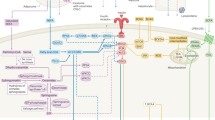
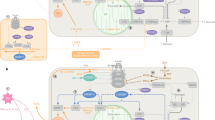
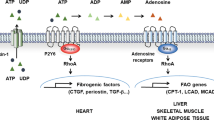
Abstract
Maintenance of systemic homeostasis and the response to nutritional and environmental challenges require the coordination of multiple organs and tissues. To respond to various metabolic demands, higher organisms have developed a system of inter-organ communication through which one tissue can affect metabolic pathways in a distant tissue. Dysregulation of these lines of communication contributes to human pathologies, including obesity, diabetes, liver disease and atherosclerosis. In recent years, technical advances such as data-driven bioinformatics, proteomics and lipidomics have enabled efforts to understand the complexity of systemic metabolic cross-talk and its underlying mechanisms. Here, we provide an overview of inter-organ signals and their roles in metabolic control, and highlight recent discoveries in the field. We review peptide, small-molecule and lipid mediators secreted by metabolic tissues, as well as the role of the central nervous system in orchestrating peripheral metabolic functions. Finally, we discuss the contributions of inter-organ signalling networks to the features of metabolic syndrome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aguilar, M., Bhuket, T., Torres, S., Liu, B. & Wong, R. J. Prevalence of the metabolic syndrome in the United States, 2003-2012. J. Am. Med. Assoc. 313, 1973–1974 (2015).
Afshin, A., Reitsma, M. B. & Murray, C. J. L. Health effects of overweight and obesity in 195 countries. N. Engl. J. Med. 377, 1496–1497 (2017).
Ogden, C. L., Carroll, M. D., Kit, B. K. & Flegal, K. M. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. J. Am. Med. Assoc. 307, 483–490 (2012).
Gregg, E. W. & Shaw, J. E. Global health effects of overweight and obesity. N. Engl. J. Med. 377, 80–81 (2017).
Saklayen, M. G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 20, 12 (2018).
Ogurtsova, K. et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 128, 40–50 (2017).
Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 3, 866–875 (2015).
Ryan, D. H. & Diabetes Prevention Program Research Group. Diet and exercise in the prevention of diabetes. Int. J. Clin. Pract. Suppl., 28–35 (2003).
Yoo, H. J. & Choi, K. M. Hepatokines as a link between obesity and cardiovascular diseases. Diabetes Metab. J. 39, 10–15 (2015).
Browning, J. D. & Horton, J. D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114, 147–152 (2004).
Bellentani, S. et al. Risk factors for alcoholic liver disease. Addict. Biol. 5, 261–268 (2000).
Ding, E. L. et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 361, 1152–1163 (2009).
Ding, L., Wendl, M. C., Koboldt, D. C. & Mardis, E. R. Analysis of next-generation genomic data in cancer: accomplishments and challenges. Hum. Mol. Genet. 19, R188–R196 (2010). R2.
Li, C. Y. et al. Recombinant human hepassocin stimulates proliferation of hepatocytes in vivo and improves survival in rats with fulminant hepatic failure. Gut 59, 817–826 (2010).
Wu, H. T. et al. The role of hepassocin in the development of non-alcoholic fatty liver disease. J. Hepatol. 59, 1065–1072 (2013).
Wang, Y. et al. Angiopoietin-like protein 4 improves glucose tolerance and insulin resistance but induces liver steatosis in high-fat-diet mice. Mol. Med. Rep. 14, 3293–3300 (2016).
Koo, B. K. et al. Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease. Liver Int. 38, 695–705 (2018).
Kim, K. H. et al. Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice. Sci. Rep. 8, 6789 (2018).
Dijk, W. et al. Angiopoietin-like 4 promotes intracellular degradation of lipoprotein lipase in adipocytes. J. Lipid Res. 57, 1670–1683 (2016).
Kutlu, O. et al. Serum adropin levels are reduced in adult patients with non-alcoholic fatty liver disease. Med. Princ. Pract. 28, 463–469 (2019).
Gao, S. et al. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol. Metab. 4, 310–324 (2015).
Meex, R. C. et al. Fetuin B is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell Metab. 22, 1078–1089 (2015).
Pal, D. et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 (2012).
Mukhopadhyay, S. & Bhattacharya, S. Plasma fetuin-A triggers inflammatory changes in macrophages and adipocytes by acting as an adaptor protein between NEFA and TLR-4. Diabetologia 59, 859–860 (2016).
Misu, H. et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 12, 483–495 (2010).
Choi, H. Y. et al. Increased selenoprotein P levels in subjects with visceral obesity and nonalcoholic fatty liver disease. Diabetes Metab. J. 37, 63–71 (2013).
Yang, Q. et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 (2005).
Moraes-Vieira, P. M. et al. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 19, 512–526 (2014).
Kotnik, P., Fischer-Posovszky, P. & Wabitsch, M. RBP4: a controversial adipokine. Eur. J. Endocrinol. 165, 703–711 (2011).
Lan, F. et al. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes 63, 1649–1664 (2014).
Kliewer, S. A. & Mangelsdorf, D. J. A dozen years of discovery: insights into the physiology and pharmacology of FGF21. Cell Metab. 29, 246–253 (2019).
Staiger, H., Keuper, M., Berti, L., Hrabe de Angelis, M. & Häring, H. U. Fibroblast growth factor 21-metabolic role in mice and men. Endocr. Rev. 38, 468–488 (2017).
Bookout, A. L. et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 19, (1147–1152 (2013).
Owen, B. M. et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 20, 670–677 (2014).
Zhang, X. et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57, 1246–1253 (2008).
Zhang, Y. et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1, e00065 (2012).
Gaich, G. et al. The effects of LY2405319, an FGF21 analogue, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340 (2013).
Wei, W. et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc. Natl Acad. Sci. USA 109, 3143–3148 (2012).
Owen, B. M. et al. FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med. 19, 1153–1156 (2013).
Inagaki, T. et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2, 217–225 (2005).
Potthoff, M. J. et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 13, 729–738 (2011).
Sinal, C. J. et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102, 731–744 (2000).
Watanabe, M. et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 113, 1408–1418 (2004).
Zhang, Y. et al. Activation of the nuclear receptor FXR improves hyperglycaemia and hyperlipidemia in diabetic mice. Proc. Natl Acad. Sci. USA 103, 1006–1011 (2006).
Calkin, A. C. & Tontonoz, P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 13, 213–224 (2012).
van Nierop, F. S. et al. Clinical relevance of the bile acid receptor TGR5 in metabolism. Lancet Diabetes Endocrinol. 5, 224–233 (2017).
Keitel, V. et al. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58, 1794–1805 (2010).
Watanabe, M. et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006).
Thomas, C. et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10, 167–177 (2009).
Simcox, J. et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab. 26, 509–522.e506 (2017).
Shah, R. V. et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc. Imaging 7, 1221–1235 (2014).
Cook, K. S. et al. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science 237, 402–405 (1987).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Cohen, P. et al. Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest. 108, 1113–1121 (2001).
Guo, K. et al. Disruption of peripheral leptin signalling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology 148, 3987–3997 (2007).
Flier, J. S. & Maratos-Flier, E. Leptin’s physiologic role: does the emperor of energy balance have no clothes? Cell Metab. 26, 24–26 (2017).
Minokoshi, Y. et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415, 339–343 (2002).
Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995).
Arita, Y. et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 257, 79–83 (1999).
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 (2001).
Qi, Y. et al. Adiponectin acts in the brain to decrease body weight. Nat. Med. 10, 524–529 (2004).
Wang, Z. V. & Scherer, P. E. Adiponectin, the past two decades. J. Mol. Cell Biol. 8, 93–100 (2016).
Combs, T. P., Berg, A. H., Obici, S., Scherer, P. E. & Rossetti, L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 108, 1875–1881 (2001).
Muoio, D. M., Dohm, G. L., Fiedorek, F. T. Jr., Tapscott, E. B. & Coleman, R. A. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes 46, 1360–1363 (1997).
Auguet, T. et al. Upregulation of lipocalin 2 in adipose tissues of severely obese women: positive relationship with proinflammatory cytokines. Obesity (Silver Spring) 19, 2295–2300 (2011).
Fernandez-García, C. E. et al. Lipocalin-2, a potential therapeutic target in advanced atherosclerosis. Atherosclerosis 278, 321–322 (2018).
Guo, H. et al. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 59, 1376–1385 (2010).
Law, I. K. et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 59, 872–882 (2010).
Wang, G. X. et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 20, 1436–1443 (2014).
Pfeifer, A. NRG4: an endocrine link between brown adipose tissue and liver. Cell Metab. 21, 13–14 (2015).
Hotamisligil, G. S. et al. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 274, 1377–1379 (1996).
Cao, H. et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 17, 768–778 (2013).
Furuhashi, M. et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 447, 959–965 (2007).
Zhang, M., Zhu, W. & Li, Y. Small molecule inhibitors of human adipocyte fatty acid binding protein (FABP4). Med. Chem. 10, 339–347 (2014).
Burak, M. F. et al. Development of a therapeutic monoclonal antibody that targets secreted fatty acid-binding protein aP2 to treat type 2 diabetes. Sci. Transl. Med. 7, 319ra205 (2015).
Barchetta, I., Cimini, F. A., Ciccarelli, G., Baroni, M. G. & Cavallo, M. G. Sick fat: the good and the bad of old and new circulating markers of adipose tissue inflammation. J. Endocrinol. Invest. 42, 1257–1272 (2019).
Steppan, C. M. et al. The hormone resistin links obesity to diabetes. Nature 409, 307–312 (2001).
Tarkowski, A., Bjersing, J., Shestakov, A. & Bokarewa, M. I. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J. Cell. Mol. Med. 14, 1419–1431 (2010). (6B).
Cao, H. et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134, 933–944 (2008).
Ferry, G. et al. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J. Biol. Chem. 278, 18162–18169 (2003).
Rancoule, C. et al. Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high-fat diet obese mice. Diabetologia 56, 1394–1402 (2013).
Yore, M. M. et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332 (2014).
Syed, I. et al. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab. 27, 419–427.e414 (2018).
Pflimlin, E. et al. Acute and repeated treatment with 5-PAHSA or 9-PAHSA isomers does not improve glucose control in mice. Cell Metab. 28, 217–227.e213 (2018).
Syed, I. et al. Methodological issues in studying PAHSA biology: masking PAHSA effects. Cell Metab. 28, 543–546 (2018).
van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Lee, P. et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19, 302–309 (2014).
Svensson, K. J. et al. A secreted Slit2 fragment regulates adipose tissue thermogenesis and metabolic function. Cell Metab. 23, 454–466 (2016).
Kong, X. et al. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab. 28, 631–643.e633 (2018).
Stanford, K. I. et al. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab. 27, 1111–1120.e1113 (2018).
Leiria, L. O. et al. 12-Lipoxygenase regulates cold adaptation and glucose metabolism by producing the omega-3 lipid 12-HEPE from brown fat. Cell Metab. 30, 768–783.e767 (2019).
Mohr, T. et al. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord 35, 1–16 (1997).
Bortoluzzi, S., Scannapieco, P., Cestaro, A., Danieli, G. A. & Schiaffino, S. Computational reconstruction of the human skeletal muscle secretome. Proteins 62, 776–792 (2006).
Pedersen, B. K. & Febbraio, M. A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406 (2008).
Fischer, C. P. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc. Immunol. Rev. 12, 6–33 (2006).
Hiscock, N., Chan, M. H., Bisucci, T., Darby, I. A. & Febbraio, M. A. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 18, 992–994 (2004).
Febbraio, M. A. et al. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J. Physiol. (Lond.) 549, 607–612 (2003).
Carey, A. L. et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55, 2688–2697 (2006).
Febbraio, M. A., Hiscock, N., Sacchetti, M., Fischer, C. P. & Pedersen, B. K. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53, 1643–1648 (2004).
Steensberg, A. et al. Acute interleukin-6 administration does not impair muscle glucose uptake or whole-body glucose disposal in healthy humans. J. Physiol. (Lond.) 548, 631–638 (2003).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
McPherron, A. C., Lawler, A. M. & Lee, S. J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83–90 (1997).
McPherron, A. C. & Lee, S. J. Suppression of body fat accumulation in myostatin-deficient mice. J. Clin. Invest. 109, 595–601 (2002).
Feldman, B. J., Streeper, R. S., Farese, R. V. Jr. & Yamamoto, K. R. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc. Natl Acad. Sci. USA 103, 15675–15680 (2006).
Hittel, D. S., Berggren, J. R., Shearer, J., Boyle, K. & Houmard, J. A. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 58, 30–38 (2009).
Hansen, J. et al. Exercise induces a marked increase in plasma follistatin: evidence that follistatin is a contraction-induced hepatokine. Endocrinology 152, 164–171 (2011).
Hansen, J. S. & Plomgaard, P. Circulating follistatin in relation to energy metabolism. Mol. Cell. Endocrinol. 433, 87–93 (2016).
Otero-Díaz, B. et al. Exercise induces white adipose tissue browning across the weight spectrum in humans. Front. Physiol. 9, 1781 (2018).
Boström, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Wrann, C. D. et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 18, 649–659 (2013).
Roberts, L. D. et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 19, 96–108 (2014).
Lee, C. et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 21, 443–454 (2015).
Du, C. et al. Circulating MOTS-c levels are decreased in obese male children and adolescents and associated with insulin resistance. Pediatr. Diabetes 19, 1058–1064 (2018).
Nielsen, A. R. & Pedersen, B. K. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl. Physiol. Nutr. Metab. 32, 833–839 (2007).
Carbó, N. et al. Interleukin-15 mediates reciprocal regulation of adipose and muscle mass: a potential role in body weight control. Biochim. Biophys. Acta 1526, 17–24 (2001).
Quinn, L. S., Strait-Bodey, L., Anderson, B. G., Argilés, J. M. & Havel, P. J. Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signalling pathway. Cell Biol. Int. 29, 449–457 (2005).
Hamrick, M. W., McNeil, P. L. & Patterson, S. L. Role of muscle-derived growth factors in bone formation. J. Musculoskelet. Neuronal Interact. 10, 64–70 (2010).
Ouchi, N. et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J. Biol. Chem. 283, 32802–32811 (2008).
Oshima, Y. et al. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation 117, 3099–3108 (2008).
Macleod, J. J. Pancreatic extract and diabetes. Can. Med. Assoc. J. 12, 423–425 (1922).
Klip, A., McGraw, T. E. & James, D. E. 30 sweet years of GLUT4. J. Biol. Chem. 294, 11369–11381 (2019).
Titchenell, P. M., Lazar, M. A. & Birnbaum, M. J. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol. Metab. 28, 497–505 (2017).
Liu, S. & Borgland, S. L. Insulin actions in the mesolimbic dopamine system. Exp. Neurol. 320, 113006 (2019).
Guo, S. Insulin signalling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J. Endocrinol. 220, T1–T23 (2014).
Mathieu, C., Gillard, P. & Benhalima, K. Insulin analogues in type 1 diabetes mellitus: getting better all the time. Nat. Rev. Endocrinol. 13, 385–399 (2017).
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 (1998).
Riddle, M., Umpierrez, G., DiGenio, A., Zhou, R. & Rosenstock, J. Contributions of basal and postprandial hyperglycaemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care 34, 2508–2514 (2011).
Wallia, A. & Molitch, M. E. Insulin therapy for type 2 diabetes mellitus. J. Am. Med. Assoc. 311, 2315–2325 (2014).
Lin, H. V. & Accili, D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 14, 9–19 (2011).
Habegger, K. M. et al. The metabolic actions of glucagon revisited. Nat. Rev. Endocrinol. 6, 689–697 (2010).
Day, J. W. et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 5, 749–757 (2009).
Kim, T. et al. Glucagon receptor signalling regulates energy metabolism via hepatic farnesoid X receptor and fibroblast growth factor 21. Diabetes 67, 1773–1782 (2018).
Nauck, M. A. & Meier, J. J. Incretin hormones: their role in health and disease. Diabetes Obes. Metab. 20, 5–21 (2018). (Suppl. 1).
Muscelli, E. et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 57, 1340–1348 (2008).
Nauck, M. A., Bartels, E., Orskov, C., Ebert, R. & Creutzfeldt, W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J. Clin. Endocrinol. Metab. 76, 912–917 (1993).
Nauck, M. A. et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Invest. 91, 301–307 (1993).
Flint, A., Raben, A., Astrup, A. & Holst, J. J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Invest. 101, 515–520 (1998).
Verdich, C. et al. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J. Clin. Endocrinol. Metab. 86, 4382–4389 (2001).
Ranganath, L. R. et al. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 38, 916–919 (1996).
Laferrère, B. Effect of gastric bypass surgery on the incretins. Diabetes Metab. 35, 513–517 (2009).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Zhong, J., Maiseyeu, A., Davis, S. N. & Rajagopalan, S. DPP4 in cardiometabolic disease: recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ. Res. 116, 1491–1504 (2015).
Svane, M. S. et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int. J. Obes. (Lond.) 40, 1699–1706 (2016).
Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999).
Tschöp, M., Smiley, D. L. & Heiman, M. L. Ghrelin induces adiposity in rodents. Nature 407, 908–913 (2000).
Colldén, G., Tschöp, M. H. & Müller, T. D. Therapeutic potential of targeting the ghrelin pathway. Int. J. Mol. Sci. 18, E798 (2017).
Yasuda, T., Masaki, T., Kakuma, T. & Yoshimatsu, H. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats. Neurosci. Lett. 349, 75–78 (2003).
Theander-Carrillo, C. et al. Ghrelin action in the brain controls adipocyte metabolism. J. Clin. Invest. 116, 1983–1993 (2006).
Gnanapavan, S. et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 87, 2988 (2002).
Gauna, C. et al. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J. Clin. Endocrinol. Metab. 90, 1055–1060 (2005).
Gauna, C. et al. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J. Clin. Endocrinol. Metab. 89, 5035–5042 (2004).
Sun, Y., Asnicar, M., Saha, P. K., Chan, L. & Smith, R. G. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 3, 379–386 (2006).
Li, Y. et al. Administration of ghrelin improves inflammation, oxidative stress, and apoptosis during and after non-alcoholic fatty liver disease development. Endocrine 43, 376–386 (2013).
Gortan Cappellari, G. et al. Unacylated ghrelin reduces skeletal muscle reactive oxygen species generation and inflammation and prevents high-fat diet-induced hyperglycemia and whole-body insulin resistance in rodents. Diabetes 65, 874–886 (2016).
Kraft, E. N., Cervone, D. T. & Dyck, D. J. Ghrelin stimulates fatty acid oxidation and inhibits lipolysis in isolated muscle from male rats. Physiol. Rep. 7, e14028 (2019).
Khatib, M. N. et al. Effect of ghrelin on mortality and cardiovascular outcomes in experimental rat and mice models of heart failure: a systematic review and meta-analysis. PLoS One 10, e0126697 (2015).
Erspamer, V. & Asero, B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169, 800–801 (1952).
Kim, H. et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 16, 804–808 (2010).
Paulmann, N. et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 7, e1000229 (2009).
Sumara, G., Sumara, O., Kim, J. K. & Karsenty, G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 16, 588–600 (2012).
Oh, C. M. et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat. Commun. 6, 6794 (2015).
Tanaka, M. & Itoh, H. Hypertension as a metabolic disorder and the novel role of the gut. Curr. Hypertens. Rep. 21, 63 (2019).
Ma, Y., He, F. J. & MacGregor, G. A. High salt intake: independent risk factor for obesity? Hypertension 66, 843–849 (2015).
Lee, M., Sorn, S. R., Lee, Y. & Kang, I. Salt induces adipogenesis/lipogenesis and inflammatory adipocytokines secretion in adipocytes. Int. J. Mol. Sci. 20, E160 (2019).
Zhu, Z., Xiong, S. & Liu, D. The gastrointestinal tract: an initial organ of metabolic hypertension? Cell. Physiol. Biochem. 38, 1681–1694 (2016).
Frigolet, M. E., Torres, N. & Tovar, A. R. The renin-angiotensin system in adipose tissue and its metabolic consequences during obesity. J. Nutr. Biochem. 24, 2003–2015 (2013).
Ohashi, K. et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 47, 1108–1116 (2006).
Zhao, Y. et al. Sodium intake regulates glucose homeostasis through the PPARδ/adiponectin-mediated SGLT2 pathway. Cell Metab. 23, 699–711 (2016).
Okamoto, Y. et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 106, 2767–2770 (2002).
Shibata, R. et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 10, 1384–1389 (2004).
Shibata, R. et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 11, 1096–1103 (2005).
Juárez-Rojas, J. G. et al. Association of adiponectin with subclinical atherosclerosis in a Mexican-mestizo population. Arch. Med. Res. 48, 73–78 (2017).
Makowski, L. et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 7, 699–705 (2001).
Makowski, L., Brittingham, K. C., Reynolds, J. M., Suttles, J. & Hotamisligil, G. S. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IκB kinase activities. J. Biol. Chem. 280, 12888–12895 (2005).
Shimba, Y. et al. Skeletal muscle-specific PGC-1α overexpression suppresses atherosclerosis in apolipoprotein E-knockout mice. Sci. Rep. 9, 4077 (2019).
Lee, M. J. et al. Irisin, a novel myokine is an independent predictor for sarcopenia and carotid atherosclerosis in dialysis patients. Atherosclerosis 242, 476–482 (2015).
Sawada, M., Yamamoto, H., Ogasahara, A., Tanaka, Y. & Kihara, S. β-aminoisobutyric acid protects against vascular inflammation through PGC-1β-induced antioxidative properties. Biochem. Biophys. Res. Commun. 516, 963–968 (2019).
Seldin, M. M. et al. Trimethylamine N-oxide promotes vascular inflammation through signalling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc. 5, e002767 (2016).
Miao, J. et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Commun. 6, 6498 (2015).
Schugar, R. C. et al. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep. 19, 2451–2461 (2017).
Dallabrida, S. M. et al. Adipose tissue growth and regression are regulated by angiopoietin-1. Biochem. Biophys. Res. Commun. 311, 563–571 (2003).
An, Y. A. et al. Angiopoietin-2 in white adipose tissue improves metabolic homeostasis through enhanced angiogenesis. eLife 6, e24071 (2017).
Voros, G. et al. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology 146, 4545–4554 (2005).
Jung, Y. J. et al. The effects of designed angiopoietin-1 variant on lipid droplet diameter, vascular endothelial cell density and metabolic parameters in diabetic db/db mice. Biochem. Biophys. Res. Commun. 420, 498–504 (2012).
Lee, S. et al. Renoprotective effect of COMP-angiopoietin-1 in db/db mice with type 2 diabetes. Nephrol. Dial. Transplant. 22, 396–408 (2007).
Tabata, M. et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 10, 178–188 (2009).
Horio, E. et al. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler. Thromb. Vasc. Biol. 34, 790–800 (2014).
Kersten, S. New insights into angiopoietin-like proteins in lipid metabolism and cardiovascular disease risk. Curr. Opin. Lipidol. 30, 205–211 (2019).
Romeo, S. et al. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 119, 70–79 (2009).
Aryal, B. et al. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight 3, 97918 (2018).
Lusis, A. J. et al. The Hybrid Mouse Diversity Panel: a resource for systems genetics analyses of metabolic and cardiovascular traits. J. Lipid Res. 57, 925–942 (2016).
Seldin, M. M. et al. A strategy for discovery of endocrine interactions with application to whole-body metabolism. Cell Metab. 27, 1138–1155.e1136 (2018).
Heron, M. Deaths: leading causes for 2010. Natl Vital Stat. Rep. 62, 1–96 (2013).
Goldstein, J. L. & Brown, M. S. A century of cholesterol and coronaries: from plaques to genes to statins. Cell 161, 161–172 (2015).
Libby, P. & Hansson, G. K. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ. Res. 116, 307–311 (2015).
Gisterå, A. & Hansson, G. K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 13, 368–380 (2017).
Lee, S. D. & Tontonoz, P. Liver X receptors at the intersection of lipid metabolism and atherogenesis. Atherosclerosis 242, 29–36 (2015).
Rahman, K. & Fisher, E. A. Insights from pre-clinical and clinical studies on the role of innate inflammation in atherosclerosis regression. Front. Cardiovasc. Med. 5, 32 (2018).
Farr, O. M., Li, C. R. & Mantzoros, C. S. Central nervous system regulation of eating: insights from human brain imaging. Metabolism 65, 699–713 (2016).
Soto, M., Cai, W., Konishi, M. & Kahn, C. R. Insulin signalling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc. Natl Acad. Sci. USA 116, 6379–6384 (2019).
He, Z. et al. Cellular and synaptic reorganization of arcuate NPY/AgRP and POMC neurons after exercise. Mol. Metab. 18, 107–119 (2018).
Tkach, M. & Théry, C. Communication by extracellular vesicles: where we are and where we need to go. Cell 164, 1226–1232 (2016).
Crewe, C. et al. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 175, 695–708.e613 (2018).
Chen, Y. et al. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat. Commun. 7, 11420 (2016).
Deng, Z. B. et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 58, 2498–2505 (2009).
Xie, Z. et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J. Am. Heart Assoc. 7, e007442 (2018).
Author information
Authors and Affiliations
Contributions
C.P. and P.T. prepared the original draft and revised the manuscript. C.P. prepared the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Elena Bellafante.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Priest, C., Tontonoz, P. Inter-organ cross-talk in metabolic syndrome. Nat Metab 1, 1177–1188 (2019). https://doi.org/10.1038/s42255-019-0145-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0145-5