Abstract
Infection of T cells with HIV-1 induces apoptosis and modulates apoptosis regulatory molecules. Similar effects occur following treatment of cells with individual HIV-1 encoded proteins. While HIV-1 protease is known to be cytotoxic, little is known of its effect on apoptosis and apoptosis regulatory molecules. The ability of HIV-1 protease to kill cells, coupled with the degenerate substrate specificity of HIV-1 protease, suggests that HIV-1 protease may activate cellular factor(s) which, in turn, induce apoptosis. We demonstrate that HIV-1 protease directly cleaves and activates procaspase 8 in T cells which is associated with cleavage of BID, mitochondrial release of cytochrome c, activation of the downstream caspases 9 and 3, cleavage of DFF and PARP and, eventually, to nuclear condensation and DNA fragmentation that are characteristic of apoptosis. The effect of HIV-1 protease is not seen in T cell extracts which have undetectable levels of procaspase 8, indicating a specificity and requirement for procaspase 8.
Similar content being viewed by others
Introduction
HIV-1 infection results in CD4 T cell apoptosis which contributes to CD4 T cell depletion in infected individuals. Multiple mechanisms have been proposed to explain enhanced CD4 T cell apoptosis in HIV-1 infected persons. HIV-1 infected accessory cells, including macrophages, develop the ability to induce apoptosis of autologous uninfected CD4 T cells by producing the apoptosis inducing ligand, Fas (APO-1/CD95) Ligand (FasL).1,2,3,4,5,6,7,8,9,10 AICD of T cells is a physiologic response to activation11,12,13,14 which is greater in HIV-1 infected individuals than in uninfected controls,15,16,17,18,19 and is potentially induced by tat and/or gp120 cross linking the CD4 receptor3,20,21,22 resulting in increased expression of Fas Ligand, TNF or TRAIL.17,23 A third form of HIV-1 induced CD4 T cell death follows direct infection of a CD4 T cell with HIV-1, and is independent of Fas receptor ligation.8,15,24,25
While numerous HIV-1 proteins, including tat,26,27,28 gp120,20,21,22 Nef,29,30,31,32 vpr,33,34,35 and protease36,37,38,39 have been implicated as direct mediators of infected CD4 T cell death, the molecular mechanisms, whereby some of these HIV-1 specific proteins induce apoptosis, including the mechanisms associated with HIV-1 protease induced death, are unclear.
HIV-1 protease, a late regulatory protein in the HIV-1 life cycle, functions as a homodimer40 to cleave HIV-1 polyprotein. While ectopic expression of HIV-1 protease induces apoptosis in a variety of cell types, including human CD4 T cells,36,37,38,39 coincubation of nuclei with HIV-1 protease does not induce the nuclear changes of apoptosis,40 suggesting that cytosolic factor(s) must be activated by HIV-1 protease which in turn either directly or indirectly causes nuclear fragmentation. The presence of active HIV-1 protease within the cytosolic fraction of infected cells4142 raises the possibility that cleavage of non viral proteins by HIV-1 protease may contribute to the cytotoxicity of HIV-1 infection. In support of this view, HIV-1 protease substrate specificity is not restricted to viral proteins, since Bcl-2, actin, laminin B and pro-interleukin-1 are cleaved by HIV-1 protease both in vitro and in vivo.36,43 Although some of the proteins cleaved by HIV-1 protease36,43 are important in the regulation of apoptosis, none alone is sufficient to induce apoptosis. We propose that HIV-1 protease cleaves alternate apoptosis regulatory molecules in such a manner that they develop the ability to induce apoptosis.
Results
HIV-1 protease induces HeLa nuclear apoptosis and DNA fragmentation in cell-free system
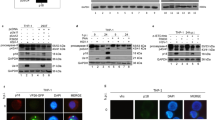
To determine if HIV-1 protease induces nuclear fragmentation, we modified a previously described cell-free system.44,45 Cytoplasmic extracts from Jurkat T cells were treated with or without HIV-1 protease and co-incubated with HeLa nuclei. The nuclear membranes and chromatin of nuclei incubated with untreated cytoplasmic extracts were intact (Figure 1A), in contrast to nuclei coincubated with HIV-1 protease treated cytoplasmic extracts which were marginated (Figure 1B) and/or fragmented (Figure 1C). These nuclear effects of HIV-1 protease were inhibited by an HIV-1-PI (Figure 1D). Similarly, nuclei incubated with HIV-1 protease treated cytoplasmic extracts developed internucleosomal DNA cleavage (as determined by DNA ladder analysis) which was also inhibited by HIV-1-PI (Figure 2A). As a control, the human aspartyl protease renin was used to treat cytoplasmic extracts, and, by contrast, the renin treated cytoplasmic extracts did not induce DNA laddering (Figure 2B), despite maintaining activity as determined by cleavage of the fluorogenic renin substrate 1 (fluorescence of control cytosols=0 relative fluorescence units, fluorescence of cytosols=22431 relative fluorescence units). Since HIV-1 protease alone does not directly induce the nuclear changes of apoptosis40 (data not shown), cytoplasmic signals must necessarily be activated by HIV-1 protease which, in turn, leads to the nuclear events of apoptosis.
HIV-1 protease induces the nuclear changes of apoptosis. Jurkat cytosols (1 mg) were treated with recombinant HIV-1 protease at 30°C for 3 h and then co-incubated with HeLa nuclei. Treated nuclei were imaged under microscopy by Hoechst 33342 staining. (A) Nuclei incubated with Jurkat cytosols without HIV-1 protease treatment. (B) Nuclei incubated with Jurkat cytosols treated with HIV-1 protease, resulting in fragmentation of the nuclear membrane and chromatin condensation or (C) margination of chromatin. (D) The induction of apoptotic changes were completely inhibited by HIV-1-PI
HIV-1 protease induces internucleosomal DNA fragmentation. (A) DNA gel from nuclei incubated with Jurkat cytosols in the presence or absence of HIV-1 protease with or without HIV-1 PI. (B) DNA gel from nuclei incubated with Jurkat cytosols and treated with or without HIV-1 protease or renin (control)
Caspase cascade is activated in cell extracts treated with HIV-1 protease
We next assessed procaspase 8 and procaspase 3 processing after treatment of Jurkat cytoplasmic extracts with HIV-1 protease. Both the 18 kd active fragment of caspase 8 and the 17 kd active fragment from caspase 346,47 were detected following HIV-1 protease treatment but not in control cytosols nor in renin treated cytoplasmic extracts (Figure 3A).
Caspase activation and mitochondrial release of cytochrome c occurs in Jurkat cytosols treated with HIV-1 protease. Jurkat cytosols were treated with HIV-1 protease and cleavage of procaspases 8, 3, 9 and the caspase substrates, BID, Bcl-2 and PARP were assessed along with mitochondrial release of cytochrome c. (A) Cleavage profiles of procaspase 8 and 3 indicating active p18 and p17 fragments respectively in the cytosols treated with HIV-1 protease, but not those treated with renin. (B) Jurkat cytosols were incubated with HIV-1 protease, recombinant active caspase 8 or Granzyme B, fractionated and analysed for cytochrome c content in the total reaction mixture, mitochondrial fractions or mitochondria free cytosolic fraction. (C) Cleavage profile of procaspase 9 indicating p35 fragment in cytosols treated with HIV-1 protease, as well as cytochrome c release from mitochondria. HSP 70 is analysed as a control mitochondria specific protein. (D) The cleavage of BID, Bcl-2 and PARP induced by HIV-1 protease treatment of cytosols. PCNA is included as an internal control. (E) Jurkat cytosols (1 mg) were treated with HIV-1 protease at 30°C, and assayed at the indicated times for analysis of caspase 8 cleavage and cytochrome c release. PCNA was used as an internal control
In HIV-1 protease treated, but not untreated cytoplasmic extracts, cytochrome c was released from mitochondria into the cytoplasmic compartment (Figure 3B) in a comparable manner to the release of cytochrome c seen with recombinant active caspase 8 or Granzyme B, indicating mitochondrial activation in treated cytoplasmic extracts. Following the mitochondrial release of cytochrome c48,49 into cytosols, cytosolic cytochrome c complexes with APAF-1 in the presence of dATP to form the apoptosome which allows the autoactivation of procaspase 9.50,51 In those samples where cytochrome c release was seen, procaspase 9 cleavage was also present, suggesting formation of the apoptosome and downstream caspase activation (Figure 3C).
We next determined whether HIV-1 protease mediated cleavage of procaspase 8 is responsible for mitochondrial activation. BID is a cytosolic member of the Bcl-2 family of apoptosis regulatory proteins52 that is cleaved by caspase 8 to create a truncated form of BID (tBID) which translocates to mitochondria and causes the release of cytochrome c into the cytosol.53 The p15 tBID form was detected in the HIV-1 protease treated cytoplasmic extracts but not in untreated cytosols (Figure 3D). Conversely, while HIV-1 protease may cleave Bcl-243 we did not detect Bcl-2 cleavage in this assay (Figure 3D) although it was observed after 4 h (data not shown). Following mitochondrial activation and downstream effector caspase activation, cellular substrates, including PARP, are cleaved. Consistent with our data indicating caspase activation in HIV-1 protease treated cytoplasmic extracts, but not untreated cytoplasmic extracts, the 85 kd fragment of activated PARP was seen only in HIV-1 protease treated cytoplasmic extracts (Figure 3D). These data suggest that activated caspase 8 cleaves BID to initiate the mitochondrial events which lead to apoptosis. Kinetic analysis of cleavage of procaspase 8 and cytochrome c release was performed at 30°C to slow the reaction, and analysed using Western blot. In these experiments cytochrome c was released after the cleavage of procaspase 8 into its 18 kd active fragments (Figure 3E).
Activation of caspase 8 leads to the activation of downstream caspases
We next determined the kinetics of caspase activation. HIV-1 protease induced the processing of procaspase 8 as early as 1 min after adding HIV-1 protease at 37°C (Figure 4A), and cleavage of procaspase 3 into its 17 kd active fragment was seen within 5 m. The relationship between caspase 8 cleavage and the cleavage of caspase 3, 9 and DFF were next evaluated in reaction mixtures incubated at 30°C. In these experiments HIV-1 cleavage of both procaspase 8 and 3 induced by HIV-1 protease was inhibited by HIV-1-PI pre-treatment, but only procaspase 3 cleavage was inhibited by the caspase 8 inhibitor (IETD-fmk) (Figure 4B). The lack of procaspase 8 inhibition by z-IETD-fmk indicates that procaspase 8 activation is a consequence of HIV-1 protease, rather than a result of autocatalysis. Thus both the timing of caspase 3 cleavage (Figure 4A) and its inhibition by z-IETD-fmk (Figure 4B) indicate that the cleavage of procaspase 3 depends upon prior caspase 8 activation. Furthermore, cleavage of procaspase 9 occurred after the cleavage of procaspase 8 (Figure 4B) and was inhibited by saquinavir and partially inhibited by z-IETD-fmk (Figure 4B). Therefore both caspase 3 and 9 activation occur after caspase 8 activation. We also determined that cleavage of DFF (a DNAse, activated by caspase 3, that contributes to nuclear fragmentation) into its 10 kd form occurred after 4 h in the treated cytosols, and its cleavage was inhibited by HIV-1-PI and by z-IETD-fmk (Figure 4B). These results demonstrate that HIV-1 protease treatment of cytoplasmic extracts results in procaspase 8 processing which precedes and contributes to processing of caspases 9 and 3 as well as DFF.
Activation of procaspase 8 by HIV-1 protease leads to cleavage of downstream caspases. (A) At the indicated times, 100 μg cytosol proteins were probed with anti-caspase 8 and 3. (B) In parallel cleavage of caspase 9 and DFF were assessed. As indicated either the caspase 8 inhibitor z-IETD-fmk or HIV-1-PI were used
HIV-1 protease cleaves caspase 8 but not caspase 3
The ability of HIV-1 protease to cleave pro-interleukin 1 into its active subunits54 infers that it may function as a caspase, a suggestion that is supported by our data in Jurkat cytoplasmic extracts showing that HIV-1 protease cleaves and activates procaspase 8. To investigate this possibility further, recombinant GST-caspase 8 was directly incubated with HIV-1 protease (Figure 5A). Within 1 min of co-incubation of recombinant GST-procaspase 8 with HIV-1 protease, caspase 8 is cleaved specifically by HIV-1 protease, as demonstrated by the lack of autocatalysis of GST-caspase 8 and the inhibition of HIV-1 protease cleavage by HIV-1-PI (HIV-1-PI does not inhibit caspase 8 activity (data not shown)). Importantly, coincubation of HIV-1 protease with full-length recombinant GST-caspase 8 generates p18 fragments, which have previously been associated with caspase 8 activity.47,55,56 To confirm the activity of the p18 caspase 8 fragments, we tested the ability of GST-caspase 8 treated with HIV-1 protease to cleave caspase 3, yet such experiments did not result in caspase 3 cleavage (data not shown). However, when HIV-1 protease was added after GST-caspase 8 was cleaved by HIV-1 protease (to inhibit remaining HIV-1 protease activity) (Figure 5B, top), and then cytoplasmic extracts added, caspase 3 was cleaved (Figure 5B, bottom), suggesting the requirement of a mitochondrial amplification step to cleave caspase 3. In these experiments the effects of GST-caspase 8 cleavage products on caspase 3 were inhibited by z-IETD-fmk (Figure 5B, bottom). In contrast to our results with GST-caspase 8, incubation of recombinant caspase 3 with HIV-1 protease did not result in cleavage, yet co-incubation of caspase 3 with Granzyme B did, as previously described57 (Figure 5C).
HIV-1 protease directly cleaves GST-procaspase 8 but not procaspase 3. Purified recombinant GST-procaspase 8 (A) was incubated for the indicated times with HIV-1 protease, with or without HIV-1-PI and analyzed for cleavage. Reactions were stopped at the indicated times by addition of gel loading buffer. (B) GST-caspase 8 was incubated with HIV-1 protease for 30 min and analyzed for caspase 8 cleavage (top). Thereafter reactions were stopped by the addition of HIV-1 PI, and Jurkat cytosols added and analyzed for caspase 3 cleavage (bottom). (C) Treatment of procaspase 3 with HIV-1 protease does not alter procaspase 3, whereas Granzyme B results in cleavage of procaspase 3
HIV-1 protease cleavage of procaspase 8 occurs at an atypical site
The pattern of procaspase 8 cleavage that follows HIV-1 protease cleavage appears distinct from that seen with active caspase 8 treatment (compare Figures 5A and B with Figures 3A, E and 4A, B), suggesting that the HIV-1 protease cleavage site is different than the usual caspase 8 cleavage site. We instead propose that HIV-1 protease generates active caspase 8 (cleaved at an atypical site, Figure 5A, B), which then activates more procaspase 8 (cleaved at the typical site) resulting in the generation of p43, p41 and p18 fragments (Figures 3A, E and 4A, B).
To assess this possibility further, two sets of experiments were performed. First we mutated the typical cleavage of caspase 8. The initial cleavage event of procaspase 8 activation occurs at ASP374,56 within the domain VETDSEEQ. Using a sequence coupled predictive method of Markov chain theory,58 this sequence would be predicted to be cleaved by HIV-1 protease with a high degree of likelihood. We therefore mutated this domain to VDPDSDKQ, using site directed mutagenesis, as this sequence is extremely unlikely to be cleaved by HIV-1 protease.58 Both WT and mutant forms of GST-procaspase 8 were then reacted with HIV-1 protease. Analysis of cleavage products by Western blot revealed identical banding patterns, suggesting that HIV-1 protease cleavage of procaspase 8 does not occur at this site.
To further address whether HIV-1 protease initiates cleavage at this site, HIV-1 protease was incubated with fluorogenic substrate z-IETD-AFC (Figure 6). Both active caspase 8 and Granzyme B caused cleavage of z-IETD-AFC, yet consistent with our mutational data, HIV-1 protease did not directly cleave z-IETD-AFC, supporting the concept that HIV-1 protease activates procaspase 8 at a site distinct from the typical activation site.
Effect of active caspase 8, Granzyme B and HIV protease on z-IETD-AFC. The caspase 8 autoactivation cleavage site fluorogenic substrate z-IETD-AFC was incubated with recombinant active caspase 8, Granzyme B, or with either 0.1 or 1 μg of HIV protease as indicated, and fluorescence measured every 2 min for 30 min
HIV-1 protease induced apoptotic signaling requires procaspase 8
Our cumulative data thus demonstrate a direct effect of HIV-1 protease on procaspase 8, which is associated with the downstream events of apoptosis including cleavage of BID, release of cytochrome c, activation of caspases 9 and 3, as well as cleavage of DFF and PARP. It remains possible that HIV-1 protease initiated cleavage of other factors (e.g. other initiator caspases) may also occur to initiate apoptotic signaling. Thus, we assessed the ability of HIV-1 protease to initiate apoptotic signaling in cells which are deficient in procaspase 8. Cytosolic extracts of JB6 cells and I9.2 cells which are a procaspase 8 deficient T cell derivatives were treated with or without HIV-1 protease and cleavage of procaspase 8, BID, procaspase 3 assessed. As expected, while JB6 and I9.2 cells had undetectable levels of procaspase 8, Jurkat T cell procaspase 8 was processed by HIV-1 protease. Only in the Jurkat T cell extracts treated with HIV-1 protease, was there any evidence of cleavage of BID or of procaspase 3 (Figure 7), indicating that the presence of procaspase 8 in Jurkat T cells is required for activation of the downstream apoptotic signaling events, since the absence of procaspase 8 in JB6 and I9.2 cells prevents downstream apoptotic signaling.
Direct infection of HIV-1 causing cell death is correlated with HIV protease expression and requires active caspase 8
To determine whether HIV-1 protease expression is correlated with the induction of apoptosis, we analyzed the expression of protease in relation to the timing of apoptosis in Jurkat T cells acutely infected with HIV-1. In this model of acute HIV-1 infection, cell death by apoptosis occurs several days following infection,15 is inhibited by z-VAD-fmk, z-IETD-fmk (Figure 8A) and by Saquinavir (data not shown), and is associated with caspase 8 and PARP cleavage (Figure 8B). Further cell death coincides with detectable expression of HIV-1 protease (Figure 8C). Freshly isolated PBL were also collected from six HIV-1 negative controls and from two untreated patients infected with HIV-1 were analyzed for expression of HIV-1 protease and for PARP cleavage into an 85 kd apoptosis characteristic fragments.59,60 In control patient 1 (who had an upper respiratory tract infection) and HIV-1 patients 2 to 6 the 85 kd PARP fragment was present, indicating that PBLs from these patients were undergoing apoptosis (Figure 8D). Conversely the 85 kd PARP was not present in control patient 2 and HIV patients 1. Expression of HIV-1 protease was seen only in HIV-1 patients 2–6 who had high levels of viral replication (>500,000 copies/ml) and importantly HIV-1 protease was not detected in HIV-1 patient 1 who had a low level of viral replication (1600 copies/ml). Thus, given previous literature which demonstrate that HIV-1 protease cleaves actin into an HIV-1 protease specific pattern in vivo, as well as in vitro,36 our observations that apoptosis in HIV-1 infection coincides with HIV-1 protease expression support a possible role for HIV-1 protease apoptosis associated with directly infected cells.
Jurkat T cells were infected or mock infected (HIV-) with HIVIIIb, in the presence or absence of z-IETD-fmk, z-VAD-fmk or z-DEVD-fmk, and assessed for viability (A). Infected cells harvested on day 8 were then analyzed for caspase 8 and PARP cleavage (B), or for HIV protease expression (C). HIV protease expression and PARP cleavage were also assessed in bulk PBL from HIV positive or negative patients, as indicated (D)
Discussion
The demonstration of caspase activation by HIV-1 protease is significant for several reasons. First, the ability of HIV-1 protease to induce T cell apoptosis represents another potential mechanism whereby HIV-1 may cause death of HIV-1 infected T cells. This mechanism applies only to cells directly infected by HIV-1, as addition of HIV-1 protease to cell cultures does not influence cell viability (data not shown). The relative importance of this mechanism, in comparison to other proposed mechanisms of HIV-1 associated T cell depletion (reviewed in61), including AICD, autologous cell mediated killing, and direct virus induced killing associated with gp120, Nef, Tat and/or Vr is however unclear. Secondly, the ability of viruses to influence apoptosis has been well characterized,62 and a number of virally encoded proteins have been shown to interact with members of the caspase family to inhibit apoptosis: these include baculovirus IAP and p35, Adenovirus E1B-19k, Cowpox Crm-A, Epstein Barr virus BHRF1,62 and gamma herpes virus FLIP.63 In contrast, HIV-1 protease is an example of a virally encoded protein that activates caspase 8 to promote apoptosis.
The HIV-1 genome is translated as polyprotein fusions that require processing by HIV-1 protease. These polyproteins are processed by HIV-1 protease in two cellular compartments: first, as membrane-associated polyproteins that are cleaved for viral assembly and maturation, and second, as free polyproteins within the cytosols41,42 of infected cells. Previous studies have shown that HIV-1 protease can induce apoptosis in both transfected and microinjected cells.43,64,65 Furthermore, a variety of cellular proteins, including the antiapoptotic regulating protein Bcl-2 and cell structure proteins such as laminin B and cytoskeleton proteins, are substrates of HIV-1 protease in vitro and in vivo.39,43,54,65,66 These observations suggest that the degenerate substrate specificity of HIV-1 protease allows protease to activate proteins which initiate apoptosis cascades.
In the present study, we have developed a cell-free system to characterize the mechanisms by which HIV-1 protease induces apoptosis. Cell-free systems have been successfully used to identify the apoptotic molecules and their signal pathways.44,45,67 In our system, treatment of cytosols with HIV-1 protease initiates a pathway that involves activation of both caspases and mitochondrial events involved in apoptosis (Figure 9). Further, we demonstrate that the apical and requisite event in this pathway is the cleavage of procaspase 8 by HIV-1 protease, which in turn activates BID, causes mitochondrial release of cytochrome c, activation of caspases 9 and 3 as well as cleavage of DFF and PARP. The requirement for mitochondria in this apoptosis cascade is demonstrated by observations that GST caspase 8 activated by HIV-1 protease does not cleave caspase 3. Only when GST caspase 8 was incubated with HIV-1-PR, PI added (to inhibit protease) and the entire reaction added to cytoplasmic extract was caspase 3 activated (Figure 5B). However, as it is now recognized that activated caspase 8 can initiate apoptosis directly via caspase 3 (type 1 pathway) or indirectly via mitochondrial activation, cytochrome-c release and caspase 9 processing (type 2 pathway),68,69 we cannot exclude the possibility that HIV-1 protease mediated apoptosis may involve both type 1 as well as type 2 signaling pathways. Indeed, when T cell extracts treated with HIV-1 protease in the presence or absence of the mitochondrial PTPC inhibitor BA were analysed for caspase 3 and caspase 9 activation, BA resulted in partial, but incomplete inhibition of caspase 3 and 9 activation, thereby indicating that both type I and type II pathways are likely involved (data not shown). The results are consistent with previous work which demonstrate that activation of procaspase 8 is sufficient to induce changes in a cell-free system that are similar to those seen during apoptosis in vivo.47
In the present study we demonstrate that procaspase 8 is required for HIV-1 protease induced apoptosis, as both JB6 and I9.2 cells which are deficient in procaspase 8 do not develop the molecular changes of apoptosis following HIV-1 protease treatment. However, since our evidence that HIV protease activates caspase 8 physiologically is indirect, it remains possible that it may also act on different substrates to initiate death pathways. Additional studies are underway to address these possibilities.
Treatment of HIV-1 infected patients with inhibitors of HIV-1 protease has dramatically reduced both morbidity and mortality associated with this infection. Thus far, two reasons for the improved outcomes are apparent: first, protease inhibitors are potent inhibitors of viral replication70 and second, this class of drugs possesses intrinsic immunomodulatory properties including antiapoptotic effects.61,71 We suggest that direct inhibition of HIV-1 protease also reduces protease induced apoptosis of infected cells to further reduce HIV-1 associated T cell death. Further research is therefore required to determine the contribution of this form of cell death on the pathogenesis of HIV-1 disease, and the effect of HIV-1 protease mutations on the pathogenesis of HIV-1 induced immunodeficiency.
Materials and Methods
Preparation of cell-free extracts
Cell-free extracts were freshly prepared from human Jurkat T lymphoblastoid cells (ATCC, Rockville, MD, USA) as described previously44,45 with some modifications. Briefly, cells (0.5×106 cells/ml) were harvested by centrifugation at 1600×g for 5 min at 4°C. The cell pellet was washed twice with ice-cold PBS (pH 7.4), followed by a single wash with ice-cold caspase buffer (20 mM PIPES, 100 mM NaCl, 10 mM DTT, 1 mM EDTA, 0.1% CHAPS, 250 mM sucrose, pH 7.2).72 After centrifugation, the cells were resuspended with two volumes of ice-cold complete caspase buffer which was supplemented with protease inhibitors (100 μm PMSF, 10 μg/ml leupeptin, 2 μg/ml aprotinin) and then transferred to a 2-ml dounce homogenizer. After sitting on ice for 15 min, the cells were disrupted with 50 strokes of B-type pestle (Fisher Scientific Ltd, Nepean, ON, Canada). Cell disruption (>95%) was confirmed by examination of 5 μl aliquot of suspension under a light microscope after staining with Trypan blue. The nuclei were removed by the centrifugation at 1000×g for 10 min at 4°C. Protein concentrations were determined with BCA protein assay kit (Pierce Chemical Co, Rockford, IL, USA). JB6 cells and I9.2 cells which are procaspase 8 deficient T cell derivatives, were a kind gift of Dr. S Nagata73 and Dr. J Blenis74 respectively. JB6 and I9.2 cells were handled in an identical manner to the method described for Jurkat T cells above.
Preparation of HeLa nuclei
HeLa cell (ATCC, Rockville, MD, USA) nuclei isolation was performed as described.75 Nuclei were freshly prepared for each experiment from the 80% confluent cultures of HeLa cells. Cells were washed three times with ice-cold PBS (pH 7.4), followed by a single wash with ice-cold nuclear buffer (10 mM PIPES, 80 mM KCL, 20 mM NaCl, 250 mM sucrose, 5 mM EGTA, 1 mM DTT, 0.5 mM spermidine, 0.2 mM spermine, 1 μg ml protease inhibitors, pH 7.4). The cell pellet was resuspended with two volumes of ice-cold nuclear buffer. The cells were disrupted with 50 strokes of B-type pestle and >95% lysis confirmed by Trypan blue exclusion. Nuclei were pelleted (1000×g for 10 min at 4°C) and washed twice with nuclear washing buffer (10 mM PIPES, 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 10 μM cytochalasin B, 1 μg ml−1 protease inhibitors, pH 7.4).
HIV-1 protease treatment of cytosolic extracts
Cell extracts treated with HIV-1 protease were carried out in 100 μl cell-free reaction buffer (complete caspase buffer supplemented with 10 mM phosphocreatine, 2 mM ATP and 150 mg/ml creatine phosphokinase). The concentration ratio of cytosol proteins and HIV-1 protease was 1000 : 1. The final concentration of HIV-1 protease was between 0.5–1 μg per reaction mixture. HIV-1 protease was purchased (Bachem Bioscience Inc - King of Prussia, PA, USA) with a specific activity of 1.81×104 mmole/min/mg at 37°C, with a purity of >96% by SDS–PAGE and a single peak by RP–HPLC. Where indicated, the HIV-1 protease inhibitors (HIV-1-PI) Saquinavir 10 μM (Roche Laboratories, Mississauga, Ontario, used for data described in Figures 3 and 4) or Nelfinavir 7 μM (Agouron Laboratories, Mississauga, Ontario, Canada), used for data described in all Figures except 3 and 4) were used. Where indicated the human aspartyl protease renin (Sigma Aldrich Canada Ltd, Oakville, ON, Canada) was used as a control. Renin substrate 1 (Molecular Probe Inc., Eugene, OR, USA) was used to measure renin activity in cytosol mixtures according to the supplied protocol. z-IETD-fmk (Enzyme Systems Products, Livermore, CA, USA) was used in some experiments as indicated, at 100 μM dissolved in DMSO (Sigma, Irvine, UK).
Nuclei incubation with HIV-1 protease treated cytoplasmic extracts
First, a mixture of cytoplasmic extracts and HIV-1 protease were incubated at 30°C for 4 h in cell-free reaction buffer. Then, aliquots of 20 μl HIV-1 protease treated cytoplasmic were incubated with 80 μl of HeLa cell nuclei (5×106 nuclei) at 37°C in nuclear apoptosis buffer (nuclear washing buffer supplemented with 2 mM ATP and 5 mM EGTA). Apoptotic nuclei were determined by Hoechst staining and DNA fragmentation assay.
Hoechst staining
HeLa nuclei were stained with Hoechst 33342 (Molecular Probes, Eugene, OR, USA) as previously described67 in fixing buffer (10% formaldehyde, 50% glycerol, 100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 0.1 mM EDTA, 1 μg/ml Hoechst 33342, 5 mM HEPES, pH 7.8). The stained nuclei were imaged under fluorescence microscopy (Zeiss AxioCAM, Jena, Germany).
DNA fragmentation assay
The DNA fragmentation assay was performed as described.76 Briefly 2–5×106 nuclei were pelleted for 20 min at 4°C, and dispersed in 30 μl of lysis buffer (10 mM Tris, 100 mM NaCl, 25 mM EDTA, 0.5% Sarkosyl) by gentle vortexing. Forty micrograms protease K (Qiagen Inc., Mississauga, ON, Canada) was added and incubated at 52°C overnight. Then, 40 μg RNase (Sigma, Irvine, UK) was added and incubated for 2 h at room temperature. The fragmented DNA in the lysates was detected by 2% agarose gel electrophoresis.
SDS–PAGE and Western blot
For Western blot analysis, 50–200 μg of cytosolic proteins were fractionated on 4–15% gradient polyacrylamide gels (Biorad Laboratories Canada Inc., Hercules, CA, USA), then transferred onto PVDF membranes (Millipore, Bedford, MA, USA) for 1 h at 100 V using transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol). The membranes were blocked by incubation in TBS buffer (20 mM Tris, 500 mM NaCl, 0.05% Tween, pH 7.5) containing 5% milk for overnight at 4°C or 2 h at room temperature and washed five times with TBS buffer. Then, the membranes were blotted for 1 h at room temperature with the various dilutions of primary antibodies, specifically, monoclonal anti-caspase 8 (Biosource International, Camarillo, CA, USA), anti-caspase 9 (Medical & Biological Laboratories Co., Watertown, MA, USA), anti-cytochrome c (BD Pharmingen, Mississauga, ON, Canada), anti-PARP (Oncogene, Darmstadt, Germany) and anti-Bcl-2 (Calbiochem, La Jolla, CA, USA), anti-PCNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-caspase 377 and rabbit anti-cFLIP (Alexis Biochemicals, San Diego, CA, USA), goat anti-BID, anti-actin and anti-DFF45 (Santa Cruz Biotechnology). The blots were washed five times with TBS and developed with HRP linked secondary antibodies, sheep anti-mouse Ig, donkey anti-rabbit Ig (Amersham Pharmacia Biotech, Oakville, ON, Canada) and anti-goat IgG (Santa Cruz Biotechnology). All the blots were developed by SuperSignal (Pierce, Rockford, IL, USA), an enhanced chemiluminescence method, following the manufacturer's protocol.
Generation of recombinant caspase 3 and caspase 8
GST-caspase 8 was made by subcloning full-length cDNA caspase 8 into pGEX-4T-1 (Amersham Pharmacia Biotech) and expression of GST–Caspase 8 performed by IPTG stimulation at 30°C, according to the manufacturers instructions, in the presence of 100 uM EGTA and EDTA. The human caspase 3 cDNA was amplified by RT–PCR with the following primers: 5-GATGGAGAACACTGAAAAACTC-3 and 5-ATCCAACCAACCATTTCTTTAGTG-3 from Jurkat total RNA and subcloned into BamHI and EcoRI sites of pBSKS+(Stratagene, Cedar Creek, TX, USA) and sequenced. To produce recombinant caspase-3, the cysteine 163 of the active site was mutated to serine in order to avoid autocatalysis. The mutagenesis was performed by overlapping PCR using PBSKS+caspase-3 as the template, and the mutation was then confirmed after cloning and sequencing of the PCR product. The caspase-3-C163S was then subcloned into pGEX2TK (Amersham Pharmacia Biotech) and transformed into DH5 alpha. Purified Caspase 3 was made as previously described, followed by removal of the GST tag by thrombin digestion.77
Cleavage reactions of recombinant caspases
Reactions to assess the ability of HIV-1 protease to cleave recombinant caspases were performed under the following conditions: 3 μl of purified recombinant GST-caspase 8 or caspase 3 were mixed with 10 μl of HIV-1 protease buffer (100 mM Na acetate, 1 mM EDTA, 1 M NaCl, 1 mM DTT, 1 mg/ml BSA pH 4.7) in the absence or presence of 0.5 μg HIV-1 protease (2 μl) preincubated for 15 min at room temperature with either 2 μl of methanol, or 2 μl of 10 mM Saquinavir in methanol. In the case of caspase 3, Granzyme B (Enzyme Systems, Livermore, CA, USA) was used as a positive control for cleavage, at the indicated concentrations. The final reaction mixtures were incubated for the indicated times at 37°C. Cleavage products were then analyzed by Western blot analysis.
Cytochrome c release assay
Cytochrome c release assay was modified according to a previous publication.78 Crude cell extracts were supplemented with an ATP regenerating system (10 mM phosphocreatine, 2 mM ATP and 150 mg/ml creatine phosphokinase). At various time points, HIV-1 protease treated cytosols were harvested and centrifuged twice at 15 000 g (4°C) for 15 min to fractionate the cytosolic (supernatant) fraction from the mitochondrial pellet. Aliquots of 20 μl cytosolic protein (200 μg) were separated by 4–15% gradient SDS–PAGE and probed with monoclonal antibody against cytochrome c. As indicated, recombinant active caspase 8 (Biomol, Plymouth Meeting, PA, USA) or Granzyme B (Enzyme Systems Products, Livermore, CA, USA) were used as positive controls.
Caspase inhibitors
The caspase consensus site inhibitors z-DEVD-fmk, z-IETD-fmk and z-VAD-fmk were purchased from Enzyme Systems. Independent experiments were performed to validate the inhibitory effects of z-DEVD-fmk, z-IETD-fmk or z-VAD-fmk on caspase activation. Jurkat T cells were stimulated with recombinant leucine zipper Fas Ligand (10 g/ml, Immunex) for 6 h at 37°C in the absence or presence of z-DEVD-fmk, z-IETD-fmk or z-VAD-fmk (Enzyme Systems), at concentrations ranging from 3 to 300 μM. Each inhibitor blocked recombinant Fas Ligand (Immunex Corp, Seattle, WA, USA) induced cell death at all concentrations, in a dose dependant manner (data not shown).
Cells and HIV infection
Jurkat T cells were purchased from ATCC and maintained in RPMI medium supplemented with 10% fetal calf serum (FCS, GIBCO). For experiments using patient peripheral blood lymphocytes (PBL), consenting patients or controls donated 20 mls of blood into heparinized tubes, and PBLs extracted using ficol hypaque density gradient centrifugation, and plastic adherence.56 Resultant PBL were cultured in RPMI 1640-10% human AB serum, supplemented with penicillin/streptamycin and glutamine (Gibco). HIV infection using HIV IIIb (NIH AIDS Reference Reagent Program) was performed as previously described;79 briefly virus containing supernatants (or mock infected supernatants) were propogated in PBMC from HIV uninfected donors. Cells are infected by overnight culture with virus containing (or mock) supernatant (45 373 pg of p24/ml). Cell viability following infection was assessed by Trypan blue exclusion.
Fluorogenic release assays
To assess the activity of different enzymes against z-IETD-AFC (Enzyme Systems), caspase 8 (Enzyme Systems), 180 μg of Granzyme B, 0.1 or 1.0_g of HIV protease were added to either caspase 8 buffer (100 mM HEPES, pH 7.5, 10% v/v sucrose, 10 mM DTT, 0.5 mM EDTA),80 Granzyme B buffer (50 mM HEPES, pH 7.4, 0.1% CHAPS, 0.1 M NaCl, 10% v/v sucrose, 10 mM DTT)81 or to HIV protease buffer (100 mM Na Acetate, 4 mM EDTA, 300 mM NaCl, pH 4.7)82 to achieve a final volume of 500 μl. After 30 min, with the reaction mixture in a fluorimeter (CytoFluor 2300, Millipore) adjusted to 400 nm excitation, 505 nm emission, 20 μl of z-IETD-AFC (20 mM stock) was added, and fluorescence release measured every 2 min until 30 min.82 Data presented representative of results obtained using all three buffers. Independent experiments using the HIV protease fluorogenic substrate. DABCYL-_-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDAN (Bachem, King of Prussia PA, USA) were performed with each buffer to confirm the activity of HIV protease under these conditions (data not shown).
Abbreviations
- AICD:
-
activation induced cell death
- ATCC:
-
American type cell culture
- ATP:
-
adenine trinucleotide phosphate
- BA:
-
bongkrekic acid
- BSA:
-
bovine serum albumin
- CHAPS:
-
cholamidopropyl dimethylammonio propane sulfonate
- DFF:
-
DNA fragmentation factor
- DMSO:
-
dimethyl sulfoxide
- DTT:
-
dithiothreitol
- EDTA:
-
ethylene diamine tetracetic acid
- EGTA:
-
ethylene glycol tetracetic acid
- FLIP:
-
FLICE-like inhibitory peptide
- HEPES:
-
hydroxyethyl piperazine ethane sulfonic acid
- HIV:
-
human immunodeficiency virus
- HIV-1 PI:
-
HIV-1 protease inhibitor
- HPLC:
-
high performance liquid chromatography
- HRP:
-
horseradish peroxidase
- PAGE:
-
poly crylamide gel electropheresis
- PARP:
-
poly (ADP Ribose) polymerase
- PBL:
-
peripheral blood lymphocyte
- PMSF:
-
phenylmethylsulfonyl fluoride
- SDS:
-
sodium dodecyl sulphate
- TRAIL:
-
TNF related apoptosis inducing ligand
References
Badley AD, McElhinny JA, Leibson PJ, Lynch DH, Alderson MR, Paya CV . 1996 Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes J. Virol. 70: 199–206
Badley AD, Dockrell D, Simpson M, Schut R, Lynch DH, Leibson P, Paya CV . 1997 Macrophage-dependent apoptosis of CD4+T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor J. Exp. Med. 185: 55–64
Banda NK, Bernier J, Kurahara DK, Kurrle R, Haigwood N, Sekaly RP, Finkel TH . 1992 Crosslinking CD4 by Human Immunodeficiency virus gp120 primes T cells for activation-induced apoptosis J. Exp. Med. 176: 1099–1106
Cottrez F, Manca F, Dalgleish AG, Arenzana-Seisdedos F, Capron A, Groux H . 1997 Priming of human CD4+antigen-specific T cells to undergo apoptosis by HIV-infected monocytes J. Clin. Invest. 99: 257–266
Herbein G, Van Lint C, Lovett JL, Verdin E . 1998 Distinct mechanisms trigger apoptosis in human immunodeficiency virus type-1 infected and in uninfected bystander T lymphocytes J. Virol. 72: 660–670
Kameoka M, Suzuki S, Kimura T, Fujinaga K, Auwanit W, Luftig RB, Ikuta K . 1997 Exposure of resting peripheral blood T cells to HIV-1 particles generates CD25+killer cells in a small subset, leading to induction of apoptosis in bystander cells Int. Immunol. 9: 1453–1462
Nardelli B, Gonzalez CJ, Schechter M, Valentine FT . 1995 CD4+blood lymphocytes are rapidly killed in vitro by contact with autologous human immunodeficiency virus-infected cells Proc. Natl. Acad. Sci. USA. 92: 7312–7316
Orlikowsky T, Wang Z-Q, Dudhane A, Horowitz H, Riethmuller G, Hoffman MK . 1997 Cytotoxic monocytes in the blood of HIV type-1 infected subjects destroy targeted T cells in a CD-95-dependent fashion AIDS Res. Hum. Retro. 13: 953–960
Mitra D, Steiner M, Lynch DH, Staiano-Coico L, Laurence J . 1996 HIV-1 upregulates Fas ligand expression in CD4+T cells in vitro and in vivo: association with Fas-mediated apoptosis and modulation by aurintricarboxylic acid Immunol. 87: 581–585
Sloand EM, Young NS, Kumar P, Weichold FF, Sato T, Maciejewski JP . 1997 Role of Fas ligand and receptor in the mechanism of T-cell depletion in acquired immunodeficiency syndrome: effect on CD4+lymphocyte depletion and human immunodeficiency virus replication Blood 89: 1357–1363
Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley A, Goodwin RG, Smith CA, Ramsdell F, Lynch DH . 1995 Fas ligand mediates activation-induced cell death in human T lymphocytes J. Exp. Med. 181: 71–77
Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH . 1995 Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature 373: 438–443
Ju ST, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A . 1995 Fas (CD95) FasL interactions required for programmed cell death after T-cell activation Nature 373: 444–448
Wesselborg S, Janssen O, Kabelitz D . 1993 Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells J. Immunol. 150: 4338–4345
Gandhi RT, Chen BK, Straus SE, Dale JK, Lenardo MJ, Baltimore D . 1998 HIV-1 directly kills CD4+T cells by a Fas-independent mechanism J. Exp. Med. 187: 1113–1122
Katsikis PD, Garcia-Ojeda ME, Wunderlich ES, Smith CA, Yagita H, Okumura K, Kayagaki N, Alderson M, Herzenberg LA, Herzenberg LA . 1996 Activation-induced peripheral blood T cell apoptosis is Fas independent in HIV-infected individuals Int. Immunol. 8: 1311–1317
Katsikis PD, Garcia-Ojeda ME, Torres-Roca JF, Tijoe IM, Smith CA, Herzenberg LA, Herzenberg LA . 1997 Interleukin-1B converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection J. Exp. Med. 186: 1365–1372
Ledru E, Lecoeur H, Garcia S, Debord T, Gougeon ML . 1998 Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets: correlation with Bcl-2 expression and consequences for AIDS pathogenesis J. Immunol. 160: 3194–3206
Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen JC . 1992 Activation-induced death by apoptosis in CD4+T cells from human immunodeficiency virus-infected asymptomatic individuals J. Exp. Med. 175: 331–340
Algeciras A, Dockrell DH, Lynch DH, Paya CV . 1998 CD4 regulates susceptibility to Fas ligand - and tumor necrosis factor-mediated apoptosis J. Exp. Med. 187: 711–720
Laurent-Crawford AG, Krust B, Riviere Y, Desgranges C, Muller S, Kieney MP, Dauguet C, Hovanessian AG . 1993 Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells AIDS Res. Hum. Retro. 9: 761–773
Oyaizu N, McCloskey TW, Coronesi M, Chirmule N, Kalyanaraman VS, Pahwa S . 1993 Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type-1 infected patients and in CD4 cross-linked PBMCs from normal individuals Blood 82: 3392–3400
Jeremias I, Herr I, Boehler T, Debatin KM . 1998 TRAIL/Apo-2-ligand-induced apoptosis in human T cells Eur. J. Immunol. 28: 143–152
Glynn JM, McElligott DL, Mosier D . 1996 Apoptosis induced by HIV infection in H9 T cells is blocked by ICE-Family protease inhibition but not by a Fas (CD95) antagonist J. Immunol. 157: 2754–2758
Noraz N, Gozlan J, Corbeil J, Brunner T, Spector SA . 1997 HIV-induced apoptosis of activated primary CD4+T lymphocytes is not mediated by Fas–Fas ligand AIDS 11: 1671–1680
Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB . 1995 Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein Science 268: 429–431
New DR, Maggirwar SB, Epstein LG, Dewhurst S, Gelbard HA . 1998 HIV-1 Tat induces neuronal death via tumor necrosis factor-α and activation of non-N-methyl-D-aspartate receptors by a NFkB-independent mechanism J. Biol. Chem. 273: 17852–17858
Patki AH, Lederman MM . 1996 HIV-1 Tat protein and its inhibitor Ro 24-7429 inhibit lymphocyte proliferation and induce apoptosis in peripheral blood mononuclear cells from healthy donors Cell Immunol. 169: 40–46
Fujii Y, Otake K, Tashiro M, Adachi Y . 1996 Human immunodeficiency virus type 1 Nef protein on the cell surface is cytocidal for human CD4+T cells FEBS Letters 393: 105–108
Fujii Y, Otake K, Tashiro M, Adachi Y . 1996 Soluble Nef antigen of HIV-1 is cytotoxic for human CD3+T cells FEBS Letters 393: 93–96
Fujii Y, Otake K, Tashiro M, Adachi Y . 1996 In vitro cytocidal effects of human immunodeficiency virus type 1 Nef on unprimed human CD4+T cells without MHC restriction J. Gen. Virol. 77: 2943–2951
Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, Jolicoeur P . 1998 Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice Cell 95: 163–175
Poon B, Grovit-Ferbas K, Stewart SA, Chen ISY . 1998 Cell cycle arrest by vpr in HIV-1 virions and insensitivity to antiretroviral agents Science 281: 266–269
Stewart SA, Poon B, Jowett JBM, Chen ISY . 1997 Human immunodeficiency virus type 1 vpr induces apoptosis following cell cycle arrest J. Virol. 71: 5579–5592
Yao X-J, Mouland AJ, Subbramanian RA, Forget J, Rougeau N, Bergeron D, Cohen EA . 1998 Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing jurkat T cells J. Virol. 72: 4686–4693
Adams LD, Tomasselli AG, Robbins P, Moss P, Heinrikson RL . 1992 HIV-1 protease cleaves actin during acute infection human T-lymphocytes AIDS Res. Hum. Retro. 8: 291–295
Buttner J, Dornmair K, Schramm HJ . 1997 Screening of inhibitors of HIV-1 protease using an Escherichia coli cell assay Biochem. Biophy. Res. Commun. 233: 36–38
Konvalinka J, Litterst MA, Welker R, Kottler H, Rippman F, Heuser A-M, Krausslich H-G . 1995 An active site mutation in the HIV type 1 proteinase (PR) causes reduced PR activity and loss of PR mediated cytotoxicity without apparent effect on virus maturation and infectivity J. Virol. 69: 7180–7186
Rivi re Y, Blank V, Kourilsky P, Israel A . 1991 Processing of the precursor of NF-kappa B by the HIV-1 protease during acute infection Nature 350: 625–626
Shimizu T, Pommier Y . 1996 DNA fragmentation induced by protease activation in p53-null human leukemia HL60 cells undergoing apoptosis following treatment with the topoisomerase 1 inhibitor campothecin: cell-free system studies Exper. Cell. Res. 226: 292–301
Kaplan AH, Swanstrom R . 1991 The HIV-1 gag precursor is processed via two pathways: implications for cytotoxicity Biomed. Biochim. Acta. 50: 647–653
Kaplan AH, Swanstrom R . 1991 Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments Proc. Natl. Acad. Sci. USA. 88: 4528–4532
Strack PR, West Frey M, Rizzo CJ, Cordova B, George HJ, Meade R, Ho W, Corman J, Tritch R, Korant BD . 1996 Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2 Proc. Natl. Acad. Sci. USA. 93: 9571–9576
Liu X, Kim CN, Yang J, Jemmerson R, Wang R . 1996 Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c Cell 86: 147–157
Cosulich SC, Green S, Clarke PR . 1996 Bcl-2 regulates activation of apoptotic proteases in a cell-free system Curr. Biology 6: 997–1005
Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang H, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ . 1999 Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspase-2,-3,-6,-7,-8, and -10 in a caspase 9-dependent manner J. Cell. Biol. 144: 281–292
Muzio M, Salveson GS, Dixit VM . 1997 FLICE induced apoptosis in a cell-free system J. Biol. Chem. 272: 2952–2956
Kroemer G, Reed JC . 1998 Mitochondrial control of cell death Nat. Med. 6: 513–519
Green DR, Reed JC . 1998 Mitochondria and apotposis Science 281: 1309–1312
Saleh A, Srinivasula SM, Acharya S, Fishel R, Alnemri ES . 1999 Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation J. Biol. Chem. 274: 17941–17945
Hu Y, Benedict MA, Ding L, Nunez G . 1999 Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase-9 activation and apoptosis EMBO J. 18: 3586–3595
Li H, Zhu H, Xu CJ, Yuan J . 1998 Cleavage of BID by caspase 8 meidates the mitochondrial damage in the Fas pathway of apoptosis Cell 94: 491–501
Wei MC, Lindstein T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ . 2000 tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c Genes Dev. 14: 2060–2071
Tomasselli AG, Hui JO, Chosay J, Lowery D, Greenberg B, Yem A, Deibel MR, Zurcher-Neely H, Heinrickson RL . 1991 Actin, troponin C, alzheimer amyloid precursor protein and pro-interleukin 1b as substrates of the protease from human immunodeficiency virus J. Biol. Chem. 266: 14548–14553
Krammer PH . 1999 CD95 (APO-1/Fas)-meidated apoptosis: live and let die Adv. Immunol. 71: 163–210
Medema JP, Scaffidi C, Kischek FC, Shevchenko A, Mann M, Krammer PH, Peter ME . 1997 FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 16: 2794–2804
Andrade F, Roy S, Nicholson D, Thornberry N, Rosen A, Casciola-Rosen L . 1998 Granzyme B directly and efficiently cleaves several downstream caspase substrates: implications for CTL-induced apoptosis Immunity 8: 451–460
Chou KC, Zhang CT . 1993 Studies on the specificity of HIV protease: an application of Markov chain theory J. Protein Chem. 12: 709–724
Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM . 1995 Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly (ADP-ribose) polymerase Cell 81: 801–809
Nicholson DW, Ali A, Thornberry N, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA . 1995 Identification and inhibition of the ICE/CED-2 protease necessary for mammalian apoptosis Nature 376: 37–43
Badley AD, Pilon AA, Landay A, Lynch DH . 2000 Mechanisms of HIV associated lymphocyte apoptosis Blood 96: 2951–2964
Barry MG, McFadden G . 1998 Apoptosis regulators from DNA viruses Curr. Opin. Immunol. 10: 422–430
Thome M, Schnelder P, Hofmann K, Foickenscher H, Neinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M, Scaffidi C, Krammer PH, Peter ME, Tschopp J . 1997 Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors Nature 386: 517–521
Honer B, Shoeman RL, Traub P . 1991 Human immunodeficiency virus type 1 protease microinjected into cultured human skin fibroblasts cleaves vimentin and affects cytoskeletal and nuclear architecture J. Cell. Sci. 100: 799–807
Shoeman RL, Honer B, Stoller TJ, Kesselmeier C, Miedel MC, Traub P, Graves MC . 1990 Human immunodeficiency virus type 1 protease cleaves the inermediate filament proteins vimentin, desmin, and glial fibrillary acidic protein Natl. Acad. Sci. USA. 87: 6336–6340
Korant B, Strack P, Frey MW, Rizzo CJ . 1998 A cellular anti-apoptosis protein is cleaved by the HIV-1 protease Adv. Exp. Med. Biol. 436: 27–29
Martin SJ, Newmeyer DD, Mathias S, Farschon D, Wang H, Reed JC, Kolesnick RN, Green DR . 1995 Cell-free reconstitution of Fas-, UV radiation and ceramide-induced apoptosis EMBO J. 14: 5191–5200
Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J . 1997 Inhibition of death receptor signals by cellular FLIP Nature 388: 190–195
Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME . 1999 Differential modulation of apoptosis sensitivity in CD95 type I and type II cells J. Biol. Chem. 274: 22532–22538
Flexner C . 1998 HIV-protease inhibitors N. Engl. J. Med. 338: 1281–1292
Phenix BN, Lum JJ, Sanchez-Dardon J, Badley AD . 2001 Antiapoptotic mechanism of HIV protease inhibitors: preventing mitochondrial potential loss Blood 98: 1078–1085
Stennicke HR, Salvesen GS . 1997 Biochemical characteristics of caspase-3,-6,-7, and -8 J. Biol. Chem. 272: 25719–25723
Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S . 1998 Caspase-independent cell killing by Fas-associated protein with death domain J. Cell. Biol. 143: 1353–1360
Juo P, Kuo CJ, Yuan J, Blenis J . 1998 Essential requirement for caspase-8/FLICE in the initiation of the Fas- induced apoptotic cascade Curr. Biol. 8: 1001–1008
Wood ER, Earnshaw WC . 1990 Mitototic chromatin condensation in vitro using somatic cell extracts as nuclei with variable levels of endogenous topoisomerese II Cell. Biol. 111: 2389–2850
Zhu N, Wang Z . 1997 An assay for DNA fragmentation in apoptosis without phenol/chloroform extraction and ethanol precipitation Analytical Biochemistry 246: 155–158
Alam A, Braun MY, Hartgers F, Lesage G, Cohen L, Hugo P, Denis F, Sekaly R-P . 1997 Specific activation of the cysteine protease CPP32 during the negative selection of T cells in the thymus J. Exp. Med. 186: 1503–1512
Evans EK, Kuwana T, Strum SL, Smith JJ, Newmeyer DD, Kornbluth S . 1997 Reaper-induced apoptosis in a vertebrate system EMBO J. 16: 7372–7381
Schmitz I, Walczak H, Krammer PH, Peter ME . 2000 The two CD95 apoptosis signaling pathways may be a way of cells to respond to different amounts and/or forms of CD95 ligand produced in different tissues Cell Death and Diff. 7: 756–758
Stennicke HR, Salvesen GS . 1999 Catalytic properties of the caspases Cell Death Differ. 6: 1054–1059
Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhand M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM . 1996 FLICE, a novel FADD/homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex Cell. 85: 817–827
Griffiths JT, Phylip LH, Konvalinka J, Strop P, Gustchina A, Wlodawer A, Davenport RJ, Briggs R, Dunn BM, Kay J . 1992 Different requirements for productive interaction between the active site of HIV-1 proteinase and substrates containing - hydrophobic*hydrophobic- or -aromatic*pro- cleavage sites Biochemistry 31: 5193–5200
Acknowledgements
Informed consent was obtained from all subjects prior to blood collection, and the study was reviewed and approved by the ethics committee of the Ottawa Hospital/University of Ottawa. The authors gratefully acknowledge the helpful discussions and advice of Dr. BWD Badley, as well as the administrative expertise of A Carisse. BN Phenix and JJ Lum are supported by a Studentship award from the Ontario HIV Treatment Network (OHTN). AD Badley is supported by an OHTN Career Scientist Award. This work is supported by grants from the Doris Duke Foundation (#T98026), the Canadian Institute of Health Research (#HOP-36047), the Canadian Foundation for AIDS Research and a Premier's Research Excellence Award.
Author information
Authors and Affiliations
Additional information
Edited by R A Knight
Rights and permissions
About this article
Cite this article
Nie, Z., Phenix, B., Lum, J. et al. HIV-1 protease processes procaspase 8 to cause mitochondrial release of cytochrome c, caspase cleavage and nuclear fragmentation. Cell Death Differ 9, 1172–1184 (2002). https://doi.org/10.1038/sj.cdd.4401094
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401094