spatially coherent noise (first level analysis). No temporal filtering or smoothing was involved. One contrast was defined in the GLM analysis, namely the CBF difference between the two cue conditions (smoking cue vs nonsmoking cue). Individual contrast images (β maps) were normalized into canonical space (Montreal Neurological Institute standard brain). Contrast images were analyzed using one-sample t-tests to obtain the statistical parametric map of the t-statistic at each voxel for the defined contrast using a random effects model that allows population inference (second-level analysis). This step is equivalent to comparing CBF values between corresponding experimental conditions within each subject.
Linear regression analyses were carried out on the normalized individual maps to obtain the activation pattern (statistic parametric map of the t-statistic) correlated with the self-reported craving difference scores (the difference between pre- and post-stimulus presentation craving scores) with the smoking and nonsmoking cue conditions as the independent variable. Change scores were used for these analyses rather than absolute craving scores, as over 25 years experience studying CIC in the Childress lab, has provided empirical data that subject's ‘anchor’ their pre-stimulus craving score differently (one person's 2 may be another's 6). In this way, the change from a subject's self-reported baseline craving before and after cue presentation describes their craving more accurately.
RESULTS
Craving and Withdrawal Questionnaire
Eight items related to craving, withdrawal, and mood were rated before and after stimulus presentations, using the CWQ. The difference scores to four questions that may reflect subjective feelings of craving and withdrawal are presented in Table 1.
Paired t-tests of difference scores revealed significant differences in craving to the smoking cues (p<0.02; Figure 2, Table 1) There were no significant differences in change scores to the remaining CWQ questions for either smoking or nonsmoking stimuli. However, relative differences in craving (0.7), anxiety (0.6), and irritability (0.6), were comparable to one another, suggesting that the brain response to smoking vs nonsmoking stimuli may be a function of all three mood states. Thus, linear regression analyses were conducted to examine the association of craving with either of these mood states. No correlations were found between craving and either mood state. Additionally, the brain response to smoking stimuli was unaltered when separate SPM analyses treating anxiety and irritability items as nuisance variables were conducted. Additionally, co-varying out for either anxiety or irritability ratings, when craving was included in the SPM model as a regressor did not affect brain response.
Primary Contrast (Smoking Cue vs Nonsmoking Cue)
In an unmasked SPM analysis, areas of significant activation were identified at the cluster level for the p-value <0.01 (uncorrected) and the cluster extent size of 20 contiguous 2 mm3 voxels. For a priori region of interest (ROI) contrasts, changes in perfusion were determined using the small volume correction (SVC) tool in SPM2 with radius r, where r was defined by the size of the cluster and is 5>r<20 mm. These included the ventral striatum, amygdala, pedunculopontine nucleus, hippocampus, thalamus, orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), anterior and posterior cingulate cortex, fusiform gyrus, ventral tegmental area, and insula (Table 2; nonsignificant ROIs not listed). Coordinates are in MNI as provided by SPM, however, can be converted to Tailarach coordinates (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html). Coordinates listed are those chosen from the approximate center of each ROI using the Duvernoy Brain Atlas as a reference (Duvernoy, 1999).
Significantly greater perfusion to smoking compared to nonsmoking stimuli was observed bilaterally, in ventral striatum, amygdala, hippocampus; ventral medial anterior thalamus and laterally, in right posterior OFC and left anterior ventral insula (see Table 2 and Figure 3; insula and OFC not pictured). There were no increases in perfusion between smoking and nonsmoking stimuli in other a priori regions (fusiform gyrus, pedunculopontine nucleus, ventral tegmental area, and anterior and posterior cingulate cortices). Further, no areas of decreased perfusion were observed in a priori or other brain regions. As there were no formal hypotheses regarding laterality, it was not examined in this study.
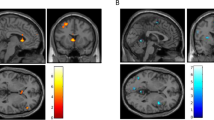
Statistical parametric maps (SPMs) of greater neural activation to smoking cues compared to nonsmoking cues, co-registered to the MNI single subject template (p<0.01 and >20 contiguous voxels per cluster). Crosshairs are centered on listed coordinates. The inset regions are enlarged for increased clarity. T-values are from 0 to 6. Sagittal, axial, and coronal views are provided.
Craving Correlations
Simple linear regression between change in craving scores and brain perfusion at each voxel for each subject was used to test for correlations between cigarette CIC and brain activity. At p<0.05 corrected, the DLPFC (r2=0.54) and posterior cingulate cortex (r2=0.53) demonstrated a positive correlation with intensity of subjective craving scores and brain perfusion (see Figure 4).
DISCUSSION
We utilized ASL perfusion fMRI to characterize regional brain activation during cigarette CIC, independent of interference from pharmacological withdrawal. This method revealed increased perfusion to smoking vs nonsmoking stimuli in several a priori brain regions. These were bilateral in amygdala, ventral striatum, thalamus, hippocampus, and lateral in insula and OFC. Perfusion correlated positively with subjective craving in DLPFC and posterior cingulate.
The selective activation of these key neural substrates, important in the detection of emotional significance, suggests that they interact as a circuit to enhance memory and assign significance to stimuli associated with cigarette dependence (Phan et al, 2005). Activation of this circuit, also activated during conditioning processes, suggests that Pavlovian mechanisms may play a role in smoking cue reactivity. The interaction of these regions with regions that evaluate rewards (OFC), regions exerting cognitive control over behavior (DLPFC), and regions supporting stimulus expectancy and attentional processes (DLPFC and posterior cingulate) may lead to the subjective experience of craving (Grant et al, 1996). These findings strongly support clinical studies of cigarette and other drug CIC and further extend our knowledge of its underlying neurobiology (McClernon et al, 2005, Wilson et al, 2005; Childress et al, 1999; Maas et al, 1998).
This report provides evidence that the DLPFC is a brain/behavior correlate of CIC, in agreement with some but not all neuroimaging drug cue studies. Participation of this region may be related to several factors, as it is a region involved in a multitude of cognitive processes. One mitigating factor may relate to whether subjects were recruited from those seeking treatment for their addiction or from nontreatment seekers. Alternatively, expecting to use drug, regardless of treatment status may partially underlie DLPFC involvement (Wilson et al, 2005). DLPFC activation was observed in 7/10 drug cue studies in subjects not currently seeking treatment but only in 1/9 studies comprised of treatment seekers. In general, expectancy of drug is not a feature of treatment CIC studies; however, in the single study showing DLPFC activation to cocaine cues, subjects were informed that drug would be available after the cue session (Grant et al, 1996). The subjects studied here were 76% treatment seekers and 24% nontreatment seekers. However, at the time of scanning none were in treatment and all were informed that a cigarette break would occur after the session. As Wilson and co-workers and others have previously reported (Grant et al, 1996; Smolka et al, 2006), DLPFC activation and intensity of craving correlated in smokers expecting to smoke after cue exposure; however, there was no direct test of expectancy on activation in these other studies, or this study. Recently, McBride et al (2006) directly tested the effect of expectancy on brain activation and found DLPFC activation in subjects expecting a cigarette after cues but not in those who did not. McBride et al (2006) also showed that activation positively correlated with self-reported craving. On reflection, expecting drug after a smoking cue session may interact with and enhance the potency or evocativeness of the cues.
An important goal of this study was to minimize the contribution of pharmacological withdrawal from brain activation patterns associated with smoking cues. Smoking and nonsmoking cue scanning sessions were temporally separated and preceded by smoking, providing subjects the opportunity to be imaged in a nondeprived state. This design feature minimized nicotine withdrawal-based craving effects that can begin as little as 20 min after smoking. Differences in cue reactivity literature may be partially related to regional activation introduced by pharmacological withdrawal. For example, Due and co-workers examined fMRI brain activation to cigarette and neutral cues in a BOLD event-related paradigm in overnight abstinent smokers and a nonsmoking comparison group. In the smokers, both craving and anxiety increased over the course of the session, whereas neither increased in the nonsmoking control group (Due et al, 2002). As anxiety is a symptom of withdrawal (Shiffman, 1979), its increase in smokers may reflect a general increase in withdrawal across the session, potentially interacting with and confounding the CIC pattern of activation. As, the work of Due et al showed, other emotions elicited either by the stimuli or the fMRI environment must be considered in interpreting brain response to smoking stimuli. We examined whether other mood states, such as anxiety and irritability were involved in mediating the brain response to smoking cues with our CWQ. Separate analyses covarying out for each mood state did not affect the brain response to smoking cues.
Like perfusion fMRI, positron emission tomography (PET) provides a quantitative baseline and is conducive to counterbalanced and split sessions. Using PET, Brody et al (2002) found positive correlations between craving intensity and relative brain metabolism in the OFC, DLPFC, and the anterior cingulate. The results found here concur with those of Brody and co-workers and further show deep limbic activation (amygdala, ventral striatum, hippocampus, insula, thalamus) to cues. The increased temporal and spatial resolution offered by perfusion fMRI in comparison to PET may have contributed to our extended findings. Alternatively, our results may reflect the use of newly developed explicit smoking cues vs the implicit cues used in our earlier studies (Droungas et al, 1995; Brody et al, 2002). The new stimuli depict demographically dissimilar actors (to aid in engaging all smokers) professing their desire and enjoyment of smoking from comfortable natural smoking environments. While smoking, the actors directly address the audience and expound upon their favorite smoking experiences, such as the after-dinner cigarette or the first cigarette of the day. Also, nonsmoking cues were comparable to smoking cues in all respects, devoid of smoking cues. This is important as brain activation to human faces, environments, and other video differences could alter specific brain activation. Both of these changes may have contributed to an enhanced brain response to cues. No direct test of the effects of our newly developed explicit vs implicit cues on brain activation was conducted.
Because separate sessions are conducted, perfusion fMRI is well suited to our study of cigarette CIC. This feature provides the opportunity to smoke a cigarette before each set of cues, minimizing withdrawal effects on brain activation. It also reduces carry over effects from one set of stimuli to the other. This is important as craving to smoking cues accrues over the course of scanning and may not abate without smoking a cigarette. BOLD event-related paradigms are challenged in this regard, as smoking and nonsmoking stimuli are interspersed across one scanning session, blurring stimulus signals as the differential between them is reduced.
Opinions differ throughout the literature regarding the measurement of subjective craving scores. It is believed by some that ratings collected immediately following stimulus presentation most accurately reflect individual craving levels. Others in the field suggest that the change in ratings from before to after cue presentation is a more accurate indicator of reactivity specifically related to the stimuli. The data presented here are measured by the latter method. Although we have not formerly tested the assertion, over 25 years of experience suggests that subject's ‘anchor’ their pre-stimulus craving score differently. Purportedly, the difference between a self-reported baseline craving and that reported after cue presentation might more accurately reflect the brain changes that occur over the scanning period. To our knowledge, demonstrative evidence to support one method of measuring craving over another does not exist.
Future studies in which smokers are stratified into subgroups may contribute to a greater understanding of CIC and the development of effective treatments. For example, some studies, including recent work in our lab suggest that sex differences in brain responses to cues exist (Franklin et al, 2004). Also, continuing to study potential differing neurobiological processes in treatment vs nontreatment seekers may uncover unique brain activity patterns. Ongoing studies are examining the impact of sex and treatment on brain response.
The study design used here and the latest ASL perfusion fMRI technology have overcome many of the challenges that were unavoidably imbedded within previous smoking cue reactivity studies and thus, at this juncture, a characteristic and comprehensive signature of smoking CIC is delineated. Encouragingly, most of the regions consistently implicated in drug craving studies are observed with the signal capture capabilities of ASL perfusion fMRI. As cue-elicited craving is a major contributor to relapse (Abrams et al, 1988; Niaura et al, 1989), the capacity to image the state sets the stage for predicting treatment outcome success without investing substantial time, expense, and labor conducting large clinical trials. Medications with suspected anticraving properties can be screened rapidly using perfusion fMRI. The necessity for such a screening tool becomes apparent when we acknowledge the heterogeneity underlying cigarette dependence. In line with other drugs of abuse, its underpinnings depend on phenotype, sex, and/or other neurobiological factors. Thus, as with depression and other psychiatric illness, successful smoking cessation interventions that target particular brain substrates in a selective and specific manner are needed to aid prospective quitters. Today's ever-advancing neuroimaging technology offers a powerful tool to reach this goal.
References
Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI (1988). Reactivity to smoking cues and relapse: two studies of discriminant validity. Behav Res Ther 26: 225–233.
Alsop DC, Detre JA (1996). Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 16: 1236–1249.
Bechara A, Tranel D, Damasio H (2000). Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123 (Part 11): 2189–2202.
Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG et al (2002). Brain metabolic changes during cigarette craving. Arch Gen Psychiat 59: 1162–1172.
Carter BL, Tiffany ST (2001). The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharmacol 9: 183–190.
Childress AR, Mozley PD, McElgin W, Fitzgeoorald J, Reivich M, O'Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiat 156: 11–18.
Corrigall WA, Coen KM, Adamson KL (1994). Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653: 278–284.
David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM et al (2005). Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiat 58: 488–494.
Detre JA, Leigh JS, Williams DS, Koretsky AP (1992). Perfusion imaging. Magn Reson Med 23: 37–45.
Dols M, van den Hout M, Kindt M, Willems B (2002). The urge to smoke depends on the expectation of smoking. Addiction 97: 87–93.
DSM IV (1994). American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Association: Washington, DC.
Droungas A, Ehrman RN, Childress AR, O'Brien CP (1995). Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict Behav 20: 657–673.
Due DL, Huettel SA, Hall WG, Rubin DC (2002). Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiat 159: 954–960.
Duvernoy HM (1999). The Human Brain: Surface, Blood Supply and Three-dimensional Sectional Anatomy (in collaboration with Bourgouin P, Cabanis EA, Cattin F, Guyot J, Iba-Zizen MT, Maeder P, Parratte B, Tatu L, and Vuillier F, with drawings by Vannson JL), 2nd edn. Springer-Verlag Wein: New York.
Fagerstrom KO, Schneider NG (1989). Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med 12: 159–182.
Franklin TR, Druhan JP (2000a). Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci 12: 2097–2106.
Franklin TR, Druhan JP (2000b). Involvement of the nucleus accumbens and medial prefrontal cortex in the expression of conditioned hyperactivity to a cocaine-associated environment in rats. Neuropsychopharmacology 23: 633–644.
Franklin TR, Listerud J, Sciortino NE, Gray J, Childress AR (2004). Perfusion fMRI of gender differences in cue-induced cigarette craving. Abstract, College on Problems of Drug Dependence, San Juan, Puerto Rico.
Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ (2005). The role of ventral frontostriatal circuitry in reward-based learning in humans. J Neurosci 25: 8650–8656.
Garavan H, Pankiewicz HJ, Bloom A, Cho JK, Sperry L, Ross TJ et al (2000). Cue-induced cocaine craving: neuroanatomical specificity for drug-users and drug stimuli. Am J Psychiat 157: 1789–1798.
Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C et al (1996). Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93: 12040–12045.
Henningfield JE, Goldberg SR (1983). Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav 19: 989–992.
Hughes JR, Goldstein MG, Hurt RD, Shiffman S (1999). Recent advances in the pharmacotherapy of smoking. JAMA 281: 72–76.
Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC et al (1997). A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 337: 1195–1202.
Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR et al (1999). A controlled trial of sustained-release bupropion, a nictoine patch, or both for smoking cessation. N Engl J Med 340: 685–691.
Laviolette SR, Alexson TO, van der Kooy D (2002). Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J Neurosci 22: 8653–8660.
Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW et al (1998). Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiat 155: 124–126.
McBride D, Barrett SP, Kelly JT, Aw A, Dagher A (2006). Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology 31: 2728–2738.
McClernon FJ, Hiott FB, Huettel SA, Rose JE (2005). Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology 30: 1940–1947.
Niaura R, Abrams D, Demuth B, Pinto R, Monti P (1989). Responses to smoking-related stimuli and early relapse to smoking. Addict Behav 14: 419–428.
Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113.
Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME (2005). Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiat 57: 210–219.
Robinson TE, Berridge KC (1993). The neural basis of drug craving: an Incentive-Sensitization Theory of Addiction. Brain Res Reviews 18: 247–291.
Rose JE (2006). Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 184: 274–285.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiat 59: 22–33, quiz 34–57.
Shiffman SM (1979). The tobacco withdrawal syndrome. NIDA Res Monogr 23: 158–184.
Smolka MN, Buhler M, Klein S, Zimmermann U, Heinz A, Braus DF (2006). Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 184: 577–588.
Teneggi V, Tiffany ST, Squassante L, Milleri S, Ziviani L, Bye A (2002). Smokers deprived of cigarettes for 72 h: effect of nicotine patches on craving and withdrawal. Psychopharmacology (Berl) 164: 177–187.
Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR et al (1999). Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 64: 775–784.
Wertz JM, Sayette MA (2001). A review of the effects of perceived drug use opportunity of self-reported urge. Exp Clin Psychopharmacol 9: 3–13.
West R, McEwen A, Bolling K, Owen L (2001). Smoking cessation and smoking patterns in the general population: a 1-year follow-up. Addiction 96: 891–902.
Williams DS, Detre JA, Leigh JS, Koretsky AP (1992). Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA 89: 212–216.
Williams LM, Barton MJ, Kemp AH, Liddell BJ, Peduto A, Gordon E et al (2005). Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. NeuroImage 28: 618–626.
Wilson SJ, Sayette MA, Delgado MR, Fiez JA (2005). Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob Res 7: 637–645.
Wise RA (1988). The neurobiology of craving: implications for the understanding and treatment of addiction. J Abnorm Psychol 97: 118–132.
Acknowledgements
This work was supported by NIH Grants DA015149, K01 DA 015426-011A1, 5-P60-DA-005186-18, NS045839, BCS-0224007, RR02305, and the GCRC of the University of Pennsylvania.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franklin, T., wang, Z., Wang, J. et al. Limbic Activation to Cigarette Smoking Cues Independent of Nicotine Withdrawal: A Perfusion fMRI Study. Neuropsychopharmacol 32, 2301–2309 (2007). https://doi.org/10.1038/sj.npp.1301371
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301371
Keywords
This article is cited by
-
Nicotine-related beliefs induce dose-dependent responses in the human brain
Nature Mental Health (2024)
-
Common hyper-entropy patterns identified in nicotine smoking, marijuana use, and alcohol use based on uni-drug dependence cohorts
Medical & Biological Engineering & Computing (2023)
-
The effect of reproductive hormones on women’s daily smoking across the menstrual cycle
Biology of Sex Differences (2021)
-
The changes of brain functional networks in young adult smokers based on independent component analysis
Brain Imaging and Behavior (2021)
-
Assessing drug cue-induced brain response in heroin dependents treated by methadone maintenance and protracted abstinence measures
Brain Imaging and Behavior (2020)