Abstract
Probiotics have been reported to ameliorate symptoms of type 2 diabetes mellitus (T2DM) in animal models and human studies. We previously demonstrated that oral administration of Lactobacillus reuteri ADR-3 reduced insulin resistance in high-fructose-fed (HFD) rats. In the present study, we first identified another L. reuteri strain, ADR-1, which displayed anti-diabetes activity that reduced the levels of serum HbA1c and cholesterol and that increased antioxidant proteins in HFD rats. We further performed a randomized, double-blinded, placebo-controlled trial with a total of 68 T2DM patients to examine the beneficial effects of oral consumption of L. reuteri strains ADR-1 and ADR-3 and to investigate the associated changes in intestinal flora using a quantitative PCR method to analyze 16 S rRNA in fecal specimens. Significant reductions in HbA1c and serum cholesterol were observed in participants in the live ADR-1 consumption group (n = 22) after 3 months of intake when compared with those in the placebo group (n = 22). Although there was no significant difference in the HbA1c serum level among participants who consumed heat-killed ADR-3 (n = 24), the systolic blood pressure and mean blood pressure were significantly decreased after 6 months of intake. There was no obvious change in serum inflammatory cytokines or antioxidant proteins in participants after intaking ADR-1 or ADR-3, except for a reduction in IL-1β in the ADR-3 consumption group after 6 months of intake. With the analysis of fecal microflora, we found that L. reuteri or Bifidobacterium spp. were significantly increased in the ADR-1 and ADR-3 consumption groups, respectively, after 6 months of intake. Interestingly, a significant reduction in HbA1c was observed in the ADR-1 and ADR-3 consumption participants who displayed at least an 8-fold increase in fecal L. reuteri. We also observed that there was a significantly positive correlation between Bifidobacterium spp. and Lactobacillus spp. in participants with increased levels of fecal L. reuteri. In the ADR-1 intake group, the fecal Lactobacillus spp. level displayed a positive correlation with Bifidobacterium spp. but was negatively correlated with Bacteroidetes. The total level of fecal L. reuteri in participants in the ADR-3 consumption group was positively correlated with Firmicutes. In conclusion, L. reuteri strains ADR-1 and ADR-3 have beneficial effects on T2DM patients, and the consumption of different strains of L. reuteri may influence changes in intestinal flora, which may lead to different outcomes after probiotic intake.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder with peripheral insulin resistance and is the most common form of diabetes, with an almost 2-fold increased incidence in the past two decades1. The complications of T2DM include hypertension, nephropathy, neuropathy, skin infections and a high risk of blindness2. There were 1.6 million deaths directly caused by diabetes, and the World Health Organization predicts that diabetes will be the seventh leading cause of death in 20301. Consequently, there is an urgent need to develop functional health care products for regulating blood sugar.
Probiotics were defined by the Joint FAO/WHO Expert Consultation in 2002 as living microorganisms with health benefits to humans when administered in adequate amounts3. It has been demonstrated that probiotics have beneficial effects on several disorders; these beneficial effects include a reduction in allergic symptoms4,5, a decrease in serum cholesterol levels6, the prevention of obesity7 and improvement of the digestive system8,9. In T2DM studies, there have been several reports demonstrating that probiotics have beneficial effects on controlling blood sugar. For example, in pregnant women, daily consumption of probiotic yogurt consisting of two strains of Lactobacilli and Bifidobacteria for 9 weeks helped to maintain serum insulin levels10. In another study, after receiving Lactobacillus gasseri BNR17, db/db mice displayed an improvement in glucose sensitivity and amelioration of diabetic symptoms such as polydipsia, hyperplasia and polyuria11. We previously demonstrated that oral administration of Lactobacillus reuteri GNML-263 (ADR-3) reduced insulin resistance and hepatic steatosis formation in high-fructose-fed (HFD) rats, an animal model of T2DM12. This finding suggests that ADR-3 has the potential to be developed as a health care product for controlling T2DM complications.
In the present study, we further selected another L. reuteri strain, ADR-1, that has been demonstrated to have beneficial effects on HDF rats, such as reductions in serum HbA1c and liver injury after oral gavage for 14 weeks. In a double-blinded, randomized, placebo-controlled trial with a total of 68 patients who consumed live ADR-1 or heat-killed ADR-3 daily for 6 months, the results indicated that oral administration of ADR-1 significantly reduced HbA1c, whereas consumption of ADR-3 significantly decreased the systolic blood pressure (SBP) and mean blood pressure (MBP) after 6 months. The analysis of fecal microflora after consumption of ADR-1 or ADR-3 indicated that the different outcomes of ADR-1 or ADR-3 consumption in T2DM patients may be associated with changes in intestinal flora. Our data suggest that L. reuteri strains ADR-1 and ADR-3 have the potential to be developed as health care products for the management of T2DM.
Materials and Methods
Examining the beneficial effects of ADR-1 on a T2DM rat model
Male Sprague-Dawley rats were purchased from the National Laboratory Animal Center (Taipei, Taiwan) and divided into three groups: (1) the control group (n = 8), which consumed a standard 65% cornstarch diet (PMI Nutrition International, Brentwood, MO, USA); (2) the high-fructose-fed (HFD) group (n = 8), which consumed a 65% fructose diet (Harlan Laboratories, Inc., USA); and (3) the HFD with oral gavage of L. reuteri ADR-1 group (n = 8), which was simultaneously fed a 65% fructose diet and ADR-1 (2 × 109 CFU/rat) every day for 14 weeks. Blood samples were collected, and HbA1c, low-density lipoprotein-cholesterol (LDL), high- density lipoprotein-cholesterol (HDL), cholesterol (CHOL), triglycerides (TG), aspartate aminotransferase (AST), and alanine transaminase (ALT) were measured by the National Laboratory Animal Center Biological Laboratory (Taipei, Taiwan). A glutathione peroxidase assay kit (Cat# 703102, CAYMAN, USA) and superoxide dismutase assay kit (Cat# 706002, CAYMAN, USA) were used in this study. The animal experiments were approved by the Institutional Animal Care and Use Committee of GenMont Biotech Inc. (Tainan, Taiwan, IACUC No. AB10009-2) and performed in accordance with the relevant guidelines and regulations.
The recruitment of participants
A total of 86 people with T2DM were recruited for eligibility assessment through local advertisements, and 12 people were excluded (Fig. 2A). The recruitment criteria were as follows: T2DM diagnosis more than 6 months ago, age ranging from 25–70 years, a body-mass index (BMI) of more than 18.5; and an HbA1c level of 7% to 10%. The exclusion criteria were as follows: pregnancy; the presence of other diseases, including cancers (with the exception of well-controlled benign tumors), kidney failure/dialysis, heart diseases, stroke, or autoimmune diseases; the use of medications, including anti-diabetes drugs, and antibiotics, or other probiotic products <4 weeks before randomization; an AST/ALT level >3-fold the normal range; participation in other clinical trials; and the presence of other medical conditions that might jeopardize compliance with the protocol (e.g., malabsorption syndrome or an inability to take orally administered drugs). All the eligible participants were given verbal and written information about the study before being asked to sign a written consent form.
Study design
A 9-month double-blinded, randomized, placebo-controlled study was performed. The study was approved by the Ethics Committee of Changhua Christian Hospital with approval No. 140703 and performed in accordance with the relevant guidelines and regulations. The trial protocol is available in the supplementary materials. This study was registered at Clinical Trials.gov with the identifier NCT02274272 on Oct 24, 2014. At the first visit, participants underwent a baseline examination, including the recording of demographic characteristics and collection of fasting blood, urine, and stool samples. A total of 74 participants were double-blinded and randomized into 3 groups: live L. reuteri ADR-1 (n = 25), heat-killed L. reuteri ADR-3 (n = 25), and placebo (n = 24). In the ADR-1 and ADR-3 consumption groups, participants were orally administered 4 × 109 CFU of ADR-1 or 2 × 1010 cells of ADR-3 every day, respectively. ADR-1 and ADR-3 were given as capsules (2 × 109 CFU/capsule or 1 × 1010 cells/capsule for ADR-1 or ADR-3, respectively), which were provided by GenMont Biotech Inc. (Taiwan). The accuracy of L. reuteri ADR-1 (China Center for Type Culture Collection [CCTCC] number: M207154) and L. reuteri ADR-3 (CCTCC number: M209263) was confirmed by 16S rRNA sequencing and random amplified polymorphic DNA (RAPD) methods (Fig. S1). All participants were called back for the collection of fasting blood and urine samples at visit 2 (3 months), visit 3 (6 months), and visit 4 (orally administered probiotic washed out for 3 months). The poststudy stool samples of participants were collected at visit 3.
Laboratory and cytokine measurements
Fasting serum samples were used for biochemical analyses, including TG, CHOL, LDL, HDL, CHOL, AST, ALT, insulin, C-peptide, free fatty acid, BUN, CRE, eGFR, HbA1c, and glucose analyses, at Union Clinical Laboratory (Taiwan, TAF No. L1447-150325/CAP No. 6979606). The cytokine profiles were determined by enzyme-linked immunosorbent assay (ELISA) kits and performed at the Department of Life Sciences (Institute of Biomedical Science, National Chung Hsing University, Taichung, Taiwan). The ELISA kits included human IL-6 (Cat# 900-K16, PeproTech, USA), human IL-10 (Cat# 900-K21, PeproTech), human IL-17A (Cat# 900-K84, PeproTech), human TNF-α (Cat# 50-114-2609, eBioscience, USA), and human IL-1β (Cat# 437005, Biolegend, USA). A glutathione peroxidase assay kit (Cat# 703102, CAYMAN, USA) and superoxide dismutase assay kit (Cat# 706002, CAYMAN) were used in this study according to the manufacturers’ standard protocol.
Analysis of fecal microbiota compositions
All 180–220 mg stool samples were collected in RNAlaterTM (Thermo Fisher Scientific, Waltham, MA, USA)-containing tubes with autoclaved glass beads, and DNA was extracted with a QIAamp DNA Stool Mini Kit (QIAGEN, Hilden, Germany) after homogenization by MagNA Lyser (Roche Diagnostics Corporation, IN, USA). Furthermore, 2 ng of extracted DNA was used for analysis of the fecal microbiota composition by quantitative PCR, which was performed with a commercial Rotor-GeneTM SYBR Green PCR Kit (QIAGEN, Germany) and measured on a Rotor-Gene Q system (QIAGEN, Germany). The primer sets are listed in Table S1. The changes in specific bacteria were calculated by the 2−ΔΔCt method, in which ΔΔCt was [Ct(target bacteria at 6 months)-Ct(total bacteria at 6 months)]-[Ct(target bacteria at the starting time point)-Ct(total bacteria at the starting time point)].
Statistical analysis
All statistical analyses were performed by a private company (Bestat Pharma services corp., Taiwan) with SAS software version 9.4 (SAS Institute, Cary, NC). Baseline information was analyzed using the chi-square test or two-sample t-test. Medical histories, medication for diabetes, and hypertension in the different groups were analyzed using Fisher’s exact test. Comparisons of the change in results between the probiotic and placebo groups were analyzed using the two-sample t-test. Correlations between the variables were computed by Spearman’s rho correlation provided by PASW Statistics 18 Software (SPSS Inc., Chicago, IL, USA). A p value less than 0.05 was considered statistically significant.
Results
L. reuteri strain ADR-1 displayed beneficial effects in a T2DM rat model
We previously reported that oral administration of live L. reuteri ADR-3 improves insulin resistance and ameliorates hepatic steatosis in HFD rats12. In addition to L. reuteri ADR-3, we further examined if another L. reuteri strain, ADR-1, displays similar effects. With daily oral gavage of live L. reuteri ADR-1 at 2 × 109 CFU/rat, the serum HbA1c level in HFD rats was significantly reduced at the 8th week and the 14th week in comparison with that in the HFD only group (Fig. 1A). The levels of CHOL and TG and the ratio of LDL/HDL were significantly reduced in HFD rats receiving ADR-1 orally (Fig. 1B). The decreased serum levels of aspartate transaminase (AST) and alanine transaminase (ALT) demonstrated that liver injury in HFD rats was ameliorated by oral gavage of ADR-1 (Fig. 1C). Reductions in serum antioxidant proteins such as glutathione reductase (GPX) and superoxide dismutase (SOD) were also observed in HFD rats with oral administration of ADR-1 (Fig. 1D). These data indicate that L. reuteri ADR-1 has clinical potential for the management of T2DM.
The beneficial effects of ADR-1 in a T2DM rat model. Male Sprague-Dawley rats were divided into three groups as control (normal diet), HFD (high fructose diet with oral gavage of water), and HDF + ADR-1 (high fructose diet with oral gavage of ADR-1 as 2 × 109 cfu/rat/day). (A) The changes of HbA1c among groups were determined. Data was shown as mean±SD. *p < 0.05, comparing with HFD group. (B) The effects of ADR-1 in blood lipids of HFD rats. (C) The effect of ADR-1 in HDF induced liver injury was determined by serum AST/ALT activity. (D) The changes of hepatic antioxidant enzymes, glutathione reductase and superoxide dismutase (SOD) were determined. The value of control group was calculated as 100%. Data was shown as mean ± SD. *p < 0.05, comparing with HFD group.
A double-blinded, randomized, placebo-controlled trial of live L. reuteri ADR-1 and heat-killed L. reuteri ADR-3 in T2DM patients
We previously demonstrated that heat-killed ADR-3 displayed a similar effect to live ADR-3 in the improvement of metabolic function in high-fat diet induced obese rats13. Heat-killed probiotics have an advantage when used in subjects with immunodeficiency14. To understand if live ADR-1 or heat-killed ADR-3 has beneficial effects on T2DM patients, we conducted a 9-month, double-blinded, randomized, placebo-controlled trial (Clinical Trial Number at ClinicalTrials.gov: NCT02274272) as described in the Materials and Methods section. The study design is shown in Fig. 2, and the times of visits are indicated as V1 (baseline before trial), V2 (3 months after consumption), V3 (6 months after consumption), and V4 (3 months after stopping consumption). At V1, a total of 74 participants underwent a baseline examination to collect demographic data and fasting blood, urine and fecal samples and to measure blood pressure. A total of 6 participants discontinued the intervention or were lost to follow-up, while the other 68 participants completed the trial. However, one participant lost a fecal sample. The baseline information of the participants is listed in Table S1. There were no significant differences in the gender ratio, body weight, blood pressure, liver function (AST/ALT level), the HbA1c level (Table S2) and medication history (Table S3) among the groups at the beginning of the trial.
Recruitment of participants and the study design of a double blinded, randomized, and placebo controlled trial. (A) A total of 86 T2DM patients came for assessment of eligibility and excluded for 12 people. A total of 74 eligible participants was randomized divided into live L. reuteri ADR-1, heat-killed L. reuteri ADR-3, or placebo group. After exclusion for participates with loss of follow up or discontinued intervention, there was 46 or 22 analyzed participates for probiotics consumption or placebo group, respectively. (B) The probiotics consumption was performed a total of 6 months and wash out the probiotics for further 3month. All participates were requested to collect fasting blood and urine samples at 4 visits (V1: starting point, V2: 3 months after consumption, V3: 6 months after consumption, and V4: 3 months after stopping consumption) as indicated. The fecal samples were collected at V1 and V3.
The effects of live L. reuteri ADR-1 or heat-killed L. reuteri ADR-3 consumption on HbA1c and other metabolic markers
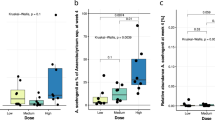
The HbA1c net change level in the L. reuteri ADR-1 consumption group was significantly reduced at V2, V3, and V4 (Table 1 and Fig. S2A, p = 0.0321 for V2, p = 0.0212 for V3, and p = 0.0285 for V4). These results indicated that participants who consumed L. reuteri ADR-1 for 6 months could maintain a stable HbA1c level for 3 months after stopping intake. For the comparison of HbA1c levels among the three groups at different times throughout the intervention, only participants from the live L. reuteri ADR-1 intake group displayed a decreased trend in the HbA1c level, but a significant difference was observed at only V4 (Fig. S2B, p = 0.0494). We did not observe any significant net change in HbA1c at any time points in the heat-killed L. reuteri ADR-3 consumption group (Table 1). The net changes in insulin, HOMA-IR, and fasting blood glucose (glucose-AC) among groups were not significant in comparison with those in the placebo group (Table 1). For other metabolic markers, we observed a trend in decreasing blood lipids in the ADR-1 consumption group. Reductions in LDL, cholesterol, and free fatty acids were observed in the ADR-1 consumption group at V2, although only cholesterol was significantly reduced (Table 1, p = 0.0467). On the other hand, there was no significant change in blood lipids for the ADR-3 consumption group, but significant reductions in systolic blood pressure (SBP) and mean blood pressure (MBP) were observed (Table 1, p = 0.0248 for SBP and p = 0.0254 for mean pressure). We also observed that bodyweight control in participants with obesity (body mass index greater than 27) was better in the ADR-3 consumption group (Fig. 3).
The effects of L. reuteri ADR-1 or ADR-3 consumption on inflammatory cytokines or antioxidant proteins
Epidemiologic studies have demonstrated a strong association between inflammatory and oxidative conditions and the occurrence of complications in T2DM patients15,16. Therefore, we next examined the changes in inflammatory cytokines and antioxidant proteins in the ADR-1 or ADR-3 consumption group in comparison with those in the placebo group. Among the inflammatory cytokines analyzed, only IL-1β displayed a significant reduction in the ADR-3 consumption group at V3 (Table 2, p = 0.0181). The SOD expression tended to be increased in the ADR-1 consumption group at V3 but did not reach a significant difference (Table 2, p = 0.0833).
The changes in intestinal flora in T2DM participants in the ADR-1 and ADR-3 consumption groups
It has been reported that the ratio of Bacteroidetes to Firmicutes is positively correlated with the serum glucose level of T2DM patients17. Metformin, an antidiabetic drug, may increase mucin-degrading Akkermansia muciniphila18,19. Bifidobacterium has been reported to be decreased in diabetic individuals20. Consequently, we were interested in using quantitative RT-PCR analysis to investigate the changes in intestinal flora, including L. reuteri, Lactobacillus spp., Bifidobacterium spp., A. muciniphila, Clostridium cluster I, Bacteroidetes, and Firmicutes, after the consumption of ADR-1 or ADR-3 by T2DM participants. We first found that there was an increased level of total L. reuteri in the ADR-1 and ADR-3 consumption groups in comparison with that in the placebo group, although a significant difference was only observed in the ADR-1 consumption group (Table 3, p = 0.017), and the increased total L. reuteri level in the ADR-1 consumption group was significantly higher than that in the ADR-3 consumption group (Table 3, p = 0.022). A significantly increased level of Bifidobacterium was observed in the ADR-3 consumption group (Table 3, p = 0.049). The levels of Lactobacillus spp., A. muciniphila, Clostridium cluster I, Bacteroidetes, and Firmicutes or the ratio of Bacteroidetes/Firmicutes showed no significant differences between the placebo and the ADR-1 or ADR-3 consumption group (Table 3). Correlation analysis was further used to identify the relationship between changes in microbiota and HbA1c changes. The net change in the HbA1c level was significantly negatively correlated with upregulation of total L. reuteri (Fig. 4A, p = 0.025) or Lactobacillus spp. (Fig. S3A, p = 0.044) and was positively correlated with the Bacteroidetes/Firmicutes ratio (Fig. S3B, p = 0.009). There was no significant correlation between the net change in the HbA1c level with total L. reuteri (Fig. 4B, p = 0.421), Lactobacillus spp. (Fig. S3C, p = 0.091), or the Bacteroidetes/Firmicutes ratio (Fig. S3D, p = 0.181) in participants with downregulation of total L. reuteri. Furthermore, in the ADR-1 consumption group with upregulated total L. reuteri (n = 16), the net change in the HbA1c level was significantly positively correlated with Bacteroidetes (Fig. S4A, p = 0.013) or the Bacteroidetes/Firmicutes ratio (Fig. S4B, p = 0.001). In the ADR-3 consumption group with upregulated total L. reuteri (n = 11), the net change in the HbA1c level was significantly negatively correlated with Lactobacillus spp. (Fig. S4E, p = 0.019). Interestingly, in all participants or in those with upregulated L. reuteri (n = 27), the reduction in the HbA1c level was observed in only those with a greater than 8-fold increase in upregulated L. reuteri (Fig. 4C, p = 0.034 or Fig. 4D, p = 0.033). In participants who consumed probiotics (n = 46), there was a significantly positive correlation between the total L. reuteri level and Lactobacillus spp. (Fig. 5A, p = 0.003). The level of Lactobacillus spp. was significantly and positively correlated with the Bifidobacterium spp. level in participants who consumed probiotics (Fig. 5B, p = 0.022). In participants with upregulated L. reuteri (n = 27), the level of Lactobacillus spp. was significantly and positively correlated with Bifidobacterium spp. (Fig. 5C, p = 0.005), and there was a negative correlation with the Bacteroidetes level (Fig. 5D, p = 0.056). In participants in the ADR-1 consumption group with upregulated L. reuteri levels (n = 16), the level of Lactobacillus spp. was significantly and positively correlated with Bifidobacterium spp. (Fig. S4C, p = 0.02) and was significantly and negatively correlated with Bacteroidetes (Fig. S4D, p = 0.023). In participants in the ADR-3 consumption group with upregulated L. reuteri levels (n = 11), there was a significant and positive correlation between Firmicutes and L. reuteri (Fig. S4F, p = 0.015). In summary, these results suggest that the degree of HbA1c reduction is affected by the level of L. reuteri upregulation in T2DM patients after ADR-1 or ADR-3 consumption and that there is a difference between ADR-1 and ADR-3 in terms of the change in intestinal flora that may lead to different outcomes for the regulation of blood sugar or T2DM-related complications.
The association between fecal L. reuteri level and the changes of HbA1c. The correlation between L. reuteri level and the change of HbA1c in participants with L. reuteri level up-regulated group (LR change>1) (A) or down-regulated group (LR change≤1) (B). The statistical analysis was calculated with Spearman’s rho correlation. (C,D) Analysis of HbA1c changes between participants with different level of upregulated L. reuteri (C), n = 27) or all cases involving in this trial (D), n = 67).
The changes of intestinal flora in probiotics consumption T2DM patients. The association between L. reuteri and Lactobacillus spp. (A) or Lactobacillus spp. and Bifidobacterium spp. (B) in probiotics consumption participants (ADR-1 or ADR-3, n = 46). The correlation between Lactobacillus and Bifidobacterium (C) or Lactobacillus spp. and Bacteriodetes (D) in participants with upregulated L. reuteri (ADR-1 or ADR-3 group, n = 27). The statistical analysis was calculated with Spearman’s rho correlation.
Discussion
In this study, we identified another L. reuteri strain, ADR-1, with beneficial effects on a T2DM rat model, and we conducted a double-blinded, randomized and placebo-controlled clinical trial to investigate the clinically beneficial effects of ADR-1 and ADR-3 on T2DM patients. The results revealed that oral consumption of ADR-1 or ADR-3 by T2DM patients was safe, without any adverse effects. Although there were two cases of severe adverse effects, these were not caused by the consumption of ADR-1 or ADR-3. Our data indicated that the consumption of ADR-1 displayed a reduction effect on serum HbA1c levels, and the intake of ADR-3 showed a beneficial effect on blood pressure reduction (Table 1). Based on previous studies, a reduction in the HbA1c level is often observed in probiotic consumption trials using a blend of probiotic genera, including Lactobacilli and Bifidobacteria21,22,23, but rare in trials using a single probiotic strain21,24. The results for ADR-1 suggest that it has great potential in T2DM management.
Another important finding of our study was the observation that the reduction in HbA1c levels was observed in only subjects with a greater than 8-fold increase in fecal L. reuteri (Fig. 4D). The difference in outcomes between ADR-1 and ADR-3 regarding the HbA1c level may have resulted from the preparation of these two L. reuteri strains: ADR-1 was supplied as a live organism but ADR-3 was supplied as a heat-killed organism. From our results, the greater increase in total fecal L. reuteri, which has been observed to be associated with glycemic control in germ-free mice25, was found in participants who consumed live ADR-1 (Table 3). Based on the RAPD and API-50 CHO tests (Fig. S1), it is clear that ADR-1 differs from ADR-3, although the BSH activity of these two strains was similar (Fig. S5). From these results, one suggestion is to increase the dosage of heat-killed ADR-3 that is consumed for glycemic control in T2DM patients. Additionally, a greater than 8-fold increase in the total fecal L. reuteri level may be used as an indicator for predicting the possible beneficial effects of probiotic products on T2DM management. Although the consumption of live probiotics products is generally considered safe for most populations, some side effects have been reported under certain conditions. For example, live probiotics may become pathogenic when used in subjects with severe immunodeficiency14. In infants with short bowel or cardiac stenosis, bacteremia has been reported in some cases26. Another advantage of heat-killed probiotics is that they are relatively easy to standardize. To develop heat-killed probiotics products is thus still worthwhile for the health care market.
The possible mechanisms of the beneficial effects of L. reuteri consumption on T2DM management might include (1) a reduction in the levels of pro-inflammatory cytokines, (2) the high bile salt hydrolase (BSH) activity of ADR-1 or ADR-3, and (3) the changes in intestinal microflora composition. It has been proposed that the intake of probiotics might improve the integrity of intestinal epithelium and diminish the Toll-like receptor 4 pathway to reduce pro-inflammatory signaling and to enhance insulin sensitivity27,28. In our study, the trend of IL-1β changes tended to be reduced after ADR-1 or ADR-3 consumption, although a significant difference was observed in only the ADR-3 consumption group at V3 (Table 2). Microbial bile salt hydrolase (BSH) has been demonstrated to increase the level of unconjugated bile acids and activate TGR5 to improve insulin sensitivity in animal studies29. We also found that ADR-1 or ADR-3 contained high BSH activity in comparison to the two reference strains, L. rhamnosus and L. plantarum (Fig. S5). On the other hand, microbial BSH has also been demonstrated to play a role in lowering cholesterol30. The high BSH activity of ADR-1 or ADR-3 may also be one of the mechanisms by which cholesterol levels are reduced in participants at the 3-month time point after consumption. However, the blood lipids profile is influenced by many factors, including diet, exercise, and stress31. To better understand the effect of ADR-1 on blood lipid management in T2DM patients requires another trial that is controlled for lifestyle factors. It has been demonstrated that administration of Bifidobacterium reduces serum lipid levels in high-fat-diet-induced obese rats32. In a human study, consumption of yogurt consisting of Bifidobacterium longum strain BL1 could reduce serum total cholesterol in subjects with moderate hypercholesterolemia33. Here, we hypothesize that the beneficial effects on the reduction in serum levels of HbA1c and lipids in T2DM patients in the ADR-1 consumption group could also be a result of increased levels of L. reuteri followed by the upregulation of Lactobacillus spp. and Bifidobacterium spp. but reduction in Bacteroidetes (Fig. 6A).
Hypothetical mechanisms of beneficial effect of ADR-1 or ADR-3 consumption in T2DM patients. (A) Intake of live ADR-1 could increase intestinal level of L. reuteri to further up-regulate Lactobacillus spp., and Bifidobacterium spp. and decrease Bacteroidetes. The changes of intestinal microflora may lead to the reduction of HbAlc and blood lipids. The reduction of blood lipids may also result from the up-regulation of serum SOD level. (B) Consumption of heat-killed could increase intestinal level of L. reuteri to further up-regulate Lactcobacillus spp. and Firmicutes which may let patients to control their HbAlc level. The increased level of Bifidobacterium spp. in ADR-3 intake participants may lead to the down-regulation of IL-1β to further reduce blood pressure.
Although the changes in HbA1c levels were not significant in the ADR-3 consumption group, decreased blood pressure, such as SBP and MBP, was observed. In intestinal flora analysis, only Bifidobacterium spp. was significantly increased in the ADR-3 consumption group. Indeed, we observed a significantly negative correlation between MBP and the Bifidobacterium level (Fig. S6) in all participants. Several reports have demonstrated that the consumption of probiotics may improve blood pressure, especially when the duration of consumption lasts for more than 8 weeks or when the daily consumption dose is more than 1011 CFU34. On the other hand, IL-1β has been known to be elevated in individuals with hypertension, which leads to renal and vascular inflammation35. It also has been demonstrated that metabolites from Bifidobacterium infantis displayed anti-inflammatory activity to reduce IL-6 or IL-8 production in IL-1β-stimulated human enterocytes36. Additionally, it has been reported that fermented milk or a mixture of probiotics called VSL#3 displayed an anti-hypertension effect. In one study, a fermented milk with three different Lactobacillus strains displayed an in vitro inhibitory effect on angiotensin-converting enzyme (ACE inhibitor), a key enzyme in blood pressure control37. Furthermore, treatment with VSL#3, which contains Streptococcus thermophilus, B. longum, B. breve, B. infantis, L. acidophilus, L. plantarum, L. casei, and L. bulgaricus, prevented endothelial dysfunction in a rat model of hypertension through the improvement of vascular oxidative stress and reduction in the levels of TNF-α, IL-1α and MCP-1 in plasma38. Our data regarding the beneficial effect of ADR-3 on reductions in SBP and MBP is the first report to use a single Lactobacillus strain to potentially achieve blood pressure control. Our results also suggest that the downregulation of IL-1β through the increased intestinal Bifidobacterium spp. level after ADR-3 consumption may lead to the control of blood pressure in T2DM patients (Fig. 6B).
In conclusion, the results from our double-blinded, randomized, placebo-controlled trial demonstrate that the L. reuteri strains ADR-1 and ADR-3 have beneficial effects on T2DM patients. The changes in intestinal flora after the consumption of different L. reuteri strains may influence the outcomes. The fecal L. reuteri level after ADR-1 or ADR-3 consumption may thus be used as a predictor of reduced HbA1c levels in T2DM patients in the future.
Change history
26 February 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Organization, W. H. Global report on diabetes. 88 (2016).
Cefalu, W. T. & Ratner, R. E. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: the “gift” that keeps on giving! Diabetes Care 37, 5–7, https://doi.org/10.2337/dc13-2369 (2014).
(WHO)., F. a. A. O. F. W. H. O. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria (2001).
Cuello-Garcia, C. A. et al. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol 136, 952–961, https://doi.org/10.1016/j.jaci.2015.04.031 (2015).
Pelucchi, C. et al. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology 23, 402–414, https://doi.org/10.1097/EDE.0b013e31824d5da2 (2012).
Guo, Z. et al. Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis 21, 844–850, https://doi.org/10.1016/j.numecd.2011.04.008 (2011).
Yoo, S. R. et al. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity (Silver Spring) 21, 2571–2578, https://doi.org/10.1002/oby.20428 (2013).
Hempel, S. et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA 307, 1959–1969, https://doi.org/10.1001/jama.2012.3507 (2012).
Johnston, B. C. et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med 157, 878–888, https://doi.org/10.7326/0003-4819-157-12-201212180-00563 (2012).
Asemi, Z. et al. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. Eur J Clin Nutr 67, 71–74, https://doi.org/10.1038/ejcn.2012.189 (2013).
Yun, S. I., Park, H. O. & Kang, J. H. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol 107, 1681–1686, https://doi.org/10.1111/j.1365-2672.2009.04350.x (2009).
Hsieh, F. C. et al. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr Metab (Lond) 10, 35, https://doi.org/10.1186/1743-7075-10-35 (2013).
Hsieh, F. C. et al. Heat-killed and live Lactobacillus reuteri GMNL-263 exhibit similar effects on improving metabolic functions in high-fat diet-induced obese rats. Food Funct 7, 2374–2388, https://doi.org/10.1039/c5fo01396h (2016).
Adams, C. A. The probiotic paradox: live and dead cells are biological response modifiers. Nutr Res Rev 23, 37–46, https://doi.org/10.1017/S0954422410000090 (2010).
Akash, M. S., Rehman, K. & Chen, S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem 114, 525–531, https://doi.org/10.1002/jcb.24402 (2013).
Rochette, L., Zeller, M., Cottin, Y. & Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta 1840, 2709–2729, https://doi.org/10.1016/j.bbagen.2014.05.017 (2014).
Larsen, N. et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5, e9085, https://doi.org/10.1371/journal.pone.0009085 (2010).
de la Cuesta-Zuluaga, J. et al. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care 40, 54–62, https://doi.org/10.2337/dc16-1324 (2017).
Shin, N. R. et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63, 727–735, https://doi.org/10.1136/gutjnl-2012-303839 (2014).
Gomes, A. C., Bueno, A. A., de Souza, R. G. & Mota, J. F. Gut microbiota, probiotics and diabetes. Nutr J 13, 60, https://doi.org/10.1186/1475-2891-13-60 (2014).
Andreasen, A. S. et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr 104, 1831–1838, https://doi.org/10.1017/S0007114510002874 (2010).
Ejtahed, H. S. et al. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 28, 539–543, https://doi.org/10.1016/j.nut.2011.08.013 (2012).
Mohamadshahi, M. et al. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 4, 83–88, https://doi.org/10.5681/bi.2014.007 (2014).
Mobini, R. et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes Metab 19, 579–589, https://doi.org/10.1111/dom.12861 (2017).
Suez, J. et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514, 181–186, https://doi.org/10.1038/nature13793 (2014).
Didari, T., Solki, S., Mozaffari, S., Nikfar, S. & Abdollahi, M. A systematic review of the safety of probiotics. Expert Opin Drug Saf 13, 227–239, https://doi.org/10.1517/14740338.2014.872627 (2014).
Lee, S. K., Yang, K. M., Cheon, J. H., Kim, T. I. & Kim, W. H. [Anti-inflammatory mechanism of Lactobacillus rhamnosus GG in lipopolysaccharide- stimulated HT-29 cell]. Korean J Gastroenterol 60, 86–93 (2012).
Amar, J. et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 3, 559–572, https://doi.org/10.1002/emmm.201100159 (2011).
Duboc, H., Tache, Y. & Hofmann, A. F. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis 46, 302–312, https://doi.org/10.1016/j.dld.2013.10.021 (2014).
Choi, S. B., Lew, L. C., Yeo, S. K., Nair Parvathy, S. & Liong, M. T. Probiotics and the BSH-related cholesterol lowering mechanism: a Jekyll and Hyde scenario. Crit Rev Biotechnol 35, 392–401, https://doi.org/10.3109/07388551.2014.889077 (2015).
Shephard, R. J., Cox, M. & West, C. Some factors influencing serum lipid levels in a working population. Atherosclerosis 35, 287–300 (1980).
An, H. M. et al. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis 10, 116, https://doi.org/10.1186/1476-511X-10-116 (2011).
Xiao, J. Z. et al. Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J Dairy Sci 86, 2452–2461, https://doi.org/10.3168/jds.S0022-0302(03)73839-9 (2003).
Khalesi, S., Sun, J., Buys, N. & Jayasinghe, R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 64, 897–903, https://doi.org/10.1161/HYPERTENSIONAHA.114.03469 (2014).
Krishnan, S. M., Sobey, C. G., Latz, E., Mansell, A. & Drummond, G. R. IL-1beta and IL-18: inflammatory markers or mediators of hypertension? Br J Pharmacol 171, 5589–5602, https://doi.org/10.1111/bph.12876 (2014).
Guo, S. et al. Secreted Metabolites of Bifidobacterium infantis and Lactobacillus acidophilus Protect Immature Human Enterocytes from IL-1beta-Induced Inflammation: A Transcription Profiling Analysis. PLoS One 10, e0124549, https://doi.org/10.1371/journal.pone.0124549 (2015).
Chen, Y. et al. Angiotensin-converting enzyme inhibitory activity of Lactobacillus helveticus strains from traditional fermented dairy foods and antihypertensive effect of fermented milk of strain H9. J Dairy Sci 97, 6680–6692, https://doi.org/10.3168/jds.2014-7962 (2014).
Rashid, S. K. et al. Probiotics (VSL#3) prevent endothelial dysfunction in rats with portal hypertension: role of the angiotensin system. PLoS One 9, e97458, https://doi.org/10.1371/journal.pone.0097458 (2014).
Acknowledgements
This research was supported by the Small Business Innovation Research (SBIR), Ministry of Economic Affairs (MOEA), Taiwan, R.O.C. under contract 1Z1030713.
Author information
Authors and Affiliations
Contributions
M.C.H., W.H.T. and Y.H.C. contributed to the concept and design of the study. W.H.T. and Y.H.C. conducted the animal study. M.C.H., Y.P.J., S.L.S. and S.Y.W. conducted the clinical study and collected the data. C.C.L. performed the serum cytokine determination. M.C.H., W.H.T., Y.H.C. and W.W.C. performed the data analysis and interpretation. W.H.T. and W.W.C. wrote the draft.
Corresponding authors
Ethics declarations
Competing Interests
This work is supported by GenMont Biotech Incorporation. W.H.T. and Y.H.C. are employed by GenMont Biotech Incorporation. M.C.H., Y.P.J., S.L.S., S.Y.W., C.C.L. and W.W.C. have no competing interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hsieh, MC., Tsai, WH., Jheng, YP. et al. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep 8, 16791 (2018). https://doi.org/10.1038/s41598-018-35014-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35014-1
Keywords
This article is cited by
-
Comparison of the efficacy of fish oil and probiotic supplementation on glucose and lipid metabolism in patients with type 2 diabetes: a systematic review and network meta-analysis
Diabetology & Metabolic Syndrome (2024)
-
Gut metagenomes of Asian octogenarians reveal metabolic potential expansion and distinct microbial species associated with aging phenotypes
Nature Communications (2024)
-
The effects of probiotics supplementation on glycaemic control among adults with type 2 diabetes mellitus: a systematic review and meta-analysis of randomised clinical trials
Journal of Translational Medicine (2023)
-
Beyond probiotics: a narrative review on an era of revolution
Food Science and Biotechnology (2023)
-
The effects of probiotic and synbiotic supplementation on inflammation, oxidative stress, and circulating adiponectin and leptin concentration in subjects with prediabetes and type 2 diabetes mellitus: a GRADE-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials
European Journal of Nutrition (2022)