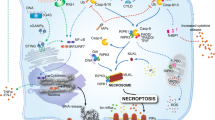
Abstract
Apoptotic cell death is executed by the caspase-mediated cleavage of various vital proteins. Elucidating the consequences of this endoproteolytic cleavage is crucial for our understanding of cell death and other biological processes. Many caspase substrates are just cleaved as bystanders, because they happen to contain a caspase cleavage site in their sequence. Several targets, however, have a discrete function in propagation of the cell death process. Many structural and regulatory proteins are inactivated by caspases, while other substrates can be activated. In most cases, the consequences of this gain-of-function are poorly understood. Caspase substrates can regulate the key morphological changes in apoptosis. Several caspase substrates also act as transducers and amplifiers that determine the apoptotic threshold and cell fate. This review summarizes the known caspase substrates comprising a bewildering list of more than 280 different proteins. We highlight some recent aspects inferred by the cleavage of certain proteins in apoptosis. We also discuss emerging themes of caspase cleavage in other forms of cell death and, in particular, in apparently unrelated processes, such as cell cycle regulation and cellular differentiation.
Similar content being viewed by others
Introduction
In 1998, we published a list of caspase substrates comprising 65 different proteins that were cleaved by proteases of the caspase family.1 Most of the substrates known at that time could be categorized into a few functional groups, including proteins involved in scaffolding of the cytoplasm and nucleus, signal transduction and transcription-regulatory proteins, cell cycle controlling components and proteins involved in DNA replication and repair. Since then, the number of caspase substrates has considerably increased, more recently in particular because of a systematic proteome analysis of apoptotic cells.2,3,4 To date, more than 280 caspase targets are identified. Various methods have been employed to search for caspase substrates, including direct cDNA pool expression strategies or two-hybrid cloning approaches.5,6 By comparative two-dimensional (2D) gel electrophoresis of healthy and apoptotic cells, often a few hundred altered protein spots can be detected. Although not all of them have been confirmed as caspase targets, such proteomic approaches will certainly lead to the identification of numerous additional substrates in the near future (Table 1).
Already now, a bewildering number of substrates are cleaved by caspases. However, it should be kept in mind that some proteins might be cleaved very late and less completely during apoptosis, or not in all cell types. For example, it has been reported that β-actin can be cleaved by caspases in pheochromocytoma and ovarian carcinoma cells,7,8 whereas in many other cell types no cleavage was detected.9 Thus, it is possible that certain protein cleavages are cell type-specific, which may be because of variations in the expression of individual caspases. Also, caspase cleavage sites are not always conserved in different species. For instance, cyclin A is cleaved during apoptosis of Xenopus oocytes,10 but the caspase cleavage site is not present in homologues of mammalian cells. Some proteins, such as DNase-X, contain one or more classical cleavage sites in their sequence. However, the protein is virtually not cleaved inside apoptotic cells despite massive caspase activation.11 Moreover, in some cases, a first cut by caspases unleashes additional cleavage sites for other types of proteases. Cleavage of acinus, for instance, by caspase-3 is necessary but not sufficient to activate its DNA-condensing activity. For full activation, an additional, still unknown serine protease has to intervene. Only the combined action of both proteases generates the mature fragment, which, when added to purified nuclei, causes chromatin condensation.12
For many of the identified substrates, the functional consequences of their cleavage are unknown and have only been inferred from their normal functions. In other cases, the role of caspase cleavage has been experimentally assessed by expressing substrate proteins that have mutant caspase cleavage sites or by expressing protein fragments of the caspase-cleaved products. Given the high conservation of the apoptotic phenotype, from worms to mammals, it is highly likely that a conserved group of crucial caspase substrates exist. Proteolysis of the latter substrates presumably leads to the stereotypical destructive alterations that we call apoptosis.
The search for caspase substrates has brought several major questions into focus. For instance, is there a critical death substrate or what is the minimal set of proteins that must be cleaved in order to induce the phenotypic hallmarks of apoptosis? How is caspase substrate cleavage coordinated with other cellular processes, such as removal of dead cells, or presumably unrelated events including cell proliferation and differentiation? Although the significance of cleavage is not well understood for many substrates, the intense study of caspase substrates has recently shed some light on these questions. Here, we discuss several topics that have emerged from the accumulating knowledge regarding the role of caspase substrates in different biological processes.
Key morphological alterations are determined by caspase substrate cleavage
For most proteins, the consequences of their cleavage are poorly understood. In a few cases, however, proteolysis of certain components can be linked to discrete morphological changes of cell death. A classical example is the DNase inhibitor ICAD. Cleavage of ICAD by caspase-3 liberates the active CAD nuclease that mediates apoptotic DNA fragmentation (for references, see Table 1). In addition, the cleavage of acinus and helicard, a DNA helicase, contributes to chromatin condensation and nuclear remodeling. The cleavage of several other substrates, including gelsolin as well as the kinases ROCK-1 and PAK2, has been implicated in membrane blebbing, a classical morphological feature. Gelsolin is cleaved by caspase-3 to generate a constitutively active fragment that can depolymerize F-actin. Gelsolin-deficient neutrophils exhibit greatly delayed membrane blebbing during apoptosis, implying that membrane blebbing requires actin reorganization mediated by caspase-activated gelsolin. Caspases also cleave and thereby activate ROCK-1 leading to the phosphorylation of myosin light chains, which finally results in membrane blebbing.
Caspases destroy several proteins involved in maintenance of the cytoskeletal architecture such as the intermediate filaments cytokeratin-18 and vimentin, or Gas2 and plectin, two proteins involved in filament organization. These cleavages may directly contribute to apoptotic changes in cell shape. Caspases attack targets of the cortical actin network such as fodrin, and several components of the focal adhesion complex which links cortical actin filaments and membrane proteins to the extracellular matrix. Examples of this kind of substrates are focal adhesion kinase, Cas or paxillin. Cleavage of these proteins presumably contributes to cell shrinkage and cell detachment and, importantly, will interrupt antiapoptotic integrin signaling. A large percentage of caspase substrates are involved in cell adhesion or mediate cell–cell commmunication in adherens and gap junctions, or in desmosomes. Examples are β-catenin, E-cadherin, plakoglobin or desmoglein.
In the course of apoptosis, disruption of the endoplasmic reticulum and Golgi apparatus also takes place. Cleavage of golgin-160 and GRASP65 was suggested to cause disassembly of the Golgi complex, and proteolysis of Bap31 disrupts the transport between the ER and the Golgi complex. During apoptosis, vesicle transport processes are also impaired, for instance by the cleavage of rabaptin-5 or kinectin.
Caspases initiate the destruction of the nucleus where a huge variety of different proteins are cleaved. By 2D gel electrophoresis it has been recently determined that approximately 70 nuclear matrix proteins are consistently degraded or translocated during apoptosis, irrespective of the cell type or apoptotic stimulus.13 Many cleavages lead to nuclear lamina disassembly, and the cleavage of several components of the nuclear pore results in impaired nuclear transport. Inhibition of DNA repair, for instance by the cleavage of PARP-1 or the kinases ATM and DNA-PK, has been long thought to promote the apoptosis process. Other targeted factors are involved in DNA synthesis and replication, such as DNA polymerase Pol ɛ, MCM3 or replication factor RFC140. In addition, various proteins that bind to chromatin, and either fulfill a transcriptional role or have structural functions in the nuclear matrix, are destroyed. In almost all cases, these cleavages result in the generation of proteins that are no longer able to bind to DNA or to stabilize chromatin in the nuclear matrix. With a few exceptions that are discussed below, virtually all pathways of macromolecular synthesis are impaired by caspases. Cleavage of RNA helicase A and multiple splicing factors, including U1 70-kDa snRNP and at least eight different heterogeneous nuclear ribonucleo-proteins (hnRNPs), leads to a general shut-off of RNA synthesis, processing and transport. Moreover, protein synthesis is blocked either by the inactivation of translation initiation factors, including eIF2α, eIF3 and eIF4G proteins, or by the activation of PKR kinase that blocks protein synthesis through eIF2-α phosphorylation.
Caspase substrates in signal transduction
A tremendous variety of proteins involved in signal transduction are cleaved by caspases. The proteolytic cleavage can either lead to the functional inhibition or to the activation of these mediators. In some cases, it has been established that caspase-mediated activation of these molecules is involved in transduction and amplification of the apoptotic signal. Caspases turn off cell-protective mechanisms and activate pathways that lead to cell destruction. Classical apoptosis inhibitors that are cleaved by caspases are Bcl-2 proteins or the caspase-8 inhibitor c-FLIP. The cleavage of Bcl-2 and Bcl-xL resulting in the removal of the N-terminal BH4 domain not only leads to a loss of their antiapoptotic function, but even converts them to proapoptotic proteins. Similarly, during death receptor-mediated apoptosis caspase-8 cleaves the Bcl-2 member Bid generating an active C-terminal fragment that induces the proapoptotic release of cytochrome c from mitochondria. The conversion of antiapoptotic into proapoptotic regulators constitutes a positive feedback loop in the terminal phase of apoptosis, removing apoptotic inhibitors and promoting caspase activation. It is interesting to note that certain viral Bcl-2 proteins can also be cleaved by caspases, but in these cases no proapoptotic fragments are generated.
Several kinases and transcription factors with antiapoptotic activity are inactivated during apoptosis. Akt and Raf-1 provide two examples of antiapoptotic kinases that are cleaved by caspase-3. As both kinases can inactivate proapoptotic molecules such as Bad, their degradation presumably constitutes a positive feedback loop in apoptosis. Antiapoptotic transcription factors inhibited by caspases include the cAMP-responsive factor CREB, heat-shock factor HSF-1 and NF-κB. The NF-κB pathway is a paradigm of how caspase cleavage may result in a complete loss of the transcription factor's antiapoptotic function: (i) Cleavage of NF-κB subunit p65 (RelA) generates a dominant-negative fragment that is still able to bind to DNA but looses its transactivating activity, and therefore functions as a dominant-negative inhibitor. (ii) The NF-κB inhibitor IκB-α is normally inducibly degraded by the proteasome. The N-terminal cleavage of IκB-α by caspases generates a constitutive super-repressor that can no longer be removed by the proteasome. (iii) The cleavage of the adapter proteins TRAF-1 and RIP-1 that are involved in receptor-mediated pathways also contributes to impaired NF-κB activation and antiapoptotic capacity. Thus, cells have elaborate mechanisms in order to interrupt antiapoptotic signaling efficiently.
While some substrates are functionally inactivated upon caspase-mediated cleavage, other proteins and enzymes can be activated, mostly by removing an inhibitory or regulatory domain within the caspase target. The physiological consequence of this gain-of-function cleavage for apoptosis remains mostly unclear. Several members of the PKC family and MAP kinase pathway are constitutively activated by the separation of an N-terminal regulatory and the C-terminal catalytic domain. Examples are the p21-activated kinase PAK2 as well as ROCK-1. As described above, activation of PAK2 and ROCK-1 is important for cytoskeletal reorganization and plasma membrane blebbing. In the case of MEKK1, expression of the caspase-cleaved kinase fragment induces caspase activation, thereby providing a positive feedback loop for apoptosis. Epithelial cells undergo apoptosis if they are detached from the basement membrane, a process called anoikis. MEKK1 is activated following cell detachment, and blockade of either MEKK1 or caspase activity blocks anoikis. Cleavage of several MST kinases by caspase-3 also yields constitutively active molecules and potent inducers of apoptosis. Apoptosis induction by all these upstream kinases in the SAPK/JNK pathway may be explained in part by their ability to activate JNK, which then phosphorylates and inactivates Bcl-2.
Most kinase pathways exert antiapoptotic functions. It is thus not unexpected that a major cellular protein phosphatase, PP2A, which counteracts the survival function of kinases, is activated by caspases. Protein phosphorylation can also protect caspase substrates from proteolysis. This has been convincingly demonstrated for Bid that is protected from caspase-8 cleavage through phosphorylation by casein kinases I and II.14 Another example is Max, a transcription factor in the c-Myc network, which can be cleaved only if dephosphorylated. A very intriguing finding has been recently made for C/EBPβ. The transcription factor itself is not cleaved by caspases, but curiously acts as caspase inhibitor upon phosphorylation.15 Threonine phosphorylation of C/EBPβ within a KTVD sequence creates a noncleavable mimic of an XEXD cleavage site, which binds caspases and thereby inhibits caspase action. Hence, such dummies of caspase substrates may represent a novel survival mechanism.
Some peculiarities of substrate cleavage
Caspase cleavage can also result in the cellular redistribution and dislocation of signaling mediators. In some cases, such as the Grb2 adapter protein GrpL or the phosphodiesterase PDE4A5, an SH3-domain within the substrate is removed causing its inability to bind to physiological interaction partners. A change of subcellular localization following caspase cleavage has also been observed for the kinases Fyn and MEKK1. Another notable example is Bid. Upon cleavage by caspase-8, the proapoptotic p15 fragment of Bid undergoes post-translational rather than the classical cotranslational N-myristoylation at a glycine residue that becomes newly exposed by the cleavage.16 This postproteolytic N-myristoylation then enables Bid to target mitochondria and serves as an activating switch, which strongly enhances cytochrome c release.
Apoptosis is generally associated with a shut-down of cap-dependent protein translation, which is mediated by caspase cleavage of several translation factors. Interestingly, it has been recently observed that during apoptosis, translation of a subset of mRNAs prevails. The reason for this is presumably a switch from cap-dependent to internal ribosome entry site (IRES)-mediated protein translation. DAP-5, a member of the eIF4G family, is activated by caspases and stimulates translation from the IRES sites of c-Myc, Apaf-1, and its own mRNA.17 Thus, DAP-5 is a rather unique caspase-activated factor that supports cap-independent translation of apoptosis-related proteins and thereby may amplify the apoptosis cascade.
Most caspase substrates identified so far are cleaved by caspase-3. This has been convincingly shown in the system of MCF-7 breast carcinoma cells that lack caspase-3, and caspase-3 re-expressing derivatives.18 Nevertheless, several substrates that are efficiently cleaved by caspase-3 can also be targeted by caspase-7, suggesting an at least partial redundance of both caspases. Caspase-7 activity is upregulated in cells of caspase-3-deficient mice, where it might compensate for the loss of caspase-3. Caspase-7 and -5, but not caspase-3, cleave transcription factor Max. Interestingly, in this case Max is not cleaved at the classical aspartate residue in the P1 position, but at an unusual glutamate residue.19 Cleavage of the cytoplasmic tail of TNF-R1, the cardiac myosin light chain vMLC and connexin 45.6 at a glutamate instead of an aspartate residue are further examples. Cleavage at these noncanonical sites suggests that the specificity of caspases may in fact be broader than generally thought. Also, the Drosophila caspase DRONC can cleave substrates following glutamate residues.20 Caspase-7 not only cleaves substrates at atypical motifs, but can be activated itself by a rather unusual processing event. It has been reported that various serine proteases can trigger the proteolytic activity of the caspase-7 zymogen.21 For instance, cathepsin G activates caspase-7 by cleaving at a glutamate bond, indicating that the cleavage specificity at aspartic acid is not strictly required for caspase activation.
The interaction of caspases with other classes of proteases, including calpains, cathepsins or the proteasome, is poorly understood. When searching for caspase substrates, it must be considered that high concentrations of caspase inhibitors, such as the fluoromethylketone zVAD-fmk, are less specific than often anticipated, because calpains are inhibited as well. Several substrates of caspases are also cleaved by calpains including structural proteins, such as fodrin, keratins and β-actin, and proteins involved in signal transduction, such as Bid, Bax, focal adhesion kinase and many others. It has been found that caspases and calpains interfere with each other, resulting in mutual protease activation. Caspases can indirectly activate calpain by cleavage and inactivation of its inhibitor calpastatin, and thereby turn on downstream events leading to cellular destruction. However, it is still controversial as to whether calpains function upstream or downstream of caspases. It has also been reported that calpains cleave procaspases to generate proteolytically inactive caspase fragments.22
Caspases are not only involved in apoptosis but also in the induction of inflammation. In fact, the former notion that apoptosis and inflammation are exclusive processes should be replaced, as both processes are linked at various levels. Caspase-1 processes and maturates the cytokine precursors pro-IL-1β and pro-IL-18, also known as IFN-γ-inducing factor. Although caspase-1 is required mainly for induction of inflammation, it can process the effector caspases-3, -6 and -7 and may initiate apoptosis under certain conditions. Effector caspases can also activate pro-IL-16 and pro-EMAP-II, an endothelial-monocyte-activating polypeptide. This precursor of EMAP-II is an intriguing substrate, because it exerts a dual function:23 Pro-EMAP-II is identical to the p43 cofactor of the aminoacyl-tRNA synthetase complex. After cleavage, preferentially by caspase-7, its t-RNA binding capacity is lost and protein translation is blocked. The translation arrest is accompanied by the release of the EMAP-II cytokine that may play a role in the engulfment of apoptotic cells by phagocytes. Caspase-mediated substrate cleavage therefore has multiple effects summarized as (i) a halt of cell cycle progression, (ii) disabling of repair mechanisms, (iii) disassembly of molecular structures, (iv) cell detachment, and (v) maturation of cytokine precursors.
Substrate cleavage at the balance between necrosis and apoptosis
Although caspases are presumably not essential for necrotic cell death, recent evidence suggests that the cleavage of certain substrates may determine the form of cell death. One of the first death substrates found to be cleaved by caspases was PARP-1, which catalyzes the transfer of ADP-ribose polymers to nuclear proteins and thus presumably facilitates DNA repair.24 Owing to its role in DNA repair, it was originally hypothesized that the cleavage of PARP may lead to lethal DNA damage and compromise most of its DNA repair activity, and thus may contribute to the demise of the cell. However, PARP(−/−) mice neither reveal a phenotype which would indicate a crucial role in apoptosis nor is the sensitivity towards CD95- and TNF-R1-mediated apoptosis affected.25 Thus, cleavage of PARP may be a characteristic event, but is presumably dispensable for most apoptotic pathways.
New evidence, however, suggests that PARP inactivation by caspase-3 is important for turning off an energetically expensive DNA repair pathway and for maintaining ATP levels that are required for the execution of apoptosis. PARP is rapidly activated during oxidative stress and DNA damage. Activated PARP then transfers more than 100 ADP-ribose moieties to each acceptor site in target proteins, and each cycle of ADP-ribosylation is coupled with consumption of one NAD molecule, which is metabolically equivalent to four ATP molecules. Hence, it can be imagined that excessive activation of PARP will quickly deplete cellular energy stores. In the absence of an energy pool sufficient to execute apoptosis or to maintain ionic homeostasis, cells can die quickly by necrosis. Indeed, when cells engineered to express caspase-resistant PARP are treated with apoptotic stimuli, they undergo extensive necrosis instead of apoptosis.26 Consistent with the requirement of maintaining cellular energy during apoptosis, cells artificially depleted of ATP undergo necrosis instead of apoptosis under conditions that would normally trigger caspase activation.27 Thus, cleavage of PARP prevents depletion of the cellular energy needed for apoptosis and thus may function as a molecular switch between apoptotic and necrotic cell death. Similar to PARP, also the cleavage of other substrates may provide a link between apoptosis and necrosis. For instance, cleavage and inactivation of the plasma membrane calcium ATPase PMCA-4, which removes calcium from the cytosol, disturbs ion homeostasis.28 The subsequent cellular calcium overload may be responsible for the secondary necrosis that is observed in the late stages of apoptosis.
Role of caspase substrates in disease progression
Increased caspase activation has been recently demonstrated in various diseases. However, the cleavage of several substrates may not only contribute to increased tissue damage, but may also play an active role in disease progression. Such a direct role of substrate cleavage has been most intensively studied in neurodegeneration and autoimmune diseases. Autoimmunity to intracellular proteins has been identified as an important factor in autoimmune diseases. Massive apoptosis or defective clearance may lead to an accumulation of apoptotic cells that concentrate caspase-cleaved proteins in their apoptotic bodies and membrane blebs. The presence of autoantibodies against caspase substrates, such as lamins, fodrin, DNA-PK, PARP or NuMA, has been demonstrated in several autoimmune diseases.29 Cleavage of these autoantigens presumably enhances their immunogenicity by exposing cryptic neoepitopes. The cleaved proteins are then processed and presented by dendritic cells to circulating autoreactive T cells, triggering an autoimmune response.
The cleavage of specific substrates can be directly linked to the pathogenesis of certain neurodegenerative disorders. Huntington's disease, a genetically determined neurodegenerative disease, results from the expansion of CAG triplets at the 5′-primed end of the gene encoding huntingtin, a protein with a long polyglutamine stretch. Huntingtin is cleaved by caspase-3 and results in an N-terminal fragment, which aggregates and forms nuclear inclusions that are directly cytotoxic for neurons.30 Huntington's disease manifests only if huntingtin exceeds 35 glutamine residues. Because the rate of caspase cleavage of huntingtin correlates with the length of the polyglutamine stretch, accumulation of the fragment may cause a vicious cycle. A pathogenic role of caspase cleavage has also been implicated in other neurodegenerative disorders. Similar to huntingtin, the polyglutamine tract proteins atrophin-1, androgen receptor and ataxin-3 are caspase substrates. Indeed, mutations of the caspase recognition sites in atrophin-1 and androgen receptor abrogate their cytotoxicity in vitro.
Alzheimer's disease is characterized by brain lesions of neurofibrillary tangles, and senile plaques built of aggregates of the β-amyloid peptide. Aggregates of β-amyloid peptide induce neuronal apoptosis, and increased production of β-amyloid peptide has been postulated as an important pathologic mechanism in early-onset familial Alzheimer's disease. Effector caspases presumably increase β-amyloid production by several mechanisms. Loss-of-function mutations in the presenilin-1 and -2 genes are responsible for the majority of familial Alzheimer's disease and are thought to increase β-amyloid production. Caspase-3 can cleave and inactivate presenilins, which may mimic the effect of pathologic presenilin mutations. The 40- to 42-amino-acid β-amyloid peptide is derived from proteolytic processing of the amyloid precursor protein (APP) at two sites by the β- and γ-secretase. Caspase-3 cleaves APP at a site different from the γ-secretase site.31 Nevertheless, the N-terminal caspase cleavage product of APP strongly facilitates the production of β-amyloid peptide, and appears itself to be a component of senile plaques found in Alzheimer patients. Because caspase-3 activation and APP cleavage are also induced in vitro after ischemic brain injury, a risk factor for Alzheimer's disease, these results provide another example of a positive feedback loop between caspase substrate cleavage and neurodegeneration. Neuronal apoptosis from ischemia or other causes activates caspase-3 and stimulates APP cleavage, which increases the propensity for β-amyloid peptide production. In turn, increased extracellular β-amyloid peptide production may induce neuronal apoptosis, leading to further deposition of senile plaques. The cytotoxic properties of their cleavage products illustrate that specific caspase substrates are not only involved in cell destruction, but also fulfill an active role in the exacerbation of disease processes.
Caspases: more than just killers?
A strikingly large number of caspase targets are involved in cell cycle regulation. This has led to speculations that caspases are not only involved in cell death but also in proliferative events.32 Supportive, yet indirect evidence for a role of caspases in cell growth is the observation that proliferation of primary T cells is inhibited by cell-permeable caspase inhibitors.33,34 Moreover, interference with pathways leading to caspase processing, as in FADD-deficient or Bcl-2-transgenic mice, also results in impaired mature T-cell proliferation.
Several negative regulators including Wee1, an inhibitor of the cell cycle-regulatory kinases CDK2 and CDC2, as well as CDC27, a component of the anaphase-promoting complex, are cleaved by caspases. Wee1 is a critical component of the G2/M cell cycle checkpoint machinery and mediates cell cycle arrest by phosphorylation of CDC2. Therefore, cleavage of Wee1 in proliferating lymphocytes could lead to its inactivation, thus allowing cell cycle progression. Of note, Wee1 processing by caspases during apoptosis in Jurkat T cells correlated with a strong decrease in Wee1 activity and an increase in CDC2 activity.35 Moreover, the cyclin inhibitors p21Waf1 and p27Kip1 are targeted by caspases resulting in increased CDK2 activity that could allow cell cycle progression.
If caspases are activated during mitosis, a critical question is then, how could caspase cleavage be restricted to those cell cycle regulators, while leaving other vital proteins intact? The answer could lie in a specific subcellular compartmentalization of caspases, the existence of scaffold proteins or a different accessibility of cleavable substrates. Some caspases are translocated to a certain organelle during activation, and in some cell types certain caspases have been localized in the nucleus. Interestingly, it has been found that, although caspases were activated and Wee1 was cleaved after mitogenic T-cell stimulation, neither DNA replication factor RFC140 nor ICAD were cleaved in proliferating T cells.33 Cleavage of RFC140 and ICAD would lead to inhibition of DNA replication and fragmentation of genomic DNA, events that are not compatible with cell proliferation. Thus, selective substrate processing could explain why nonapoptotic cells survive and proliferate despite caspases being activated.
Certainly, there exist many links, also at the morphological level, between the processes of cell death and proliferation. However, it must be emphasized that the view of a potential involvement of caspases in proliferation is largely based on indirect evidence and therefore remains highly speculative. Because cleavage of cell cycle regulators occurs late in apoptosis by caspase-3-like activities in parallel with the dismantling of the transcription and translation machinery, caspase activation cannot trigger the normal mitotic program. For example, mitotic spindles do not form in apoptotic cells, distinguishing apoptosis from a mitotic catastrophe.
Limited substrate cleavage in terminal differentiation and hematopoiesis
In contrast to the rather speculative involvement of caspases in proliferation, there is an increasing body of evidence suggesting that caspases might act in cellular differentiation. A physiological role of caspases in this process has first been suggested for keratinocytes and lens fiber cells, in which the characteristic enucleation of the cells could be regarded somehow as a caspase-mediated incomplete apoptotic process.36,37 Caspases have also been implicated in erythropoiesis, because caspase inhibitors suppressed the nuclear extrusion process and consequent erythrocyte formation.38 Furthermore, caspase activation can be detected during thrombopoiesis and the fragmentation of proplatelets from megakaryocytes, without a concomitant induction of cell death.39 Both the incubation with peptide caspase inhibitors and the overexpression of Bcl-2 blocked proplatelet formation. Interestingly, in transgenic mice overexpressing Bcl-2 under the control of a hematopoietic cell-specific promoter, also a reduction in platelet formation is found, whereas the number of megakaryocytes remains unchanged. Finally, caspases might be required for differentiation processes also of nucleated cells such as macrophages and muscle cells. Elevated caspase activation is detectable in monocytes when they undergo M-CSF-stimulated macrophage differentiation.40 This is not only prevented by pharmacological caspase inhibitors, but also by the overexpression of Bcl-2 and p35. In myoblasts, homologous deletion of caspase-3 leads to a dramatic reduction in myofiber formation and decreased expression of muscle-specific proteins.41 Thus, all these lines of evidence suggest that caspases are not only required for cell death processes, but might also be capable of regulating nonapoptotic functions in certain cell types.
It is obvious that differentiation-related caspase activation must be tightly regulated to prevent cells from dying by apoptosis. During cellular differentiation, caspase activation is apparently either very limited, transient or localized. For instance, during megakaryocyte differentiation, the limited caspase activation is confined to dot-like structures.39 When senescent megakaryocytes die, however, caspase activation switches from a localized to a diffused and largely increased cytosolic activation. Also, little is known about the proteins cleaved by caspases during differentiation processes. Only a limited number of distinct substrates seem to be cleaved. For instance, in erythroblasts cleavage of PARP, lamin B and acinus was found, while the ICAD and GATA-1, a transcription factor essential for erythrocyte formation, remained intact. Interestingly, MST1 kinase was identified as a crucial caspase-3 effector in myoblast differentiation.41 As mentioned above, MST1 is cleaved and activated by caspase-3, and serves to enhance the activity of downstream MAP kinases that promote skeletal muscle differentiation. Expression of the truncated active kinase restored the differentiation phenotype in caspase-3 deficient myoblasts.
As discussed above, it remains currently unexplained as to how caspases could selectively cleave some targets without cleaving others. The compartmentalization of caspases, the duration of the caspase signal, or the coordinated expression of antiapoptotic molecules might play a role in the selectivity of caspase cleavage. It is also conceivable that low levels of caspase activity, such as those observed in differentiating cells, are associated with protective mechanisms. For instance, it was reported that the partial cleavage of Ras-GAP, a GTPase in the Ras signaling pathway, owing to low caspase activity first generates an N-terminal fragment that is antiapoptotic by activating the PI3K pathway.42 Increased caspase levels, in contrast, result in the further cleavage of Ras-GAP into two proapoptotic fragments. Thus, caspase cleavage of intracellular target proteins may strongly depend on the cellular context including the differentiation status. Clearly, much remains to be learned about a potential dual role of caspases in apoptosis and cellular differentiation. Characterization of the molecules that regulate this limited caspase activation and the relevant substrates will certainly provide exciting new insights into processes that, beyond cell death, might link caspase cleavage to important nonapoptotic biological processes.
Abbreviations
- Abbreviations:
-
See Table 1
References
Stroh C and Schulze-Osthoff K (1998) Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 5: 997–1000
Brockstedt E, Rickers A, Kostka S, Laubersheimer A, Dorken B, Wittmann-Liebold B, Bommert K and Otto A (1998) Identification of apoptosis-associated proteins in a human Burkitt lymphoma cell line. Cleavage of heterogeneous nuclear ribonucleoprotein A1 by caspase 3. J. Biol. Chem. 273: 28057–28064
Gerner C, Frohwein U, Gotzmann J, Bayer E, Gelbmann D, Bursch W and Schulte-Hermann R (2000) The Fas-induced apoptosis analyzed by high throughput proteome analysis. J. Biol. Chem. 275: 39018–39026
Thiede B, Dimmler C, Siejak F and Rudel T (2001) Predominant identification of RNA-binding proteins in Fas-induced apoptosis by proteome analysis. J. Biol. Chem. 276: 26044–26050
Cryns VL, Byun Y, Rana A, Mellor H, Lustig KD, Ghanem L, Parker PJ, Kirschner MW and Yuan J (1997) Specific proteolysis of the kinase protein kinase C-related kinase 2 by caspase-3 during apoptosis. Identification by a novel, small pool expression cloning strategy. J. Biol. Chem. 272: 29449–29453
Kamada S, Kusano H, Fujita H, Ohtsu M, Koya RC, Kuzumaki N and Tsujimoto Y (1998) A cloning method for caspase substrates that uses the yeast two-hybrid system: cloning of the antiapoptotic gene gelsolin. Proc. Natl. Acad. Sci. USA 95: 8532–8537
Kayalar C, Ord T, Testa MP, Zhong LT and Bredesen DE (1996) Cleavage of actin by interleukin 1 beta-converting enzyme to reverse DNase I inhibition. Proc. Natl. Acad. Sci. USA 93: 2234–2238
Chen Z, Naito M, Mashima T and Tsuruo T (1996) Activation of actin-cleavable interleukin 1beta-converting enzyme (ICE) family protease CPP-32 during chemotherapeutic agent-induced apoptosis in ovarian carcinoma cells. Cancer Res. 56: 5224–5229
Song Q, Wei T, Lees-Miller S, Alnemri E, Watters D and Lavin MF (1997) Resistance of actin to cleavage during apoptosis. Proc. Natl. Acad. Sci. USA 94: 157–162
Stack JH and Newport JW (1997) Developmentally regulated activation of apoptosis early in xenopus gastrulation results in cyclin A degradation during interphase of the cell cycle. Development 124: 3185–3195
Los M, Neubuser D, Coy JF, Mozoluk M, Poustka A and Schulze-Osthoff K (2000) Functional characterization of DNase X, a novel endonuclease expressed in muscle cells. Biochemistry 39: 7365–7373
Sahara S, Aoto M, Eguchi Y, Imamoto N, Yoneda Y and Tsujimoto Y (1999) Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature 401: 168–173
Gerner C, Gotzmann J, Frohwein U, Schamberger C, Ellinger A, and Sauermann G (2002) Proteome analysis of nuclear matrix proteins during apoptotic chromatin condensation. Cell Death Differ. 9: 671–681
Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B and Martinou JC (2001) Phosphorylation of Bid by casein kinases I and II regulates its cleavage by caspase 8. Mol. Cell 8: 601–611
Buck M, Poli V, Hunter T and Chojkier M (2001) C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol. Cell 8: 807–816
Zha J, Weiler S, Oh KJ, Wei MC and Korsmeyer SJ (2000) Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science 290: 1761–1765
Henis-Korenblit S, Shani G, Sines T, Marash L, Shohat G and Kimchi A (2002) The caspase-cleaved DAP5 protein supports internal ribosome entry site-mediated translation of death proteins. Proc. Natl. Acad. Sci. USA 99: 5400–5405
Jänicke RU, Sprengart ML, Wati MR and Porter AG (1998) Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 273: 9357–9360
Krippner-Heidenreich A, Talanian RV, Sekul R, Kraft R, Thole H, Ottleben H and Luscher B (2001) Targeting of the transcription factor Max during apoptosis: phosphorylation-regulated cleavage by caspase-5 at an unusual glutamic acid residue in position P1. Biochem. J. 358: 705–715
Hawkins CJ, Yoo SJ, Peterson EP, Wang SL, Vemooy SY and Hay BA (2000) The Drosophila caspase DRONC cleaves following glutamate or aspartate and is regulated by DIAP1, HID, and GRIM. J. Biol. Chem. 275: 27084–27093
Zhou Q and Salvesen GS (1997) Activation of pro-caspase-7 by serine proteases includes a non-canonical specificity. Biochem. J. 324: 361–364
Chua BT, Guo K and Li P (2000) Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J. Biol. Chem. 275: 5131–5135
Shalak V, Kaminska M, Mitnacht-Kraus R, Vandenabeele P, Clauss M and Mirande M (2001) The EMAPII cytokine is released from the mammalian multisynthetase complex after cleavage of its p43/proEMAPII component. J. Biol. Chem. 276: 23769–23776
Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS and Dixit VM (1995) Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 81: 801–809
Wang ZQ, Stingl L, Morrison C, Jantsch M, Los M, Schulze-Osthoff K and Wagner EF (1997) PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 11: 2347–2358
Los M, Mozoluk M, Ferrari D, Stepczynska A, Stroh C, Renz A, Herceg Z, Wang ZQ and Schulze-Osthoff K (2002) Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 13: 978–988
Ferrari D, Stepczynska A, Los M, Wesselborg S and Schulze-Osthoff K (1998) Differential regulation and ATP requirement for caspase-8 and caspase-3 activation during CD95- and anticancer drug-induced apoptosis. J. Exp. Med. 188: 979–984
Schwab BL, Guerini D, Didszun C, Bano D, Ferrando-May E, Fava E, Tam J, Xu D, Xanthoudakis S, Nicholson DW, Carafoli E and Nicotera P (2002) Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis. Cell Death Differ. 9: 818–831
Rosen A and Casciola-Rosen L (1999) Autoantigens as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 6: 6–12
Goldberg YP, Nicholson DW, Rasper DM, Kalchman MA, Koide HB, Graham RK, Bromm M, Kazemi-Esfarjani P, Thornberry NA, Vaillancourt JP and Hayden MR (1996) Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nat. Genet. 13: 442–449
Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S and Nicholson DW (1999) Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell 97: 395–406
Los M, Stroh C, Jänicke RU, Engels IH and Schulze-Osthoff K (2001) Caspases: more than just killers? Trends Immunol. 22: 31–34
Alam A, Cohen LY, Aouad S and Sekaly RP (1999) Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J. Exp. Med. 190: 1879–1890
Kennedy NJ, Kataoka T, Tschopp J and Budd RC (1999) Caspase activation is required for T cell proliferation. J. Exp. Med. 190: 1891–1896
Zhou BB, Li H, Yuan J and Kirschner MW (1998) Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat cells. Proc. Natl. Acad. Sci. USA 95: 6785–6790
Weil M, Raff MC and Braga VM (1999) Caspase activation in the terminal differentiation of human epidermal keratinocytes. Curr. Biol. 9: 361–364
Ishizaki Y, Jacobson MD and Raff MC (1998) A role for caspases in lens fiber differentiation. J. Cell Biol. 140: 153–158
Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E and Hermine O (2001) Caspase activation is required for terminal erythroid differentiation. J. Exp. Med. 193: 247–254
De Botton S, Sabri S, Daugas E, Zermati Y, Guidotti JE, Hermine O, Kroemer G, Vainchenker W and Debili N (2002) Platelet formation is the consequence of caspase activation within megakaryocytes. Blood 100: 1310–1317
Sordet O, Rebe C, Plenchette S, Zermati Y, Hermine O, Vainchenker W, Garrido C, Solary E and Dubrez-Daloz L . (2002) Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood 97: 3931–3340
Femando P, Kelly JF, Balazsi K, Slack RS and Megeney LA (2002) Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl. Acad. Sci. USA 99: 11025–11030
Yang JY and Widmann C (2002) The RasGAP N-terminal fragment generated by caspase cleavage protects cells in a Ras/PI3K/Akt-dependent manner that does not rely on NFkappa B activation. J. Biol. Chem. 277: 14641–14646
Bratton SB, Walker G, Roberts DL, Cain K and Cohen GM (2001) Caspase-3 cleaves Apaf-1 into an approximately 30 kDa fragment that associates with an inappropriately oligomerized and biologically inactive approximately 1.4 MDa apoptosome complex. Cell Death Differ. 8: 425–433
Lauber K, Appel HA, Schlosser SF, Gregor M, Schulze-Osthoff K and Wesselborg S (2001) The adapter protein apoptotic protease-activating factor-1 (Apaf-1) is proteolytically processed during apoptosis. J. Biol. Chem. 276: 29772–29781
Condorelli F, Salomoni P, Cotteret S, Cesi V, Srinivasula SM, Alnemri ES and Calabretta B (2001) Caspase cleavage enhances the apoptosis-inducing effects of BAD. Mol. Cell. Biol. 21: 3025–3036
Wood DE and Newcomb EW (1999) Caspase-dependent activation of calpain during drug-induced apoptosis. J. Biol. Chem. 274: 8309–8315
Yanase N, Ohshima K, Ikegami H and Mizuguchi J (2000) Cytochrome c release, mitochondrial membrane depolarization, caspase-3 activation, and Bax-alpha cleavage during IFN-alpha-induced apoptosis in Daudi B lymphoma cells. J. Interferon Cytokine Res. 20: 1121–1129
Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K and Hardwick JM (1997) Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 278: 1966–1968
Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA and Hardwick JM (1998) Modulation of cell death by Bcl-XL through caspase interaction. Proc. Natl. Acad. Sci. USA 95: 554–559
Bellows DS, Chau BN, Lee P, Lazebnik Y, Bums WH and Hardwick JM (2000) Antiapoptotic herpesvirus Bcl-2 homologs escape caspase-mediated conversion to proapoptotic proteins. J. Virol. 74: 5024–5031
Li H, Zhu H, Xu CJ and Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501
Luo X, Budihardjo I, Zou H, Slaughter C and Wang X (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481–490
Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Bums K, Mattmann C, Rimoldi D, French LE and Tschopp J (1997) Inhibition of death receptor signals by cellular FLIP. Nature 388: 190–195
Clem RJ, Sheu TT, Richter BW, He WW, Thomberry NA, Duckett CS and Hardwick JM (2001) c-IAP1 is cleaved by caspases to produce a proapoptotic C-terminal fragment. J. Biol. Chem. 276: 7602–7608
Earnshaw WC, Martins LM and Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68: 383–424
Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS and Reed JC (1999) Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 18: 5242–5251
Levkau B, Garton KJ, Ferri N, Kloke K, Nofer JR, Baba HA, Raines EW and Breithardt G (2001) XIAP induces cell-cycle arrest and activates nuclear factor-kappaB: new survival pathways disabled by caspase-mediated cleavage during apoptosis of human endothelial cells. Circ. Res. 88: 282–290
Browne SJ, MacFarlane M, Cohen GM and Paraskeva C (1998) The adenomatous polyposis coli protein and retinoblastoma protein are cleaved early in apoptosis and are potential substrates for caspases. Cell Death Differ. 5: 206–213
Webb SJ, Nicholson D, Bubb VJ and Wyllie AH (1999) Caspase-mediated cleavage of APC results in an amino-terminal fragment with an intact armadillo repeat domain. FASEB J. 13: 339–346
Kim JA and Kim HL (2001) Cleavage of purified neuronal clathrin assembly protein (CALM) by caspase 3 and calpain. Exp. Mol. Med. 33: 245–250
Bannerman DD, Sathyamoorthy M and Goldblum SE (1998) Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J. Biol. Chem. 273: 35371–35380
Kook S, Shim SR, Choi SJ, Ahnn J, Kim JI, Eom SH, Jung YK, Paik SG and Song WK (2000) Caspase-mediated cleavage of p130cas in etoposide-induced apoptotic Rat-1 cells. Mol. Biol. Cell 11: 929–939
Brancolini C, Lazarevic D, Rodriguez J and Schneider C (1997) Dismantling cell-cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of beta-catenin. J. Cell Biol. 139: 759–771
Herren B, Levkau B, Raines EW and Ross R (1998) Cleavage of beta-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol. Biol. Cell 9: 1589–1601
Steinhusen U, Badock V, Bauer A, Behrens J, Wittman-Liebold B, Dorken B and Bommert K (2000) Apoptosis-induced cleavage of beta-catenin by caspase-3 results in proteolytic fragments with reduced transactivation potential. J. Biol. Chem. 275: 16345–16353
Brancolini C, Sgorbissa A and Schneider C (1998) Proteolytic processing of the adherens junctions components beta-catenin and gamma-catenin/plakoglobin during apoptosis. Cell Death Differ. 5: 1042–1050
Weiske J, Schoneberg T, Schroder W, Hatzfeld M, Tauber R and Huber O (2001) The fate of desmosomal proteins in apoptotic cells. J. Biol. Chem. 276: 41175–41181
Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K and Huber O (2001) Cleavage and shedding of E-cadherin after induction of apoptosis. J. Biol. Chem. 276: 4972–4980
Schmeiser K and Grand RJ (1999) The fate of E- and P-cadherin during the early stages of apoptosis. Cell Death Differ. 6: 377–386
Hunter I, McGregor D and Robins SP (2001) Caspase-dependent cleavage of cadherins and catenins during osteoblast apoptosis. J. Bone Miner. Res. 16: 466–477
Crouch DH, Fincham VJ and Frame MC (1996) Targeted proteolysis of the focal adhesion kinase pp125 FAK during c-MYC-induced apoptosis is suppressed by integrin signalling. Oncogene 12: 2689–2696
Gervais FG, Thomberry NA, Ruffolo SC, Nicholson DW and Roy S (1998) Caspases cleave focal adhesion kinase during apoptosis to generate a FRNK-like polypeptide. J. Biol. Chem. 273: 17102–17108
Levkau B, Herren B, Koyama H, Ross R and Raines EW (1998) Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J. Exp. Med. 187: 579–586
Wen LP, Fahmi JA, Troie S, Guan JL, Orth K and Rosen GD (1997) Cleavage of focal adhesion kinase by caspases during apoptosis. J. Biol. Chem. 272: 26056–26061
Law SF, O'Neill GM, Fashena SJ, Einarson MB and Golemis EA (2000) The docking protein HEF1 is an apoptotic mediator at focal adhesion sites. Mol. Cell. Biol. 20: 5184–5195
O'Neill GM and Golemis EA (2001) Proteolysis of the docking protein HEF1 and implications for focal adhesion dynamics. Mol. Cell. Biol. 21: 5094–5108
Yin X, Gu S and Jiang JX (2001) The development-associated cleavage of lens connexin 45.6 by caspase-3-like protease is regulated by casein kinase II-mediated phosphorylation. J. Biol. Chem. 276: 34567–34572
Lesay A, Hickman JA and Gibson RM (2001) Disruption of focal adhesions mediates detachment during neuronal apoptosis. Neuroreport 12: 2111–2115
Chay KO, Park SS and Mushinski JF (2002) Linkage of caspase-mediated degradation of paxillin to apoptosis in Ba/F3 murine pro-B lymphocytes. J. Biol. Chem. 277: 14521–14529
Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ and Hajjar RJ (2002) Functional consequences of caspase activation in cardiac myocytes. Proc. Natl. Acad. Sci. USA 99: 6252–6256
Mashima T, Naito M, Noguchi K, Miller DK, Nicholson DW and Tsuruo T (1997) Actin cleavage by CPP-32/apopain during the development of apoptosis. Oncogene 14: 1007–1012
van de Water B, Tijdens IB, Verbrugge A, Huigsloot M, Dihal AA, Stevens JL, Jaken S and Mulder GJ (2000) Cleavage of the actin-capping protein alpha-adducin at Asp–Asp–Ser–Asp633–Ala by caspase-3 is preceded by its phosphorylation on serine 726 in cisplatin-induced apoptosis of renal epithelial cells. J. Biol. Chem. 275: 25805–25813
Lane JD, Vergnolle MA, Woodman PG and Allan VJ (2001) Apoptotic cleavage of cytoplasmic dynein intermediate chain and p150(Glued) stops dynein-dependent membrane motility. J. Cell Biol. 153: 1415–1426
Chen YR, Kori R, John B and Tan TH (2001) Caspase-mediated cleavage of actin-binding and SH3-domain-containing proteins cortactin, HS1, and HIP-55 during apoptosis. Biochem. Biophys. Res. Commun. 288: 981–989
Browne KA, Johnstone RW, Jans DA and Trapani JA (2000) Filamin (280-kDa actin-binding protein) is a caspase substrate and is also cleaved directly by the cytotoxic T lymphocyte protease granzyme B during apoptosis. J. Biol. Chem. 275: 39262–39266
Umeda T, Kouchi Z, Kawahara H, Tomioka S, Sasagawa N, Maeda T, Sorimachi H, Ishiura S and Suzuki K (2001) Limited proteolysis of filamin is catalyzed by caspase-3 in U937 and Jurkat cells. J. Biochem. 130: 535–542
Martin SJ, Finucane DM, Amarante-Mendes GP, O'Brien GA and Green DR (1996) Phosphatidylserine externalization during CD95-induced apoptosis of cells and cytoplasts requires ICE/CED-3 protease activity. J. Biol. Chem. 271: 28753–28756
Cryns VL, Bergeron L, Zhu H, Li H and Yuan J (1996) Specific cleavage of alpha-fodrin during Fas- and tumor necrosis factor-induced apoptosis is mediated by an interleukin-1beta-converting enzyme/Ced-3 protease distinct from the poly(ADP-ribose) polymerase protease. J. Biol. Chem. 271: 31277–31282
Vanags DM, Porn-Ares MI, Coppola S, Burgess DH and Orrenius S (1996) Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J. Biol. Chem. 271: 31075–31085
Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H, Talanian RV, Yuen P, Gilbertsen RB and Wang KK (1996) Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem. J. 319: 683–690
Brancolini C, Benedetti M and Schneider C (1995) Microfilament reorganization during apoptosis: the role of Gas2, a possible substrate for ICE-like proteases. EMBO J. 14: 5179–5190
Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ and Williams LT (1997) Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science 278: 294–298
Geng YJ, Azuma T, Tang JX, Hartwig JH, Muszynski M, Wu Q, Libby P and Kwiatkowski DJ (1998) Caspase-3-induced gelsolin fragmentation contributes to actin cytoskeletal collapse, nucleolysis, and apoptosis of vascular smooth muscle cells exposed to proinflammatory cytokines. Eur. J. Cell Biol. 77: 294–302
Ku NO, Liao J and Omary MB (1997) Apoptosis generates stable fragments of human type I keratins. J. Biol. Chem. 272: 33197–33203
Caulin C, Salvesen GS and Oshima RG (1997) Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J. Cell Biol. 138: 1379–1394
Prasad S, Soldatenkov VA, Srinivasarao G and Dritschilo A (1998) Identification of keratins 18, 19 and heat-shock protein 90 beta as candidate substrates of proteolysis during ionizing radiation-induced apoptosis of estrogen-receptor negative breast tumor cells. Int. J. Oncol. 13: 757–764
Badock V, Steinhusen U, Bommert K, Wittmann-Liebold B and Otto A (2001) Apoptosis-induced cleavage of keratin 15 and keratin 17 in a human breast epithelial cell line. Cell Death Differ. 8: 308–315
Moretti A, Weig HJ, Ott T, Seyfarth M, Holthoff HP, Grewe D, Gillitzer A, Bott-Flugel L, Schomig A, Ungerer M and Laugwitz KL (2002) Essential myosin light chain as a target for caspase-3 in failing myocardium. Proc. Natl. Acad. Sci. USA 99: 11860–11865
Stegh AH, Herrmann H, Lampel S, Weisenberger D, Andra K, Seper M, Wiche G, Krammer PH and Peter ME (2000) Identification of the cytolinker plectin as a major early in vivo substrate for caspase 8 during CD95- and tumor necrosis factor receptor-mediated apoptosis. Mol. Cell. Biol. 20: 5665–5679
Wang KK, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian RV, Glantz SB and Morrow JS (1998) Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J. Biol. Chem. 273: 22490–22497
Canu N, Dus L, Barbato C, Ciotti MT, Brancolini C, Rinaldi AM, Novak M, Cattaneo A, Bradbury A and Calissano P (1998) Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. J. Neurosci. 18: 7061–7074
Fasulo L, Ugolini G, Visintin M, Bradbury A, Brancolini C, Verzillo V, Novak M and Cattaneo A (2000) The neuronal microtubule-associated protein tau is a substrate for caspase-3 and an effector of apoptosis. J. Neurochem. 75: 624–633
Byun Y, Chen F, Chang R, Trivedi M, Green KJ and Cryns VL (2001) Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 8: 443–450
Nakanishi K, Maruyama M, Shibata T and Morishima N (2001) Identification of a caspase-9 substrate and detection of its cleavage in programmed cell death during mouse development. J. Biol. Chem. 276: 41237–41244
Columbaro M, Mattioli E, Lattanzi G, Rutigliano C, Ognibene A, Maraldi NM and Squarzoni S (2001) Staurosporine treatment and serum starvation promote the cleavage of emerin in cultured mouse myoblasts: involvement of a caspase-dependent mechanism. FEBS Lett. 509: 423–429
Duband-Goulet I, Courvalin JC and Buendia B (1998) LBR, a chromatin and lamin binding protein from the inner nuclear membrane, is proteolyzed at late stages of apoptosis. J. Cell. Sci. 111: 1441–1451
Lazebnik YA, Takahashi A, Poirier GG, Kaufmann SH and Earnshaw WC (1995) Characterization of the execution phase of apoptosis in vitro using extracts from condemned-phase cells. J. Cell. Sci. 19: 41–49
Orth K, Chinnaiyan AM, Garg M, Froelich CJ and Dixit VM (1996) The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J. Biol. Chem. 271: 16443–16446
Rao L, Perez D and White E (1996) Lamin proteolysis facilitates nuclear events during apoptosis. J. Cell Biol. 135: 1441–1455
Gotzmann J, Vlcek S and Foisner R (2000) Caspase-mediated cleavage of the chromosome-binding domain of lamina-associated polypeptide 2 alpha. J. Cell. Sci. 113: 3769–3780
Buendia B, Santa-Maria A and Courvalin JC (1999) Caspase-dependent proteolysis of integral and peripheral proteins of nuclear membranes and nuclear pore complex proteins during apoptosis. J. Cell. Sci. 112: 1743–1753
Ferrando-May E, Cordes V, Biller-Ckovric I, Mirkovic J, Gorlich D and Nicotera P (2001) Caspases mediate nucleoporin cleavage, but not early redistribution of nuclear transport factors and modulation of nuclear permeability in apoptosis. Cell Death Differ. 8: 495–505
Gohring F, Schwab BL, Nicotera P, Leist M and Fackelmayer FO (1997) The novel SAR-binding domain of scaffold attachment factor A (SAF-A) is a target in apoptotic nuclear breakdown. EMBO J. 16: 7361–7371
Kipp M, Schwab BL, Przybylski M, Nicotera P and Fackelmayer FO (2000) Apoptotic cleavage of scaffold attachment factor A (SAF-A) by caspase-3 occurs at a noncanonical cleavage site. J. Biol. Chem. 275: 5031–5036
Gotzmann J, Meissner M and Gerner C (2000) The fate of the nuclear matrix-associated-region-binding protein SATB1 during apoptosis. Cell Death Differ. 7: 425–438
Galande S, Dickinson LA, Mian IS, Sikorska M and Kohwi-Shigematsu T (2001) SATB1 cleavage by caspase 6 disrupts PDZ domain-mediated dimerization, causing detachment from chromatin early in T-cell apoptosis. Mol. Cell. Biol. 21: 5591–5604
Ng FW, Nguyen M, Kwan T, Branton PE, Nicholson DW, Cromlish JA and Shore GC (1997) p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum. J. Cell Biol. 139: 327–338
Granville DJ, Carthy CM, Jiang H, Shore GC, McManus BM and Hunt DW (1998) Rapid cytochrome c release, activation of caspases 3, 6, 7 and 8 followed by Bap31 cleavage in HeLa cells treated with photodynamic therapy. FEBS Lett. 437: 5–10
Maatta J, Hallikas O, Welti S, Hilden P, Schroder J and Kuismanen E (2000) Limited caspase cleavage of human BAP31. FEBS Lett. 484: 202–206
Mancini M, Machamer CE, Roy S, Nicholson DW, Thomberry NA, Casciola-Rosen LA and Rosen A (2000) Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J. Cell Biol. 149: 603–612
Lane JD, Lucocq J, Pryde J, Barr FA, Woodman PG, Allan VJ and Lowe M (2002) Caspase-mediated cleavage of the stacking protein GRASP65 is required for Golgi fragmentation during apoptosis. J. Cell Biol. 156: 495–509
Machleidt T, Geller P, Schwandner R, Scherer G and Kronke M (1998) Caspase 7-induced cleavage of kinectin in apoptotic cells. FEBS Lett. 436: 51–54
Di Bacco AM and Cotter TG (2002) p53 expression in K562 cells is associated with caspase-mediated cleavage of c-ABL and BCR-ABL protein kinases. Br. J. Haematol. 117: 588–597
Pelizon C, D'Adda Di Fagagna F, Farrace L and Laskey RA (2002) Human replication protein Cdc6 is selectively cleaved by caspase 3 during apoptosis. EMBO Rep. 3: 780–784
Mazumder S, Gong B, Chen Q, Drazba JA, Buchsbaum JC and Almasan A (2002) Proteolytic cleavage of cyclin e leads to inactivation of associated kinase activity and amplification of apoptosis in hematopoietic cells. Mol. Cell. Biol. 22: 2398–2409
Chen L, Marechal V, Moreau J, Levine AJ and Chen J (1997) Proteolytic cleavage of the mdm2 oncoprotein during apoptosis. J. Biol. Chem. 272: 22966–22973
Erhardt P, Tomaselli KJ and Cooper GM (1997) Identification of the MDM2 oncoprotein as a substrate for CPP32-like apoptotic proteases. J. Biol. Chem. 272: 15049–15052
Gentiletti F, Mancini F, D'Angelo M, Sacchi A, Pontecorvi A, Jochemsen AG and Moretti F (2002) MDMX stability is regulated by p53-induced caspase cleavage in NIH3T3 mouse fibroblasts. Oncogene 21: 867–877
Hsu HL and Yeh NH (1996) Dynamic changes of NuMA during the cell cycle and possible appearance of a truncated form of NuMA during apoptosis. J. Cell. Sci. 109: 277–288
Casiano CA, Martin SJ, Green DR and Tan EM (1996) Selective cleavage of nuclear autoantigens during CD95 (Fas/APO-1)-mediated T cell apoptosis. J. Exp. Med. 184: 765–770
Gervais JL, Seth P and Zhang H (1998) Cleavage of CDK inhibitor p21(Cip1/Waf1) by caspases is an early event during DNA damage-induced apoptosis. J. Biol. Chem. 273: 19207–19212
Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth K, Roberts JM and Ross R (1998) Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol. Cell 1: 553–563
Eymin B, Sordet O, Droin N, Munsch B, Haugg M, Van de Craen M, Vandenabeele P and Solary E (1999) Caspase-induced proteolysis of the cyclin-dependent kinase inhibitor p27Kip1 mediates its anti-apoptotic activity. Oncogene 18: 4839–4847
Lahti JM, Xiang J, Heath LS, Campana D and Kidd VJ (1995) PITSLRE protein kinase activity is associated with apoptosis. Mol. Cell. Biol. 15: 1–11
Beyaert R, Kidd VJ, Comelis S, Van de Craen M, Denecker G, Lahti JM, Gururajan R, Vandenabeele P and Fiers W (1997) Cleavage of PITSLRE kinases by ICE/CASP-1 and CPP32/CASP-3 during apoptosis induced by tumor necrosis factor. J. Biol. Chem. 272: 11694–11697
Evstafieva AG, Belov GA, Kalkum M, Chichkova NV, Bogdanov AA, Agol VI and Vartapetian AB (2000) Prothymosin alpha fragmentation in apoptosis. FEBS Lett. 467: 150–154
Enkemann SA, Wang RH, Trumbore MW and Berger SL (2000) Functional discontinuities in prothymosin alpha caused by caspase cleavage in apoptotic cells. J. Cell Physiol. 182: 256–268
Jänicke RU, Walker PA, Lin XY and Porter AG (1996) Specific cleavage of the retinoblastoma protein by an ICE-like protease in apoptosis. EMBO J. 15: 6969–6978
Smith GC, d'Adda di Fagagna F, Lakin ND and Jackson SP (1999) Cleavage and inactivation of ATM during apoptosis. Mol. Cell. Biol. 19: 6076–6084
Freire R, d'Adda Di Fagagna F, Wu L, Pedrazzi G, Stagljar I, Hickson ID and Jackson SP (2001) Cleavage of the Bloom's syndrome gene product during apoptosis by caspase-3 results in an impaired interaction with topoisomerase IIIalpha. Nucleic Acids Res. 29: 3172–3180
Bischof O, Galande S, Farzaneh F, Kohwi-Shigematsu T and Campisi J (2001) Selective cleavage of BLM, the bloom syndrome protein, during apoptotic cell death. J. Biol. Chem. 276: 12068–12075
Zhan Q, Jin S, Ng B, Plisket J, Shangary S, Rathi A, Brown KD and Baskaran R (2002) Caspase-3 mediated cleavage of BRCA1 during UV-induced apoptosis. Oncogene 21: 5335–5345
Casciola-Rosen LA, Anhalt GJ and Rosen A (1995) DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J. Exp. Med. 182: 1625–1634
Song Q, Lees-Miller SP, Kumar S, Zhang Z, Chan DW, Smith GC, Jackson SP, Alnemri ES, Litwack G, Khanna KK and Lavin MF (1996) DNA-dependent protein kinase catalytic subunit: a target for an ICE-like protease in apoptosis. EMBO J. 15: 3238–3246
Liu X, Zou H, Slaughter C and Wang X (1997) DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175–184
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A and Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43–50
Kovacsovics M, Martinon F, Micheau O, Bodmer JL, Hofmann K and Tschopp J (2002) Overexpression of helicard, a CARD-containing helicase cleaved during apoptosis, accelerates DNA degradation. Curr. Biol. 12: 838–843
Schwab BL, Leist M, Knippers R and Nicotera P (1998) Selective proteolysis of the nuclear replication factor MCM3 in apoptosis. Exp. Cell Res. 238: 415–421
Affar EB, Germain M, Winstall E, Vodenicharov M, Shah RG, Salvesen GS and Poirier GG (2001) Caspase-3-mediated processing of poly(ADP-ribose) glycohydrolase during apoptosis. J. Biol. Chem. 276: 2935–2942
Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE and Poirier GG (1993) Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 53: 3976–3985
Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG and Earnshaw WC (1994) Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371: 346–347
Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA and et al. (1995) Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376: 37–43
Benchoua A, Couriaud C, Guegan C, Tartier L, Couvert P, Friocourt G, Chelly J, Menissier-De Murcia J and Onteniente B (2002) Active caspase-8 translocates into the nucleus of apoptotic cells to inactivate poly(ADP-ribose) polymerase-2. J. Biol. Chem. 277: 34217–34222
Liu W and Linn S (2000) Proteolysis of the human DNA polymerase varepsilon catalytic subunit by caspase-3 and calpain specifically during apoptosis. Nucleic Acids Res. 28: 4180–4188
Chen F, Kamradt M, Mulcahy M, Byun Y, Xu H, McKay MJ and Cryns VL (2002) Caspase proteolysis of the cohesin component RAD21 promotes apoptosis. J. Biol. Chem. 277: 16775–16781
Flygare J, Armstrong RC, Wennborg A, Orsan S and Hellgren D (1998) Proteolytic cleavage of HsRad51 during apoptosis. FEBS Lett. 427: 247–251
Rheaume E, Cohen LY, Uhlmann F, Lazure C, Alam A, Hurwitz J, Sekaly RP and Denis F (1997) The large subunit of replication factor C is a substrate for caspase-3 in vitro and is cleaved by a caspase-3-like protease during Fas-mediated apoptosis. EMBO J. 16: 6346–6354
Song Q, Lu H, Zhang N, Luckow B, Shah G, Poirier G and Lavin M (1997) Specific cleavage of the large subunit of replication factor C in apoptosis is mediated by CPP32-like protease. Biochem. Biophys. Res. Commun. 233: 343–348
Ubeda M and Habener JF (1997) The large subunit of the DNA replication complex C (DSEB/RF-C140) cleaved and inactivated by caspase-3 (CPP32/YAMA) during Fas-induced apoptosis. J. Biol. Chem. 272: 19562–19568
Casiano CA, Ochs RL and Tan EM (1998) Distinct cleavage products of nuclear proteins in apoptosis and necrosis revealed by autoantibody probes. Cell Death Differ. 5: 183–190
Samejima K, Svingen PA, Basi GS, Kottke T, Mesner PW, Jr., Stewart L, Durrieu F, Poirier GG, Alnemri ES, Champoux JJ, Kaufmann SH and Earnshaw WC (1999) Caspase-mediated cleavage of DNA topoisomerase I at unconventional sites during apoptosis. J. Biol. Chem. 274: 4335–4340
Bortul R, Zweyer M, Billi AM, Tabellini G, Ochs RL, Bareggi R, Cocco L and Martelli AM (2001) Nuclear changes in necrotic HL-60 cells. J. Cell. Biochem. 81: 19–31
Matsumoto Y, Suzuki N, Namba N, Umeda N, Ma XJ, Morita A, Tomita M, Enomoto A, Serizawa S, Hirano K, Sakaia K, Yasuda H and Hosoi Y (2000) Cleavage and phosphorylation of XRCC4 protein induced by X-irradiation. FEBS Lett. 478: 67–71
Nyormoi O, Wang Z, Doan D, Ruiz M, McConkey D and Bar-Eli M (2001) Transcription factor AP-2alpha is preferentially cleaved by caspase 6 and degraded by proteasome during tumor necrosis factor alpha-induced apoptosis in breast cancer cells. Mol. Cell. Biol. 21: 4856–4867
Francois F, Godinho MJ and Grimes ML (2000) CREB is cleaved by caspases during neural cell apoptosis. FEBS Lett. 486: 281–284
Barkett M, Dooher JE, Lemonnier L, Simmons L, Scarpati JN, Wang Y and Gilmore TD (2001) Three mutations in v-Rel render it resistant to cleavage by cell-death protease caspase-3. Biochim. Biophys. Acta 1526: 25–36
van Criekinge W, Comelis S, Van De Craen M, Vandenabeele P, Fiers W and Beyaert R (1999) GAL4 is a substrate for caspases: implications for two-hybrid screening and other GAL4-based assays. Mol. Cell. Biol. Res. Commun. 1: 158–161
De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, Srinivasula SM, Alnemri ES, Testa U and Peschle C (1999) Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature 401: 489–493
Zhang M, Blake MJ, Gout PW, Buckley DJ and Buckley AR (1999) Proteolysis of heat shock transcription factor is associated with apoptosis in rat Nb2 lymphoma cells. Cell Growth Differ. 10: 759–767
Bell B, Scheer E and Tora L (2001) Identification of hTAF(II)80 delta links apoptotic signaling pathways to transcription factor TFIID function. Mol. Cell 8: 591–600
Barkett M, Xue D, Horvitz HR and Gilmore TD (1997) Phosphorylation of IkappaB-alpha inhibits its cleavage by caspase CPP32 in vitro. J. Biol. Chem. 272: 29419–29422
Wu X, Daniels T, Molinaro C, Lilly MB and Casiano CA (2002) Caspase cleavage of the nuclear autoantigen LEDGF/p75 abrogates its pro-survival function: implications for autoimmunity in atopic disorders. Cell Death Differ. 9: 915–925
Li M, Linseman DA, Allen MP, Meintzer MK, Wang X, Laessig T, Wierman ME and Heidenreich KA (2001) Myocyte enhancer factor 2A and 2D undergo phosphorylation and caspase-mediated degradation during apoptosis of rat cerebellar granule neurons. J. Neurosci. 21: 6544–6552
Okamoto Si S, Li Z, Ju C, Scholzke MN, Mathews E, Cui J, Salvesen GS, Bossy-Wetzel E and Lipton SA (2002) Dominant-interfering forms of MEF2 generated by caspase cleavage contribute to NMDA-induced neuronal apoptosis. Proc. Natl. Acad. Sci. USA 99: 3974–3979
Ravi R, Bedi A and Fuchs EJ (1998) CD95 (Fas)-induced caspase-mediated proteolysis of NF-kappaB. Cancer Res. 58: 882–886
Levkau B, Scatena M, Giachelli CM, Ross R and Raines EW (1999) Apoptosis overrides survival signals through a caspase-mediated dominant-negative NF-kappa B loop. Nat. Cell Biol. 1: 227–233
Ohtsubo T, Kamada S, Mikami T, Murakami H and Tsujimoto Y (1999) Identification of NRF2, a member of the NF-E2 family of transcription factors, as a substrate for caspase-3(-like) proteases. Cell Death Differ. 6: 865–872
Nervi C, Ferrara FF, Fanelli M, Rippo MR, Tomassini B, Ferrucci PF, Ruthardt M, Gelmetti V, Gambacorti-Passerini C, Diverio D, Grignani F, Pelicci PG and Testi R (1998) Caspases mediate retinoic acid-induced degradation of the acute promyelocytic leukemia PML/RARalpha fusion protein. Blood 92: 2244–2251
Gianni M and de The H (1999) In acute promyelocytic leukemia NB4 cells, the synthetic retinoid CD437 induces contemporaneously apoptosis, a caspase-3-mediated degradation of PML/RARalpha protein and the PML retargeting on PML-nuclear bodies. Leukemia 13: 739–749
Stoven S, Ando I, Kadalayil L, Engstrom Y and Hultmark D (2000) Activation of the Drosophila NF-kappaB factor Relish by rapid endoproteolytic cleavage. EMBO Rep. 1: 347–352
Piedrafita FJ and Pfahl M (1997) Retinoid-induced apoptosis and Sp1 cleavage occur independently of transcription and require caspase activation. Mol. Cell. Biol. 17: 6348–6358
Wang X, Pai JT, Wiedenfeld EA, Medina JC, Slaughter CA, Goldstein JL and Brown MS (1995) Purification of an interleukin-1 beta converting enzyme-related cysteine protease that cleaves sterol regulatory element-binding proteins between the leucine zipper and transmembrane domains. J. Biol. Chem. 270: 18044–18050
Bertolotto C, Ricci JE, Luciano F, Mari B, Chambard JC and Auberger P (2000) Cleavage of the serum response factor during death receptor-induced apoptosis results in an inhibition of the c-FOS promoter transcriptional activity. J. Biol. Chem. 275: 12941–12947
King P and Goodboum S (1998) STAT1 is inactivated by a caspase. J. Biol. Chem. 273: 8699–8704
Waterhouse N, Kumar S, Song Q, Strike P, Sparrow L, Dreyfuss G, Alnemri ES, Litwack G, Lavin M and Watters D (1996) Heteronuclear ribonucleoproteins C1 and C2, components of the spliceosome, are specific targets of interleukin 1beta-converting enzyme-like proteases in apoptosis. J. Biol. Chem. 271: 29335–29341
Back SH, Shin S and Jang SK (2002) Polypyrimidine tract-binding proteins are cleaved by caspase-3 during apoptosis. J. Biol. Chem. 277: 27200–27209
Takeda Y, Caudell P, Grady G, Wang G, Suwa A, Sharp GC, Dynan WS and Hardin JA (1999) Human RNA helicase A is a lupus autoantigen that is cleaved during apoptosis. J. Immunol. 163: 6269–6274
Myohanen S and Baylin SB (2001) Sequence-specific DNA binding activity of RNA helicase A to the p16INK4a promoter. J. Biol. Chem. 276: 1634–1642
Utz PJ and Anderson P (2000) Life and death decisions: regulation of apoptosis by proteolysis of signaling molecules. Cell Death Differ. 7: 589–602
Rutjes SA, Utz PJ, van der Heijden A, Broekhuis C, van Venrooij WJ and Pruijn GJ (1999) The La (SS-B) autoantigen, a key protein in RNA biogenesis, is dephosphorylated and cleaved early during apoptosis. Cell Death Differ. 6: 976–986
Casciola-Rosen LA, Miller DK, Anhalt GJ and Rosen A (1994) Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J. Biol. Chem. 269: 30757–30760
Casciola-Rosen L, Nicholson DW, Chong T, Rowan KR, Thomberry NA, Miller DK and Rosen A (1996) Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J. Exp. Med. 183: 1957–1964
Degen WG, Aarssen Y, Pruijn GJ, Utz PJ and van Venrooij WJ (2000) The fate of U1 snRNP during anti-Fas induced apoptosis: specific cleavage of the U1 snRNA molecule. Cell Death Differ. 7: 70–79
Henis-Korenblit S, Strumpf NL, Goldstaub D and Kimchi A (2000) A novel form of DAP5 protein accumulates in apoptotic cells as a result of caspase cleavage and internal ribosome entry site-mediated translation. Mol. Cell. Biol. 20: 496–506
Satoh S, Hijikata M, Handa H and Shimotohno K (1999) Caspase-mediated cleavage of eukaryotic translation initiation factor subunit 2alpha. Biochem. J. 342: 65–70
Marissen WE, Guo Y, Thomas AA, Matts RL and Lloyd RE (2000) Identification of caspase 3-mediated cleavage and functional alteration of eukaryotic initiation factor 2alpha in apoptosis. J. Biol. Chem. 275: 9314–9323
Bushell M, Wood W, Clemens MJ and Morley SJ (2000) Changes in integrity and association of eukaryotic protein synthesis initiation factors during apoptosis. Eur. J. Biochem. 267: 1083–1091
Bushell M, Wood W, Carpenter G, Pain VM, Morley SJ and Clemens MJ (2001) Disruption of the interaction of mammalian protein synthesis eukaryotic initiation factor 4B with the poly(A)-binding protein by caspase- and viral protease-mediated cleavages. J. Biol. Chem. 276: 23922–23928
Tee AR and Proud CG (2002) Caspase cleavage of initiation factor 4E-binding protein 1 yields a dominant inhibitor of cap-dependent translation and reveals a novel regulatory motif. Mol. Cell. Biol. 22: 1674–1683
Marissen WE and Lloyd RE (1998) Eukaryotic translation initiation factor 4G is targeted for proteolytic cleavage by caspase 3 during inhibition of translation in apoptotic cells. Mol. Cell. Biol. 18: 7565–7574
Morley SJ, McKendrick L and Bushell M (1998) Cleavage of translation initiation factor 4G (eIF4G) during anti-Fas IgM-induced apoptosis does not require signalling through the p38 mitogen-activated protein (MAP) kinase. FEBS Lett. 438: 41–48
Bushell M, Poncet D, Marissen WE, Flotow H, Lloyd RE, Clemens MJ and Morley SJ (2000) Cleavage of polypeptide chain initiation factor eIF4GI during apoptosis in lymphoma cells: characterisation of an internal fragment generated by caspase-3-mediated cleavage. Cell Death Differ. 7: 628–636
Gradi A, Svitkin YV, Imataka H and Sonenberg N (1998) Proteolysis of human eukaryotic translation initiation factor eIF4GII, but not eIF4GI, coincides with the shutoff of host protein synthesis after poliovirus infection. Proc. Natl. Acad. Sci. USA 95: 11089–11094
Svitkin YV, Gradi A, Imataka H, Morino S and Sonenberg N (1999) Eukaryotic initiation factor 4GII (eIF4GII), but not eIF4GI, cleavage correlates with inhibition of host cell protein synthesis after human rhinovirus infection. J. Virol. 73: 3467–3472
Marissen WE, Gradi A, Sonenberg N and Lloyd RE (2000) Cleavage of eukaryotic translation initiation factor 4GII correlates with translation inhibition during apoptosis. Cell Death Differ. 7: 1234–1243
Utz PJ, Hottelet M, Le TM, Kim SJ, Geiger ME, van Venrooij WJ and Anderson P (1998) The 72-kDa component of signal recognition particle is cleaved during apoptosis. J. Biol. Chem. 273: 35362–35370
Mosley B, Urdal DL, Prickett KS, Larsen A, Cosman D, Conlon PJ, Gillis S and Dower SK (1987) The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J. Biol. Chem. 262: 2941–2944
Thomberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR and Aunins J et al. (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356: 768–774
Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T and Cannizzaro LA et al. (1992) Molecular cloning of the interleukin-1 beta converting enzyme. Science 256: 97–100
Zhang Y, Center DM, Wu DM, Cruikshank WW, Yuan J, Andrews DW and Komfeld H (1998) Processing and activation of pro-interleukin-16 by caspase-3. J. Biol. Chem. 273: 1144–1149
Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D and Allen H (1997) Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386: 619–623
Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ and Su MS (1997) Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 275: 206–209
Akita K, Ohtsuki T, Nukada Y, Tanimoto T, Namba M, Okura T, Takakura-Yamamoto R, Torigoe K, Gu Y, Su MS, Fujii M, Satoh-Itoh M, Yamamoto K, Kohno K, Ikeda M and Kurimoto M (1997) Involvement of caspase-1 and caspase-3 in the production and processing of mature human interleukin 18 in monocytic THP.1 cells. J. Biol. Chem. 272: 26595–26603
Knies UE, Behrensdorf HA, Mitchell CA, Deutsch U, Risau W, Drexler HC, and Clauss M (1998) Regulation of endothelial monocyte-activating polypeptide II release by apoptosis. Proc. Natl. Acad. Sci. USA 95: 12322–12327
Behrensdorf HA, van de Craen M, Knies UE, Vandenabeele P and Clauss M (2000) The endothelial monocyte-activating polypeptide II (EMAP II) is a substrate for caspase-7. FEBS Lett. 466: 143–147
Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS and Bredesen DE (1998) The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 395: 801–804
Bae SS, Choi JH, Oh YS, Perry DK, Ryu SH and Suh PG (2001) Proteolytic cleavage of epidermal growth factor receptor by caspases. FEBS Lett. 491: 16–20
Tikhomirov O and Carpenter G (2001) Caspase-dependent cleavage of ErbB-2 by geldanamycin and staurosporin. J. Biol. Chem. 276: 33675–33680
Meyer EL, Gahring LC and Rogers SW (2002) Nicotine preconditioning antagonizes activity-dependent caspase proteolysis of a glutamate receptor. J. Biol. Chem. 277: 10869–10875
Chan SL, Griffin WS and Mattson MP (1999) Evidence for caspase-mediated cleavage of AMPA receptor subunits in neuronal apoptosis and Alzheimer's disease. J. Neurosci. Res. 57: 315–323
Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, Bredesen DE, Edery P and Mehlen P (2000) The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J. 19: 4056–4063
Gastman BR, Johnson DE, Whiteside TL and Rabinowich H (1999) Caspase-mediated degradation of T-cell receptor zeta-chain. Cancer Res. 59: 1422–1427
Ethell DW, Bossy-Wetzel E and Bredesen DE (2001) Caspase 7 can cleave tumor necrosis factor receptor-I (p60) at a non-consensus motif, in vitro. Biochim. Biophys. Acta 1541: 231–238
Yankee TM, Draves KE, Ewings MK, Clark EA and Graves JD (2001) CD95/Fas induces cleavage of the GrpL/Gads adaptor and desensitization of antigen receptor signaling. Proc. Natl. Acad. Sci. USA 98: 6789–6793
Berry DM, Benn SJ, Cheng AM and McGlade CJ (2001) Caspase-dependent cleavage of the hematopoietic specific adaptor protein Gads alters signalling from the T cell receptor. Oncogene 20: 1203–1211
Irmler M, Steiner V, Ruegg C, Wajant H and Tschopp J (2000) Caspase-induced inactivation of the anti-apoptotic TRAF1 during Fas ligand-mediated apoptosis. FEBS Lett. 468: 129–133
Jang HD, Chung YM, Baik JH, Choi YG, Park IS, Jung YK and Lee SY (2001) Caspase-cleaved TRAF1 negatively regulates the antiapoptotic signals of TRAF2 during TNF-induced cell death. Biochem. Biophys. Res. Commun. 281: 499–505
Leo E, Deveraux QL, Buchholtz C, Welsh K, Matsuzawa S, Stennicke HR, Salvesen GS and Reed JC (2001) TRAF1 is a substrate of caspases activated during tumor necrosis factor receptor-alpha-induced apoptosis. J. Biol. Chem. 276: 8087–8093
Lee ZH, Lee SE, Kwack K, Yeo W, Lee TH, Bae SS, Suh PG and Kim HH (2001) Caspase-mediated cleavage of TRAF3 in FasL-stimulated Jurkat-T cells. J. Leukoc. Biol. 69: 490–496
De Valck D, Jin DY, Heyninck K, Van de Craen M, Contreras R, Fiers W, Jeang KT and Beyaert R (1999) The zinc finger protein A20 interacts with a novel anti-apoptotic protein which is cleaved by specific caspases. Oncogene 18: 4182–4190
Wu YM, Huang CL, Kung HJ and Huang CY (2001) Proteolytic activation of ETK/Bmx tyrosine kinase by caspases. J. Biol. Chem. 276: 17672–17678
Ricci JE, Maulon L, Luciano F, Guerin S, Livolsi A, Mari B, Breittmayer JP, Peyron JF and Auberger P (1999) Cleavage and relocation of the tyrosine kinase P59FYN during Fas-mediated apoptosis in T lymphocytes. Oncogene 18: 3963–3969
Luciano F, Ricci JE and Auberger P (2001) Cleavage of Fyn and Lyn in their N-terminal unique regions during induction of apoptosis: a new mechanism for Src kinase regulation. Oncogene 20: 4935–4941
Kami R and Levitzki A (2000) pp60(cSrc) is a caspase-3 substrate and is essential for the transformed phenotype of A431 cells. Mol. Cell. Biol. Res. Commun. 3: 98–104
Widmann C, Gibson S and Johnson GL (1998) Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J. Biol. Chem. 273: 7141–7147
Bachelder RE, Ribick MJ, Marchetti A, Falcioni R, Soddu S, Davis KR and Mercurio AM (1999) p53 inhibits alpha 6 beta 4 integrin survival signaling by promoting the caspase 3-dependent cleavage of AKT/PKB. J. Cell Biol. 147: 1063–1072
Bachelder RE, Wendt MA, Fujita N, Tsuruo T and Mercurio AM (2001) The cleavage of Akt/protein kinase B by death receptor signaling is an important event in detachment-induced apoptosis. J. Biol. Chem. 276: 34702–34707
Xu J, Liu D and Songyang Z (2002) The role of Asp462 in regulating Akt activity. J. Biol. Chem. 277: 35561–35566
McGinnis KM, Whitton MM, Gnegy ME and Wang KK (1998) Calcium/calmodulin-dependent protein kinase IV is cleaved by caspase-3 and calpain in SH-SY5Y human neuroblastoma cells undergoing apoptosis. J. Biol. Chem. 273: 19993–20000
Kruidering M, Schouten T, Evan GI and Vreugdenhil E (2001) Caspase-mediated cleavage of the Ca2+/calmodulin-dependent protein kinase-like kinase facilitates neuronal apoptosis. J. Biol. Chem. 276: 38417–38425
Chen YR, Meyer CF, Ahmed B, Yao Z and Tan TH (1999) Caspase-mediated cleavage and functional changes of hematopoietic progenitor kinase 1 (HPK1). Oncogene 18: 7370–7377
Amold R, Liou J, Drexler HC, Weiss A and Kiefer F (2001) Caspase-mediated cleavage of hematopoietic progenitor kinase 1 (HPK1) converts an activator of NFkappaB into an inhibitor of NFkappaB. J. Biol. Chem. 276: 14675–14684
Dan I, Ong SE, Watanabe NM, Blagoev B, Nielsen MM, Kajikawa E, Kristiansen TZ, Mann M and Pandey A (2002) Cloning of MASK, a novel member of the mammalian germinal center kinase III subfamily, with apoptosis-inducing properties. J. Biol. Chem. 277: 5929–5939
McGuire TF, Trump DL and Johnson CS (2001) Vitamin D(3)-induced apoptosis of murine squamous cell carcinoma cells. Selective induction of caspase-dependent MEK cleavage and up-regulation of MEKK-1. J. Biol. Chem. 276: 26365–26373
Cardone MH, Salvesen GS, Widmann C, Johnson G and Frisch SM (1997) The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell 90: 315–323
Deak JC, Cross JV, Lewis M, Qian Y, Parrott LA, Distelhorst CW and Templeton DJ (1998) Fas-induced proteolytic activation and intracellular redistribution of the stress-signaling kinase MEKK1. Proc. Natl. Acad. Sci. USA 95: 5595–5600
Widmann C, Gerwins P, Johnson NL, Jarpe MB and Johnson GL (1998) MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis. Mol. Cell. Biol. 18: 2416–2429
Graves JD, Gotoh Y, Draves KE, Ambrose D, Han DK, Wright M, Chemoff J, Clark EA and Krebs EG (1998) Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 17: 2224–2234
Kakeya H, Onose R and Osada H (1998) Caspase-mediated activation of a 36-kDa myelin basic protein kinase during anticancer drug-induced apoptosis. Cancer Res. 58: 4888–4894
Lee KK, Murakawa M, Nishida E, Tsubuki S, Kawashima S, Sakamaki K, and Yonehara S (1998) Proteolytic activation of MST/Krs, STE20-related protein kinase, by caspase during apoptosis. Oncogene 16: 3029–3037
Huang CY, Wu YM, Hsu CY, Lee WS, Lai MD, Lu TJ, Huang CL, Leu TH, Shih HM, Fang HI, Robinson DR, Kung HJ and Yuan CJ (2002) Caspase activation of mammalian sterile 20-like kinase 3 (Mst3): Nuclear translocation and induction of apoptosis. J. Biol. Chem. 277: 34367–34374
Rudel T, Zenke FT, Chuang TH and Bokoch GM (1998) p21-activated kinase (PAK) is required for Fas-induced JNK activation in Jurkat cells. J. Immunol. 160: 7–11
Lee N, MacDonald H, Reinhard C, Halenbeck R, Roulston A, Shi T and Williams LT (1997) Activation of hPAK65 by caspase cleavage induces some of the morphological and biochemical changes of apoptosis. Proc. Natl. Acad. Sci. USA 94: 13642–13647
Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong WW, Kamen R and Weichselbaum R et al. (1995) Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J. 14: 6148–6156
Ghayur T, Hugunin M, Talanian RV, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W and Kufe D (1996) Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J. Exp. Med. 184: 2399–2404
Bras A, Martinez AC and Baixeras E (1997) B cell receptor cross-linking prevents Fas-induced cell death by inactivating the IL-1 beta-converting enzyme protease and regulating Bcl-2/Bcl-x expression. J. Immunol. 159: 3168–3177
Mizuno K, Noda K, Araki T, Imaoka T, Kobayashi Y, Akita Y, Shimonaka M, Kishi S and Ohno S (1997) The proteolytic cleavage of protein kinase C isotypes, which generates kinase and regulatory fragments, correlates with Fas-mediated and 12-O-tetradecanoyl-phorbol-13-acetate-induced apoptosis. Eur. J. Biochem. 250: 7–18
Hoppe J, Hoppe V and Schafer R (2001) Selective degradation of the PKC-epsilon isoform during cell death in AKR-2B fibroblasts. Exp. Cell Res. 266: 64–73
Morrow TA, Muljo SA, Zhang J, Hardwick JM and Schlissel MS (1999) Pro-B-cell-specific transcription and proapoptotic function of protein kinase Ceta. Mol. Cell. Biol. 19: 5608–5618
Haussermann S, Kittstein W, Rincke G, Johannes FJ, Marks F and Gschwendt M (1999) Proteolytic cleavage of protein kinase Cmu upon induction of apoptosis in U937 cells. FEBS Lett. 462: 442–446
Endo K, Oki E, Biedermann V, Kojima H, Yoshida K, Johannes FJ, Kufe D and Datta R (2000) Proteolytic cleavage and activation of protein kinase C [micro] by caspase-3 in the apoptotic response of cells to 1-beta-D-arabino-furanosylcytosine and other genotoxic agents. J. Biol. Chem. 275: 18476–18481
Datta R, Kojima H, Yoshida K and Kufe D (1997) Caspase-3-mediated cleavage of protein kinase C theta in induction of apoptosis. J. Biol. Chem. 272: 20317–20320
Frutos S, Moscat J and Diaz-Meco MT (1999) Cleavage of zetaPKC but not lambda/iotaPKC by caspase-3 during UV-induced apoptosis. J. Biol. Chem. 274: 10765–10770
Smith L, Chen L, Reyland ME, DeVries TA, Talanian RV, Omura S and Smith JB (2000) Activation of atypical protein kinase C zeta by caspase processing and degradation by the ubiquitin–proteasome system. J. Biol. Chem. 275: 40620–40627
Saelens X, Kalai M and Vandenabeele P (2001) Translation inhibition in apoptosis: caspase-dependent PKR activation and eIF2-alpha phosphorylation. J. Biol. Chem. 276: 41620–41628
Takahashi M, Mukai H, Toshimori M, Miyamoto M and Ono Y (1998) Proteolytic activation of PKN by caspase-3 or related protease during apoptosis. Proc. Natl. Acad. Sci. USA 95: 11566–11571
Koh H, Lee KH, Kim D, Kim S, Kim JW and Chung J (2000) Inhibition of Akt and its anti-apoptotic activities by tumor necrosis factor-induced protein kinase C-related kinase 2 (PRK2) cleavage. J. Biol. Chem. 275: 34451–34458
Lin Y, Devin A, Rodriguez Y and Liu ZG (1999) Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13: 2514–2526
Kim JW, Choi EJ and Joe CO (2000) Activation of death-inducing signaling complex (DISC) by pro-apoptotic C-terminal fragment of RIP. Oncogene 19: 4491–4499
Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A and Olson MF (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 3: 339–345
Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J and Breard J (2001) Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol. 3: 346–352
Sabourin LA, Tamai K, Seale P, Wagner J and Rudnicki MA (2000) Caspase 3 cleavage of the Ste20-related kinase SLK releases and activates an apoptosis-inducing kinase domain and an actin-disassembling region. Mol. Cell. Biol. 20: 684–696
Johnston AM, Naselli G, Gonez LJ, Martin RM, Harrison LC and DeAizpurua HJ (2000) SPAK, a STE20/SPS1-related kinase that activates the p38 pathway. Oncogene 19: 4290–4297
Morley SJ, Jeffrey I, Bushell M, Pain VM and Clemens MJ (2000) Differential requirements for caspase-8 activity in the mechanism of phosphorylation of eIF2alpha, cleavage of eIF4GI and signaling events associated with the inhibition of protein synthesis in apoptotic Jurkat T cells. FEBS Lett. 477: 229–236
Mukerjee N, McGinnis KM, Park YH, Gnegy ME and Wang KK (2000) Caspase-mediated proteolytic activation of calcineurin in thapsigargin-mediated apoptosis in SH-SY5Y neuroblastoma cells. Arch. Biochem. Biophys. 379: 337–343
Mukerjee N, McGinnis KM, Gnegy ME and Wang KK (2001) Caspase-mediated calcineurin activation contributes to IL-2 release during T cell activation. Biochem. Biophys. Res. Commun. 285: 1192–1199
Santoro MF, Annand RR, Robertson MM, Peng YW, Brady MJ, Mankovich JA, Hackett MC, Ghayur T, Walter G, Wong WW and Giegel DA (1998) Regulation of protein phosphatase 2A activity by caspase-3 during apoptosis. J. Biol. Chem. 273: 13119–13128
Kim KW, Chung HH, Chung CW, Kim IK, Miura M, Wang S, Zhu H, Moon KD, Rha GB, Park JH, Jo DG, Woo HN, Song YH, Kim BJ, Yuan J and Jung YK (2001) Inactivation of farnesyltransferase and geranylgeranyltransferase I by caspase-3: cleavage of the common alpha subunit during apoptosis. Oncogene 20: 358–366
Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A and Hart GW (2001) Dynamic O-glycosylation of nuclear and cytosolic proteins: Further characterization of the nucleocytoplasmic {beta}-N-acetylglucosaminidase, O-GlcNAcase. J. Biol. Chem. 277: 1755–1761
Fabbi M, Marimpietri D, Martini S, Brancolini C, Amoresano A, Scaloni A, Bargellesi A and Cosulich E (1999) Tissue transglutaminase is a caspase substrate during apoptosis. Cleavage causes loss of transamidating function and is a biochemical marker of caspase 3 activation. Cell Death Differ. 6: 992–1001
Porn-Ares MI, Samali A and Orrenius S (1998) Cleavage of the calpain inhibitor, calpastatin, during apoptosis. Cell Death Differ. 5: 1028–1033
Wang KK, Posmantur R, Nadimpalli R, Nath R, Mohan P, Nixon RA, Talanian RV, Keegan M, Herzog L and Allen H (1998) Caspase-mediated fragmentation of calpain inhibitor protein calpastatin during apoptosis. Arch. Biochem. Biophys. 356: 187–196
Harvey KF, Harvey NL, Michael JM, Parasivam G, Waterhouse N, Alnemri ES, Watters D and Kumar S (1998) Caspase-mediated cleavage of the ubiquitin-protein ligase Nedd4 during apoptosis. J. Biol. Chem. 273: 13524–13530
Araya R, Takahashi R and Nomura Y (2002) Yeast two-hybrid screening using constitutive-active caspase-7 as bait in the identification of PA28gamma as an effector caspase substrate. Cell Death Differ. 9: 322–328
Jensen PH, Fladmark KE, Gjertsen BT and Vintermyr OK (1999) Caspase I-related protease inhibition retards the execution of okadaic acid- and camptothecin-induced apoptosis and PAI-2 cleavage, but not commitment to cell death in HL-60 cells. Br. J. Cancer 79: 1685–1691
Mahoney JA, Odin JA, White SM, Shaffer D, Koff A, Casciola-Rosen L and Rosen A (2002) The human homologue of the yeast polyubiquitination factor Ufd2p is cleaved by caspase 6 and granzyme B during apoptosis. Biochem. J. 361: 587–595
Tu S and Cerione RA (2001) Cdc42 is a substrate for caspases and influences Fas-induced apoptosis. J. Biol. Chem. 276: 19656–19663
Danley DE, Chuang TH and Bokoch GM (1996) Defective Rho GTPase regulation by IL-1 beta-converting enzyme-mediated cleavage of D4 GDP dissociation inhibitor. J. Immunol. 157: 500–503
Na S, Chuang TH, Cunningham A, Turi TG, Hanke JH, Bokoch GM and Danley DE (1996) D4-GDI, a substrate of CPP32, is proteolyzed during Fas-induced apoptosis. J. Biol. Chem. 271: 11209–11213
Cosulich SC, Horiuchi H, Zerial M, Clarke PR and Woodman PG (1997) Cleavage of rabaptin-5 blocks endosome fusion during apoptosis. EMBO J. 16: 6182–6191
Faleiro L and Lazebnik Y (2000) Caspases disrupt the nuclear–cytoplasmic barrier. J. Cell Biol. 151: 951–959
Wen LP, Madani K, Martin GA and Rosen GD (1998) Proteolytic cleavage of ras GTPase-activating protein during apoptosis. Cell Death Differ. 5: 729–734
Yang JY and Widmann C (2001) Antiapoptotic signaling generated by caspase-induced cleavage of RasGAP. Mol. Cell. Biol. 21: 5346–5358
Qi H, Juo P, Masuda-Robens J, Caloca MJ, Zhou H, Stone N, Kazanietz MG and Chou MM (2001) Caspase-mediated cleavage of the TIAM1 guanine nucleotide exchange factor during apoptosis. Cell Growth Differ. 12: 603–611
Hofmann TG, Hehner SP, Droge W and Schmitz ML (2000) Caspase-dependent cleavage and inactivation of the Vav1 proto-oncogene product during apoptosis prevents IL-2 transcription. Oncogene 19: 1153–1163
Lagace TA, Miller JR and Ridgway ND (2002) Caspase processing and nuclear export of CTP:phosphocholine cytidylyltransferase alpha during farnesol-induced apoptosis. Mol. Cell. Biol. 22: 4851–4862
Hirota J, Furuichi T and Mikoshiba K (1999) Inositol 1,4,5-trisphosphate receptor type 1 is a substrate for caspase-3 and is cleaved during apoptosis in a caspase-3-dependent manner. J. Biol. Chem. 274: 34433–34437
Diaz F and Bourguignon LY (2000) Selective down-regulation of IP(3)receptor subtypes by caspases and calpain during TNF alpha-induced apoptosis of human T-lymphoma cells. Cell. Calcium 27: 315–328
Haug LS, Walaas SI and Ostvold AC (2000) Degradation of the type I inositol 1,4,5-trisphosphate receptor by caspase-3 in SH-SY5Y neuroblastoma cells undergoing apoptosis. J. Neurochem. 75: 1852–1861
Mejillano M, Yamamoto M, Rozelle AL, Sun HQ, Wang X and Yin HL (2001) Regulation of apoptosis by phosphatidylinositol 4,5-bisphosphate inhibition of caspases, and caspase inactivation of phosphatidylinositol phosphate 5-kinases. J. Biol. Chem. 276: 1865–1872
Huston E, Beard M, McCallum F, Pyne NJ, Vandenabeele P, Scotland G and Houslay MD (2000) The cAMP-specific phosphodiesterase PDE4A5 is cleaved downstream of its SH3 interaction domain by caspase-3. Consequences for altered intracellular distribution. J. Biol. Chem. 275: 28063–28074
Frame M, Wan KF, Tate R, Vandenabeele P and Pyne NJ (2001) The gamma subunit of the rod photoreceptor cGMP phosphodiesterase can modulate the proteolysis of two cGMP binding cGMP-specific phosphodiesterases (PDE6 and PDE5) by caspase-3. Cell. Signal 13: 735–741
Paszty K, Verma AK, Padanyi R, Filoteo AG, Penniston JT and Enyedi A (2002) Plasma membrane Ca2+ATPase isoform 4b is cleaved and activated by caspase-3 during the early phase of apoptosis. J. Biol. Chem. 277: 6822–6829
Atsumi G, Murakami M, Kojima K, Hadano A, Tajima M and Kudo I (2000) Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. Proteolytic fragment of type IVA cytosolic phospholipase A2alpha inhibits stimulus-induced arachidonate release, whereas that of type VI Ca2+-independent phospholipase A2 augments spontaneous fatty acid release. J. Biol. Chem. 275: 18248–18258
Adam-Klages S, Schwandner R, Luschen S, Ussat S, Kreder D and Kronke M (1998) Caspase-mediated inhibition of human cytosolic phospholipase A2 during apoptosis. J. Immunol. 161: 5687–5694
Atsumi G, Tajima M, Hadano A, Nakatani Y, Murakami M and Kudo I (1998) Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J. Biol. Chem. 273: 13870–13877
Bae SS, Perry DK, Oh YS, Choi JH, Galadari SH, Ghayur T, Ryu SH, Hannun YA and Suh PG (2000) Proteolytic cleavage of phospholipase C-gamma1 during apoptosis in Molt-4 cells. FASEB J. 14: 1083–1092
Wellington CL, Ellerby LM, Hackam AS, Margolis RL, Trifiro MA, Singaraja R, McCutcheon K, Salvesen GS, Propp SS, Bromm M, Rowland KJ, Zhang T, Rasper D, Roy S, Thomberry N, Pinsky L, Kakizuka A, Ross CA, Nicholson DW, Bredesen DE and Hayden MR (1998) Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J. Biol. Chem. 273: 9158–9167
Ellerby LM, Hackam AS, Propp SS, Ellerby HM, Rabizadeh S, Cashman NR, Trifiro MA, Pinsky L, Wellington CL, Salvesen GS, Hayden MR and Bredesen DE (1999) Kennedy's disease: caspase cleavage of the androgen receptor is a crucial event in cytotoxicity. J. Neurochem. 72: 185–195
Galvan V, Chen S, Lu D, Logvinova A, Goldsmith P, Koo EH and Bredesen DE (2002) Caspase cleavage of members of the amyloid precursor family of proteins. J. Neurochem. 82: 283–294
Weidemann A, Paliga K, Durrwang U, Reinhard FB, Schuckert O, Evin G and Masters CL (1999) Proteolytic processing of the Alzheimer's disease amyloid precursor protein within its cytoplasmic domain by caspase-like proteases. J. Biol. Chem. 274: 5823–5829
Ellerby LM, Andrusiak RL, Wellington CL, Hackam AS, Propp SS, Wood JD, Sharp AH, Margolis RL, Ross CA, Salvesen GS, Hayden MR and Bredesen DE (1999) Cleavage of atrophin-1 at caspase site aspartic acid 109 modulates cytotoxicity. J. Biol. Chem. 274: 8730–8736
Miyashita T, Okamura-Oho Y, Mito Y, Nagafuchi S and Yamada M (1997) Dentatorubral pallidoluysian atrophy (DRPLA) protein is cleaved by caspase-3 during apoptosis. J. Biol. Chem. 272: 29238–29242
Choi EK, Zaidi NF, Miller JS, Crowley AC, Merriam DE, Lilliehook C, Buxbaum JD and Wasco W (2001) Calsenilin is a substrate for caspase-3 that preferentially interacts with the familial Alzheimer's disease-associated C-terminal fragment of presenilin 2. J. Biol. Chem. 276: 19197–19204
Wellington CL, Leavitt BR and Hayden MR (2000) Huntington disease: new insights on the role of huntingtin cleavage. J. Neural Transm. 58: 1–17
Kahns S, Lykkebo S, Jakobsen LD, Nielsen MS and Jensen PH (2002) Caspase-mediated parkin cleavage in apoptotic cell death. J. Biol. Chem. 277: 15303–15308
Kim TW, Pettingell WH, Jung YK, Kovacs DM and Tanzi RE (1997) Alternative cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science 277: 373–376
Loetscher H, Deuschle U, Brockhaus M, Reinhardt D, Nelboeck P, Mous J, Grunberg J, Haass C and Jacobsen H (1997) Presenilins are processed by caspase-type proteases. J. Biol. Chem. 272: 20655–20659
Vito P, Ghayur T and D'Adamio L (1997) Generation of anti-apoptotic presenilin-2 polypeptides by alternative transcription, proteolysis, and caspase-3 cleavage. J. Biol. Chem. 272: 28315–28320
Komiyama T, Ray CA, Pickup DJ, Howard AD, Thomberry NA, Peterson EP and Salvesen G (1994) Inhibition of interleukin-1 beta converting enzyme by the cowpox virus serpin CrmA. An example of cross-class inhibition. J. Biol. Chem. 269: 19331–19337
Zhirnov OP, Konakova TE, Wolff T and Klenk HD (2002) NS1 protein of influenza A virus down-regulates apoptosis. J. Virol. 76: 1617–1625
Zhirnov OP, Konakova TE, Garten W and Klenk H (1999) Caspase-dependent N-terminal cleavage of influenza virus nucleocapsid protein in infected cells. J. Virol. 73: 10158–10163
Satoh S, Hirota M, Noguchi T, Hijikata M, Handa H and Shimotohno K (2000) Cleavage of hepatitis C virus nonstructural protein 5A by a caspase-like protease(s) in mammalian cells. Virology 270: 476–487
Goh PY, Tan YJ, Lim SP, Lim SG, Tan YH and Hong WJ (2001) The hepatitis C virus core protein interacts with NS5A and activates its caspase-mediated proteolytic cleavage. Virology 290: 224–236
Eleouet JF, Slee EA, Saurini F, Castagne N, Poncet D, Garrido C, Solary E and Martin SJ (2000) The viral nucleocapsid protein of transmissible gastroenteritis coronavirus (TGEV) is cleaved by caspase-6 and -7 during TGEV-induced apoptosis. J. Virol. 74: 3975–3983
Bump NJ, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P and et al. (1995) Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science 269: 1885–1888
Xue D and Horvitz HR (1995) Inhibition of the Caenorhabditis elegans cell-death protease CED-3 by a CED-3 cleavage site in baculovirus p35 protein. Nature 377: 248–251
Skoldberg F, Ronnblom L, Thomemo M, Lindahl A, Bird PI, Rorsman F, Kampe O and Landgren E (2002) Identification of AHNAK as a novel autoantigen in systemic lupus erythematosus. Biochem. Biophys. Res. Commun. 291: 951–958
Huang M, Kozlowski P, Collins M, Wang Y, Haystead TA and Graves LM (2002) Caspase-dependent cleavage of carbamoyl phosphate synthetase II during apoptosis. Mol. Pharmacol. 61: 569–577
Chan SL, Tan KO, Zhang L, Yee KS, Ronca F, Chan MY and Yu VC (1999) F1Aalpha, a death receptor-binding protein homologous to the Caenorhabditis elegans sex-determining protein, FEM-1, is a caspase substrate that mediates apoptosis. J. Biol. Chem. 274: 32461–32468
Chan SL, Yee KS, Tan KM and Yu VC (2000) The Caenorhabditis elegans sex determination protein FEM-1 is a CED-3 substrate that associates with CED-4 and mediates apoptosis in mammalian cells. J. Biol. Chem. 275: 17925–17928
Ahmad M, Srinivasula SM, Wang L, Litwack G, Fernandes-Alnemri T and Alnemri ES (1997) Spodoptera frugiperda caspase-1, a novel insect death protease that cleaves the nuclear immunophilin FKBP46, is the target of the baculovirus antiapoptotic protein p35. J. Biol. Chem. 272: 1421–1424
Franklin CC, Krejsa CM, Pierce RH, White CC, Fausto N and Kavanagh TJ (2002) Caspase-3-dependent cleavage of the glutamate-L-cysteine ligase catalytic subunit during apoptotic cell death. Am. J. Pathol. 160: 1887–1894
Matsumura S, Van De Water J, Kita H, Coppel RL, Tsuji T, Yamamoto K, Ansari AA and Gershwin ME (2002) Contribution to antimitochondrial antibody production: cleavage of pyruvate dehydrogenase complex-E2 by apoptosis-related proteases. Hepatology 35: 14–22.
Morita A, Suzuki N, Matsumoto Y, Hirano K, Enomoto A, Zhu J and Sakai K (2000) p41 as a possible marker for cell death is generated by caspase cleavage of p42/SETbeta in irradiated MOLT-4 cells. Biochem. Biophys. Res. Commun. 278: 627–632.
Lu Y, Luo Z and Bregman DB (2002) RNA polymerase II large subunit is cleaved by caspases during DNA damage-induced apoptosis. Blochem. Biophys. Res. Commun. 296: 954–961
Chiu R, Novikov L, Mukherjee S and Shields D (2002) A caspase cleavage fragment of p115 induces fragmentation of the Golgi apparatus and apoptosis. J. Cell Biol. published online November 18
Ye B, Sugo N, Hurn P and Huganir R (2002) Physiological and pathological caspase cleavage of the neuronal RasGEF GRASP-1 as detected using a cleavage site-specific antibody. Neuroscience 114: 217
Sarrazin S, Bonod-Bidaud C, Remy P, Mehlen P and Morle F (2002) Caspase cleavage of the transcription factor FLl-1 during preB leukemic cell death. Biochim. Biophys. Acta 1592: 123
Kamachi M, Le TM, Kim SJ, Geiger ME, Anderson P and Utz PJ (2002) Human autoimmune sera as molecular probes for the identification of an autoantigen kinase signaling pathway. J. Exp. Med. 196: 1213–1226
Beyer TD, Kolowos W, Dumitriu IE, Voll RE, Heyder P, Gaipl US, Kalden JR and Herrmann M (2002) Apoptosis of the teratocarcinoma cell line Tera-1 leads to the cleavage of HERV-K10gag proteins by caspases and/or granzyme B. Scand. J. Immunol. 56: 303–309
Grand R, Schmeiser K, Gordon E, Zhang X, Gallimore P and Turnell A (2002) Caspase-mediated cleavage of adenovirus early region 1A proteins. Virology 301: 255
Zoog SJ, Schiller JJ, Wetter JA, Chejanovsky N and Friesen PD (2002) Baculovirus apoptotic suppressor P49 is a substrate inhibitor of initiator caspases resistant to P35 in vivo. EMBO J. 21: 5130–5140
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Mellino
Rights and permissions
About this article
Cite this article
Fischer, U., Jänicke, R. & Schulze-Osthoff, K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ 10, 76–100 (2003). https://doi.org/10.1038/sj.cdd.4401160
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401160