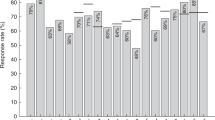
Summary:
Influenza vaccine is recommended yearly for recipients after the sixth month of BMT. Although a higher risk of complications of influenza is expected to occur in BMT patients, no study has addressed the clinical efficacy of influenza vaccination in this setting. Focusing on the clinical benefits of influenza vaccination, we evaluated the risk factors for influenza infection in a cohort of 177 BMT recipients followed up for 1 year. Influenza was diagnosed in 39 patients. Multivariate analyses showed that seasonal exposure and more aggressive conditioning regimens were independently associated with increased risk for influenza. Influenza vaccination and steroid use showed a protective role. Of the 43 patients who had received BMT longer than 6 months, 19 were vaccinated (compliance rate= 44.2%) and vaccine efficacy was 80%. We conclude that influenza vaccination plays an important role in protecting BMT recipients against influenza and all efforts should be made to ensure good compliance with vaccination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Whimbey E, Elting LS, Cough RB et al. Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant 1994; 13: 437–440.
Bowden RA . Respiratory virus infections after marrow transplant: The Fred Hutchinson Cancer Research Center experience. Am J Med 1997; 102: 27–30.
Cough RB, Englund JA, Whimbley E . Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med 1997; 102: 2–9.
Ljungman P, Ward KN, Crooks BNA et al. Respiratory virus infection after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2001; 28: 479–484.
Bridges CB, Harper SA, Fukuda K et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP); Advisory Committee on Immunization Practices. MMWR 2003; 52: 1–34.
Engelhard D, Nagler A, Hardan I et al. Antibody response to a two-dose regimen of influenza vaccine in allogeneic T cell-depleted and autologous BMT recipients. Bone Marrow Transplant 2001; 28: 775–781.
Gandhi MK, Egner W, Sizer L et al. Antibody responses to vaccinations given within the first two years after transplant are similar between autologous peripheral blood stem cell and bone marrow transplant recipients. Bone Marrow Transplant 1993; 11: 1–5.
Dykewics CA, Jaffe HW . Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Morbid Mortal Wkly Rep 2000; 49: 1–128.
Machado CM, Boas LSV, Mendes AVA et al. Use of oseltamivir to control influenza complications after bone marrow transplantation. Bone Marrow Transplant 2004; 34: 111–114.
Nichols WG, Guthrie KA, Corey L, Boeckh M . Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis 2004; 39: 1300–1306.
van Essen GA, Palache AM, Forleo E, Fedson DS . Influenza vaccination in 2000: recommendations and vaccine use in 50 developed and rapidly developing countries. Vaccine 2003; 21: 1780–1785.
Doebbeling BN, Edmond MB, Davis CS et al. Influenza vaccination of health care workers: evaluation of factors that are important in acceptance. Prev Med 1997; 26: 68–77.
Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2004; 53: 1–40.
Acknowledgements
We thank Drs AVA Mendes, MCA Macedo, RL Silva and RS Saboya from the BMT Program for providing excellent patient care, and Dr MH Lopes, from the Centro de Referência para Imunobiológicos Especiais (CRIE), University of São Paulo Medical School.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Machado, C., Cardoso, M., da Rocha, I. et al. The benefit of influenza vaccination after bone marrow transplantation. Bone Marrow Transplant 36, 897–900 (2005). https://doi.org/10.1038/sj.bmt.1705159
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705159
Keywords
This article is cited by
-
Immunogenicity of the inactivated influenza vaccine in children who have undergone allogeneic haematopoietic stem cell transplant
Bone Marrow Transplantation (2020)
-
A pilot randomized trial of adjuvanted influenza vaccine in adult allogeneic hematopoietic stem cell transplant recipients
Bone Marrow Transplantation (2017)
-
Serum IgM levels independently predict immune response to influenza vaccine in long-term survivors vaccinated at >1 year after undergoing allogeneic hematopoietic stem cell transplantation
International Journal of Hematology (2017)
-
Low-Cost Intervention to Increase Influenza Vaccination Rate at a Comprehensive Cancer Center
Journal of Cancer Education (2017)