Abstract
We review data supporting a model in which activated tBID results in an allosteric activation of BAK, inducing its intramembranous oligomerization into a proposed pore for cytochrome c efflux. The BH3 domain of tBID is not required for targeting but remains on the mitochondrial surface where it is required to trigger BAK to release cytochrome c. tBID functions not as a pore-forming protein but as a membrane targeted and concentrated death ligand. tBID induces oligomerization of BAK, and both Bid and Bak knockout mice indicate the importance of this event in the release of cytochrome c. In parallel, the full pro-apoptotic member BAX, which is highly homologous to BAK, rapidly forms pores in liposomes that release intravesicular FITC-cytochrome c ∼20Å. A definable pore progressed from ∼11Å consisting of two BAX molecules to a ∼22Å pore comprised of four BAX molecules, which transported cytochrome c. Thus, an activation cascade of pro-apoptotic proteins from BID to BAK or BAX integrates the pathway from surface death receptors to the irreversible efflux of cytochrome c. Cell Death and Differentiation (2000) 7, 1166–1173
Similar content being viewed by others
Introduction
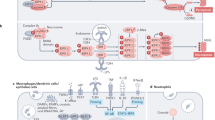
Apoptosis manifests in two major execution programs downstream of the death signal: the caspase pathway and organelle dysfunction, of which mitochondrial dysfunction is best characterized.1,2 As the BCL-2 family members reside upstream of irreversible cellular damage and focus much of their efforts at the level of mitochondria, they play a pivotal role in deciding whether a cell will live or die (Figure 1).
Schematic model of mammalian cell death pathway. A major checkpoint in the common portion of this pathway is the ratio of pro-apoptotic (BAX) to anti-apoptotic (BCL-2) members. Downstream of this checkpoint are two major execution programs: the caspase pathway and mitochondria dysfunction. Mitochondrial dysfunction includes a change in the mitochondrial membrane potential (ΔΨm), production of reactive oxygen species (ROS), permeability transition (PT), and the release of the intermembrane space protein, cytochrome c (Cyt c). Released cytochrome c activates Apaf-1, which in turn activates a downstream caspase program. Activated caspases can also effect the function of mitochondria. Caspases could be activated through Apaf-1/cytochrome c or directly by activation of cell surface death receptors. Caspases (e.g. caspase-3) are activated by cleavage events and the activated caspases cleave death substrates (e.g. PARP), which ultimately lead to cell death
The BCL-2 family of proteins has expanded significantly and includes both pro- as well as anti-apoptotic molecules. Indeed, the ratio between these two subsets helps determine, in part, the susceptibility of cells to a death signal3 (Figure 1). An additional characteristic of the members of this family is their frequent ability to form multimers, including heterodimers, suggesting neutralizing competition between these proteins. A further characteristic of probable functional significance is their ability to become integral oligomeric membrane proteins.
BCL-2 family members possess up to four conserved BCL-2 homology (BH) domains designated BH1, BH2, BH3, and BH4, which correspond to α-helical segments4,5,6 (Figure 2). In general, the anti-apoptotic members display sequence conservation in all four domains. The pro-apoptotic molecules frequently display less sequence conservation of the first α-helical segment, BH4. Deletion and mutagenesis studies argue that the amphipathic α-helical BH3 domain serves as a critical death domain in the pro-apoptotic members. This concept is supported by an emerging subset of ‘BH3-domain-only’ members who display sequence homology only within the BH3 domain and to date are all pro-apoptotic. However, the three-dimensional structure of at least one BH3-domain-only molecule, BID, demonstrates a very similar overall α-helical content to the anti-apoptotic molecule BCL-XL.7,8
Upstream of mitochondria: activation of BCL-2 family members
A considerable portion of the pro- versus anti-apoptotic BCL-2 members localize to separate subcellular compartments in the absence of a death signal. Anti-apoptotic members are initially integral membrane proteins found in the mitochondria, endoplasmic reticulum (ER), or nuclear membrane.9,10,11,12 In contrast, a substantial fraction of certain pro-apoptotic members localize to cytosol or cytoskeleton prior to a death signal.13,14,15 Following a death signal, these pro-apoptotic members undergo a conformational change that enables them to target and integrate into membranes, especially the mitochondrial outer membrane.
Post-translational modifications determine active/inactive conformations
Oligomerization
Activation of the pro-apoptotic molecule BAX involves subcellular translocation and dimerization. In viable cells a substantial portion of BAX is monomeric and found either in the cytosol or loosely attached to membranes. Following a death stimulus, the cytosolic, monomeric BAX translocates to the mitochondria where it becomes an integral membrane protein and cross-linkable as homodimers and higher order oligomers.14,16 Experiments using an FKBP-BAX fusion molecule indicated that enforced dimerization by the bivalent ligand FK1012 results in translocation of the dimer to mitochondria, where it was capable of killing cells despite the presence of survival factor and BCL-XL. However, it is still uncertain whether the dimerization of BAX normally occurs in the cytosol or is coincident with membrane insertion. Of note, nonionic detergents that might mimic the membrane environment also induce the dimerization of monomeric BAX and other family members.17 Following death signals, membrane associated BAX becomes a more integral-membrane protein and alters the exposure of its amino-terminal domain.18,19 Using antibodies directed against an amino-terminal epitope, it was demonstrated that alterations of the amino terminus following a death signal were also observed for BAK.17,19,20 Taken together, one model holds that the amino terminus is concealed to keep the molecule in a closed configuration until an activation stimulus results in a conformational change in BAX or BAK that manifests in its release.
The presence of an anti-apoptotic molecule such as BCL-2 or BCL-XL can inhibit the activation of BAX following a death signal.14 In contrast to inactive BAX, which is monomeric and in the cytosol or loosely associated with membranes, BCL-2 is an integral membrane protein heavily localized to mitochondria.
Activation of BID
BID is a member of the ‘BH3 domain only’ subgroup of BCL-2 family members proposed to connect proximal death and survival signals to the core apoptotic pathway at the level of the classic family members which bear multiple BH domains.4,21 This set of pro-apoptotic proteins shares their only sequence homology within the BH3 amphipathic α-helical domain that is essential for killing activity and heterodimerization with other BCL-2 family members. Evidence that these proteins reside within a conserved cell death pathway was supported by the demonstration that egl-1, the upstream negative regulator of the anti-apoptotic ced-9 gene in C. elegans, encodes a ‘BH3 domain only’ protein.22 Several of these proteins appear to exist in an inactive conformation in viable cells but undergo a post-translational modification in response to select death signals to assume an active conformation. These modifications dictate the subcellular location and the binding partners of such proteins. For example, BAD in response to survival factor signaling is robustly phosphorylated on serine residues, which inactivates the molecule. Phosphorylated BAD does not bind BCL-2 or BCL-XL and is sequestered in the cytosol bound to 14-3-3.23 BAD connects the core death pathway to upstream signaling in that survival pathways that activate the PI3-K pathway phosphorylate BAD on Ser136.24,25 Whereas, survival factors which activate a mitochondrial anchored PKA holoenzyme complex result in phosphorylation of the Ser112 site.26 BIM in response to several death stimuli moves from microtubules to the mitochondria where it appears to bind BCL-XL to promote cell death.15
BID is a ‘BH3 domain only’ pro-apoptotic member first noted for its capacity to bind either BCL-2 or BAX and promote cell death. Mutational analysis indicated that an intact BH3 domain was required for binding BCL-2 and BAX, and this activity correlated with BID's ability to induce cell death. This suggested a model in which BID served as a ‘death ligand’ which moved from the cytosol to the mitochondrial membrane to inactivate BCL-2 or activate BAX.27 More recently this model has been refined by the recognition that cytosolic p22 BID is activated by caspase-8 cleavage following engagement of Fas or TNFR1 receptors on cells.28,29,30 The truncated p15 BID (tBID) translocates to mitochondria where it inserts into the mitochondrial outer membrane. Immunodepletion of BID from cytosolic preparations argued tBID is required for the release of cytochrome c from mitochondria.28,30 The release of cytochrome c from mitochondria has been shown to promote the oligomerization of a cytochrome c/Apaf-1/Caspase-9 complex that activates caspase-9 to result in the cleavage of downstream effector caspases-3, -7.31,32,33
Bid-deficient mice revealed that BID was a critical caspase substrate in vivo.34 BID proved important in hepatocytes for the release of cytochrome c, dysfunction of mitochondria and even the death of cells following Fas activation in vivo. Other cell types which do not absolutely require BID for FasL or TNFα induced death still demonstrate lack of cytochrome c release, diminished effector caspase activity, and an altered pattern of substrate cleavage in Bid−/− mice. Thus, certain cell types such as hepatocytes appear to require a BID-dependent mitochondrial amplification loop that releases cytochrome c, oligomerizing Apaf-1 and caspase-9 to activate sufficient effector caspases to execute apoptosis.
However, the precise mechanism whereby cytochrome c is released from mitochondria remains uncertain, and observations have varied with different cell types and death signals. Following growth factor withdrawal mitochondrial swelling has been noted. In this model, defective exchange of ADP results in hyperpolarization of the inner membrane, an increase in matrix volume, and non-specific rupture of the outer membrane, releasing intermembrane space proteins including cytochrome c.35 Following death signals the pro-apoptotic protein BAX has been shown to translocate to mitochondria where it inserts as an apparent homo-oligomerized integral membrane protein.14,16 Other studies of BAX or BID suggest they could result in a more global permeabilization of the outer mitochondrial membrane, releasing multiple intermembrane space proteins.36,37
Two broad categories of mechanisms might account for how BID results in cytochrome c release. As noted BID might serve as a ‘death ligand’ to activate other resident mitochondrial ‘receptor’ proteins to release cytochrome c. Alternatively, it is also conceivable that BID would itself function as a downstream effector participating in an intramembranous pore which released cytochrome c. To date, BID is the one molecule absolutely required for the release of cytochrome c in loss-of-function approaches including immunodepletion and gene knockout. Moreover, tBID becomes an alkali resistant, integral membrane protein following translocation to mitochondria. Despite sequence homology limited to only the BH3 domain, BID's overall α-helical content and three dimensional structure proved remarkably similar to the anti-apoptotic BCL-XL protein.7,8,38 This includes the presence of two central hydrophobic core helices which constitute potential pore-forming domains, as they are similar to those in BCL-XL and the bacterial pore-forming toxins of diphtheria toxin fragment B and colicin. Finally, p15 BID39 like BCL-XL, BCL-2 and BAX40,41,42 has been noted to form ion conductive pores in vitro in artificial lipid bilayers. This constellation of findings lends credence to the hypothesis that tBID itself could be a downstream death effector.
BH3 domain of tBID is not required for mitochondrial targeting, but is required for cytochrome c release
In TNFα-activated cells, BID cleavage products of p15, p13 and p11 are all found in mitochondria.28 Similar to p15 BID, the p13 cleavage is also within the unstructured loop of BID and retains the BH3 domain, while p11 is cleaved at Asp98 and thus lacks the BH3 domain (Figure 3). p13 and p11 BID also target mitochondria and become integral membrane proteins, indicating that BH3 is not required for mitochondrial targeting. However, while p13 BID caused cytochrome c release comparable to p15 BID, p11 BID did not release cytochrome c (Figure 3).43 The G94E substitution mutant of the BH3 domain (mIII.4), incapable of binding to family members BAX, BAK, or BCL-2 or causing cell death, was able to target mitochondria but was defective in cytochrome c release (Figure 3). Moreover, topographic mapping indicates the BH3 domain of p15 BID is on the cytoplasmic surface of the mitochondrial outer membrane.
A chimeric molecule fusing BIDα3–5 with the mitochondrial signal-anchor sequence from BCL-244 restored mitochondrial targeting and exposed the BH3 domain of BID on the mitochondrial surface. The chimeric BIDα3–5/TM restored cytochrome c release in the absence of the potential pore-forming α6, α7 helices (Figure 3).43 Thus, directing the BID BH3 domain to the mitochondrial surface appears sufficient to cause cytochrome c release.
BAK is required for tBID induced cytochrome c release
Candidates for BID's potential ‘receptor’ on the surface of mitochondria included its documented binding partners: selected BCL-2 family members. While mouse liver mitochondria have no substantial BCL-2 or BAX, they do possess BAK, a pro-apoptotic member structurally similar to BAX. Even in viable liver cells, BAK is present on the mitochondrial outer membrane as an alkali-resistant, integral membrane protein.
Of note, tBID does not require BAK for mitochondrial targeting, as do recombinant p15 BID targeted wild-type and Bak-deficient mitochondria. However, BAK proved necessary for cytochrome c release as p15 BID could not cause cytochrome c release from Bak-deficient mitochondria.43 Although Bak-deficient mitochondria proved competent to release cytochrome c by the independent stimulus Ca2+ that induces permeability transition (PT), resulting in mitochondrial swelling and cytochrome c release. The swelling and cytochrome c release by Ca2+ was blocked by the PT inhibitor cyclosporin A. In contrast, p15 BID releases cytochrome c before any substantial mitochondrial swelling and the p15 BID induced cytochrome c release is not inhibited by cyclosporin A.43 These studies suggest BAK is not required for PT and that p15 BID requires BAK to release cytochrome c in a PT-independent manner.
Blocking tBID/BAK interaction prevents cytochrome c release
The wild-type, but not mutant p15 BID, can be co-immunoprecipitated with BAK. When mitochondria were incubated with a blocking Ab to BAK the antibody failed to prevent p15 BID targeting to mitochondria, but did inhibit p15 BID induced cytochrome c release.43 The BAK Ab appears to prevent p15 BID from binding BAK, supporting the importance of a tBID/BAK interaction in the release of cytochrome c.
tBID induces a conformational change and oligomerization of BAK
Ligand binding often causes a conformational change of a receptor protein, regulating the activity of this partner and initiating downstream signaling events.45 Mitochondria bearing targeted tBID displayed an altered pattern of trypsin digested BAK, revealing a new BAK conformation. A series of chemical cross-linkers revealed further evidence for a tBID induced conformational change in BAK consisting of the formation of higher order BAK complexes within the mitochondrial membrane. Bismaleimidohexane (BMH), a 16 Å, membrane permeable, homobifunctional maleimide that covalently cross-links sulfhydryl groups, proved instructive. Upon addition of p15 BID to isolated mitochondria, the BMH irreversible crosslinker shifted BAK into three distinct complexes at ∼48 kD (major species) and ∼72 and ∼96 kD (minor species).43 The ability of various BID chimeric and mutant proteins to induce the oligomerization of BAK mirrored their ability to bind BAK, cause a conformational change in BAK, and release cytochrome c. Thus, the targeting of an intact BH3 domain to the mitochondrial surface causes the formation of BAK complexes.
The ∼48 kD complex was most predominant and its size consistent with that of a BAK dimer, the ∼72 kD complex a trimer, and the ∼96 kD complex a tetramer. We tested these BAK cross-linked complexes for other known mitochondrial protein candidates, but did not detect the presence of any BID, VDAC, ANT, or BCL-XL to date. The estimated sizes of these BAK cross-linked complexes are consistent with an evolving homo-oligomerization of BAK similar to what we have observed for recombinant BAX in pure liposomes;46 although, we can not formally exclude the presence of other non-identified proteins.
Injection of anti-Fas Ab into mice results in the cleavage and translocation of tBID to mitochondria and subsequent massive hepatocellular apoptosis, in a process that requires BID as evidenced by Bid-deficient mice.34,47 Treatment of mitochondria from such Fas-activated hepatocytes with the crosslinker BMH revealed the movement of BAK into higher molecular weight complexes again consistent with trimers (72 kd) and ⩾tetramers (⩾96 kd). Importantly, no alteration in BAK conformation was noted in mitochondria from the anti-Fas Ab treated livers of Bid-deficient mice.43
Selected death signals also activate pro-apoptotic BAX, a molecule highly analogous to BAK, resulting in translocation to mitochondria where it inserts as an integral membrane, oligomeric protein.14,16,21 Mitochondrial dysfunction follows with the release of cytochrome c and the activation of caspases.48,49,50,51 In planar lipid bilayers BAX forms ion conducting channels and will release chloride or carboxyfluorescein from artificial liposomes.41,42 The ion transmitting pore formed by BAX can progress to a conductance of ∼1.5 nS with low ion selectivity. Recombinant, purified, functionally identical BAXΔC19 molecules that were monodispersed at pH 7.2 were able to release 6-carboxyfluorescein from 200 nm unilamellar vesicles.
Rapid exponential dequenching of carboxyfluorescein, which was strongly dependent on BAX concentrations, occurred at nanomolar levels and was sensitive to heat or protease inactivation. The strong concentration dependence enabled a Hill plot of the velocity of dequenching to be analyzed to determine the molecularity of pore activation as BAX concentration increased (Figure 4A).52 Note the Hill plot is curved with the slope increasing from ∼2 to an apparent maximum of ∼4.46 Thus, the number of BAX molecules participating in the active membrane pore increases in a concentration dependent fashion up to an apparent maximum of four. The capacity of BCL-2 family members to form dimers has been a prominent characteristic of their activity in cells, and death signals increase the fraction of BAX dimers and higher order oligomers recovered from mitochondria.14,21
BAX concentration dependence of carboxyfluorescein dequenching and pore sizing by dextran inhibition. Carboxyfluorescein vesicles were prepared and diluted as described.46 At t=0 the indicated concentration of BAXΔC19 was added with mixing and the fluorescence followed at excitation of 497 nm and emission of 520 nm. (A) Hill plot of the velocity of dequenching with increasing BAX concentrations. The slope of this plot at 0.2 Vmax is 2.0±0.19 rising to 3.9±0.24 at 0.8 Vmax. The inset indicates the continuous slope across the studied BAX concentrations. Error bars indicate the standard deviation. (B) Comparison of functional pore diameter determined by size-dependent dextran inhibition of dequenching at three BAX concentrations; 5 nM (▿), 9 nM (□) and 20 nM (▵). Time constants are averaged (n=3) and plotted with standard deviation at each dextran size. At each BAX concentration size-dependent dextran inhibition was fitted to a Gaussian peak and the peak center±1 standard deviation was determined (5 nM: 10.7±3.2 Å, 9 nM: 22.1±3.1 Å, and 21.4±5.2 Å). The vertical hatched column for each BAX concentration reflects the fitted peak with a width of two standard deviations. (C) Characterization of BAX-mediated cytochrome c release. Vesicles were prepared with 10 mg/ml FCC as described in Methods and dequenching was initiated by the addition of BAX. The size–specific dextran block, τblocked of BAX mediated FCC dequenching was determined. The peak and standard deviation were estimated by Gaussian fitting to be 28.9±6.0 Å and dextrans with a Stokes diameter greater than this show decreasing inhibition
Transport kinetics are used to characterize the diffusion pathway in classic channels. Restricted diffusion in the pore increases transit time as the penetrating molecule approximates the pore diameter.53 Accordingly, unlabeled dextran molecules can be used as blockers of carboxyfluorescein passage through the BAX pore. The effectiveness of various sized dextrans represent a measure of the pore diameter.53,54 A dominant pore size of 10.7±3.2 Å was calculated at 5 nM BAX where the slope of the Hill plot is ∼2 indicating a bimolecular mode of pore activation. The pore size reached a maximum of ∼22Å at 9 nM BAX (Figure 4B) where the slope of the Hill plot is ∼4 reflecting the shift to the maximum number of participating BAX molecules.46 No further increase in pore size was noted at 20 nM BAX, consistent with the plateau of the molecularity of pore activation at ∼4.
The large homotypic BAX pore provides a diffusion pathway for the movement of appropriately-sized macromolecules as well. Liposomes were prepared containing ∼25 μM FITC-labeled cytochrome c (FCC), estimated at a Stokes diameter for FCC of 20±5 Å, while native cytochrome c is 17±3 Å. A Hill plot of the velocity of FCC dequenching at increasing concentrations of BAX revealed a uniform slope of 4 across the effective concentrations of BAX, indicating that only the large 22Å BAX pore is responsible for release of cytochrome c. Extra-vesicular unlabeled cytochrome c proved capable of inhibiting the release of FCC. This confirms a pore mechanism of release.46 The FCC pore by size-specific dextran inhibition of FCC release at a BAX concentration with a pore activation molecularity of 4 indicated a pore size of 29.8±6.0 Å (Figure 4C). This is consistent with the large BAX pore observed for carboxyfluorescein release. From these data it can be concluded that BAX at nanomolar concentrations is capable of forming a pathway for cytochrome c release from liposomes that does not require additional proteins.46
The biologic importance of BAX multimerization was supported by experiments in which the enforced dimerization of an FKBP-BAX chimeric molecule proved sufficient to kill cells.14 Other models suggest BAX interacts with resident mitochondrial membrane pores, such as VDAC, or propose the lytic disruption of the outer mitochondrial membrane.37,55 However, BAX alone can form a membrane pathway sufficient to transport cytochrome c. The subsequent release of larger proteins, such as sulfite oxidase, suggests that further oligomerization of BAX in vivo or secondary effects may follow.56 Based on the data here, we hypothesize that the BAX or BAK mediated release of cytochrome c from mitochondria requires the establishment of a sufficient density of BAX or BAK molecules in the mitochondrial membrane to form the large pore.
Conclusion
These studies integrate an apoptotic pathway from a surface death receptor, through the sequential activation of pro-apoptotic BCL-2 family members, to the release of cytochrome c and caspase activation. Further biophysical characterization of the BAX homo-oligomerized pore and in vivo evidence for the importance of the highly related BAK molecule provide support for their allosteric conformational activation to form pores responsible for the initial release of cytochrome c. Two loss-of-function mouse models, Bid-deficiency and Bak-deficiency, were used to establish a cascade in which Fas engagement on hepatocytes activates BID which activates BAK to release cytochrome c (Figure 5). The ‘BH3 domain only’ tBID serves as an upstream death ligand which functions to allosterically regulate the full pro-apoptotic molecule BAK constitutively present on mitochondria. The BH3 domain of tBID must be intact and able to interact with resident mitochondrial BAK to release cytochrome c. The hydrophobic α-helices confer an integral mitochondrial membrane position to tBID which does not itself form a pore capable of releasing cytochrome c. Instead the membrane targeting of tBID appears to represent a localized, concentrating mechanism to present the BH3 domain to resident BAK.
References
Green DR and Reed JC . 1998 Mitochondria and apoptosis. Science 281: 1309–1312
Thornberry NA and Lazebnik Y . 1998 Caspases: enemies within. Science 281: 1312–1316
Oltvai ZN, Milliman CL and Korsmeyer SJ . 1993 Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74: 609–619
Adams JM and Cory S . 1998 The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326
Kelekar A and Thompson CB . 1998 Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell. Biol. 8: 324–330
Reed JC . 1998 Bcl-2 family proteins. Oncogene 17: 3225–3236
Chou JJ, Li H, Salvesen GS, Yuan J and Wagner G . 1999 Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96: 615–624
McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ and Cowburn D . 1999 Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell 96: 625–634
Hockenbery D, Nunez G, Milliman C, Schreiber RD and Korsmeyer SJ . 1990 Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348: 334–336
Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W and Reed JC . 1993 Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 53: 4701–4714
de Jong D, Prins FA, Mason DY, Reed JC, van Ommen GB and Kluin PM . 1994 Subcellular localization of the bcl-2 protein in malignant and normal lymphoid cells. Cancer Res. 54: 256–260
Zhu W, Cowie A, Wasfy GW, Penn LZ, Leber B and Andrews DW . 1996 Bcl-2 mutants with restricted subcellular location reveal spatially distinct pathways for apoptosis in different cell types. EMBO J. 15: 4130–4141
Hsu YT, Wolter KG and Youle RJ . 1997 Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA 94: 3668–3672
Gross A, Jockel J, Wei MC and Korsmeyer SJ . 1998 Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 17: 3878–3885
Puthalakath H, Huang DC, O'Reilly LA, King SM and Strasser A . 1999 The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell. 3: 287–296
Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG and Youle RJ . 1997 Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell. Biol. 139: 1281–1292
Hsu YT and Youle RJ . 1997 Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272: 13829–13834
Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ and Shore GC . 1998 Regulated targeting of BAX to mitochondria. J. Cell. Biol. 143: 207–215
Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B and Martinou JC . 1999 Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell. Biol. 144: 891–901
Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, Dive C and Hickman JA . 1999 Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell. Biol. 144: 903–914
Gross A, McDonnell JM and Korsmeyer SJ . 1999 BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13: 1899–1911
Conradt B and Horvitz HR . 1998 The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell 93: 519–529
Zha J, Harada H, Yang E, Jockel J and Korsmeyer SJ . 1996 Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-XL . Cell 87: 619–628
del Peso L, Gonzalez-Garcia M, Page C, Herrera R and Nunez G . 1997 Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278: 687–689
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y and Greenberg ME . 1997 Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241
Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, Scott JD and Korsmeyer SJ . 1999 Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol. Cell. 3: 413–422
Wang K, Yin XM, Chao DT, Milliman CL and Korsmeyer SJ . 1996 BID: a novel BH3 domain-only death agonist. Genes Dev. 10: 2859–2869
Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P and Korsmeyer SJ . 1999 Caspase cleaved BID targets mitochondrial and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274: 1156–1163
Li H, Zhu H, Xu CJ and Yuan J . 1998 Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501
Luo X, Budihardjo I, Zou H, Slaughter C and Wang X . 1998 Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481–490
Liu X, Kim CN, Yang J, Jemmerson R and Wang X . 1996 Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157
Zou H, Henzel WJ, Liu X, Lutschg A and Wang X . 1997 Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90: 405–413
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES and Wang X . 1997 Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489
Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA and Korsmeyer SJ . 1999 Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400: 886–891
Vander Heiden MG, Chandel NS, Schumacker PT and Thompson CB . 1999 Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell. 3: 159–167
Kluck RM, Esposti MD, Perkins G, Renken C, Kuwana T, Bossy-Wetzel E, Goldberg M, Allen T, Barber MJ, Green DR and Newmeyer DD . 1999 The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J. Cell. Biol. 147: 809–822
Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood KA, Hsu Y, Zimmerberg J and Youle RJ . 1999 Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. USA 96: 5492–5497
Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL and Fesik SW . 1996 X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381: 335–341
Schendel SL, Azimov R, Pawlowski K, Godzik A, Kagan BL and Reed JC . 1999 Ion channel activity of the BH3 only Bcl-2 family member, BID. J. Biol. Chem. 274: 21932–21936
Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M and Thompson CB . 1997 Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature 385: 353–357
Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R and Martinou JC . 1997 Inhibition of Bax channel-forming activity by Bcl-2. Science 277: 370–372
Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G and Korsmeyer SJ . 1997 Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc. Natl. Acad. Sci. USA 94: 11357–11362
Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB and Korsmeyer SJ . 2000 tBID, a membrane targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14: 2060–2071
Nguyen M, Millar DG, Yong VW, Korsmeyer SJ and Shore GC . 1993 Targeting of Bcl-2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence. J. Biol. Chem. 268: 25265–25268
Schlessinger J . 1988 Signal transduction by allosteric receptor oligomerization. Trends Biochem. Sci. 13: 443–447
Saito M, Korsmeyer SJ and Schlesinger P . 2000 BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat. Cell Biol. 2: 553–555
Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T and Nagata S . 1993 Lethal effect of the anti-Fas antibody in mice. Nature 364: 806–809
Zou H, Li Y, Liu X and Wang X . 1999 An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274: 11549–11556
Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG and Green DR . 1999 Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 274: 2225–2233.
Pastorino JG, Chen ST, Tafani M, Snyder JW and Farber JL . 1998 The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J. Biol. Chem. 273: 7770–7775
Xiang J, Chao DT and Korsmeyer SJ . 1996 BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA 93: 14559–14563
Segel IH. . Behavior and analysis of rapid equilibrium and steady-state enzyme systems John Wiley & Sons 1975 pp. 371–385
Levitt DG . 1984 Kinetics of movement in narrow channels. Curr. Topics In Membranes and Transport 21: 181
Hille B . 1968 Pharmacological modifications of the sodium channels of frog nerve. J. Gen. Physiol. 51: 199–219
Shimizu S, Narita M and Tsujimoto Y . 1999 Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487
Antonsson B, Montessuit S, Lauper S, Eskes R and Martinou JC . 2000 Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 345: 271–278
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Kroemer
Rights and permissions
About this article
Cite this article
Korsmeyer, S., Wei, M., Saito, M. et al. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 7, 1166–1173 (2000). https://doi.org/10.1038/sj.cdd.4400783
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400783