Abstract
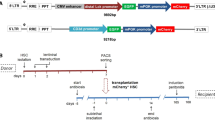
Receptor targeting of vector particles is a key technology to enable cell type–specific in vivo gene delivery. For example, T cells in humanized mouse models can be modified by lentiviral vectors (LVs) targeted to human T-cell markers to enable them to express chimeric antigen receptors (CARs). Here, we provide detailed protocols for the generation of CD4- and CD8-targeted LVs (which takes ~9 d in total). We also describe how to humanize immunodeficient mice with hematopoietic stem cells (which takes 12–16 weeks) and precondition (over 5 d) and administer the vector stocks. Conversion of the targeted cell type is monitored by PCR and flow cytometry of blood samples. A few weeks after administration, ~10% of the targeted T-cell subtype can be expected to have converted to CAR T cells. By closely following the protocol, sufficient vector stock for the genetic manipulation of 10–15 humanized mice is obtained. We also discuss how the protocol can be easily adapted to use LVs targeted to other types of receptors and/or for delivery of other genes of interest.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Code availability
Two NTA software scripts, the continuous flow script and the stop flow script, have been deposited in figshare (10.6084/m9.figshare.13221602) and are available as Supplementary Information.
References
Radek, C. et al. Vectofusin-1 improves transduction of primary human cells with diverse retroviral and lentiviral pseudotypes, enabling robust, automated closed-system manufacturing. Hum. Gene Ther. 30, 1477–1493 (2019).
Zhang, Z., Qiu, S., Zhang, X. & Chen, W. Optimized DNA electroporation for primary human T cell engineering. BMC Biotechnol. 18, 4 (2018).
Dayball, K., Millar, J., Miller, M., Wan, Y. H. & Bramson, J. Electroporation enables plasmid vaccines to elicit CD8+ T cell responses in the absence of CD4+ T cells. J. Immunol. 171, 3379–3384 (2003).
Naldini, L. Gene therapy returns to centre stage. Nature 526, 351–360 (2015).
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Gattinoni, L., Klebanoff, C. A. & Restifo, N. P. Paths to stemness: building the ultimate antitumour T cell. Nat. Rev. Cancer 12, 671–684 (2012).
Fraietta, J. A. et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 24, 563–571 (2018).
Xu, Y. et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123, 3750–3759 (2014).
Frank, A. M. & Buchholz, C. J. Surface-engineered lentiviral vectors for selective gene transfer into subtypes of lymphocytes. Mol. Ther. Methods Clin. Dev. 12, 19–31 (2018).
Agarwal, S., Weidner, T., Thalheimer, F. B. & Buchholz, C. J. In vivo generated human CAR T cells eradicate tumor cells. Oncoimmunology 8, e1671761 (2019).
Pfeiffer, A. et al. In vivo generation of human CD19-CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol. Med. 10, e9158 (2018).
Agarwal, S. et al. In vivo generation of CAR T cells selectively in human CD4+ lymphocytes. Mol. Ther. 28, 1783––1794 (2020).
Buchholz, C. J., Friedel, T. & Büning, H. Surface-engineered viral vectors for selective and cell type-specific gene delivery. Trends Biotechnol. 33, 777–790 (2015).
Funke, S. et al. Targeted cell entry of lentiviral vectors. Mol. Ther. 16, 1427–1436 (2008).
Anliker, B., Longhurst, S. & Buchholz, C. J. Environmental risk assessment for medicinal products containing genetically modified organisms. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 53, 52–57 (2010).
Zhou, Q. et al. T-cell receptor gene transfer exclusively to human CD8+ cells enhances tumor cell killing. Blood 120, 4334–4342 (2012).
Zhou, Q. et al. Exclusive transduction of human CD4+ T cells upon systemic delivery of CD4-targeted lentiviral vectors. J. Immunol. 195, 2493–2501 (2015).
Frank, A. M. et al. CD8-specific DARPins improve selective gene delivery into human and primate T lymphocytes. Hum. Gene Ther. 31, 679–691 (2020).
Bender, R. R. et al. Receptor-targeted Nipah virus glycoproteins improve cell-type selective gene delivery and reveal a preference for membrane-proximal cell attachment. PLoS Pathog. 12, e1005641 (2016).
Weidner, T. et al. Genetic in vivo engineering of human T lymphocytes in mouse models. Preprint at https://protocolexchange.researchsquare.com/article/pex-1282/v1 (2020).
Seif, M., Einsele, H. & Löffler, J. CAR T cells beyond cancer: hope for immunomodulatory therapy of infectious diseases. Front. Immunol. 10, 2711 (2019).
Akkina, R. New generation humanized mice for virus research: comparative aspects and future prospects. Virology 435, 14–28 (2013).
Ito, M. et al. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100, 3175–3182 (2002).
Hiramatsu, H. et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/γcnull mice model. Blood 102, 873–880 (2003).
Ishikawa, F. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chain null mice. Blood 106, 1565–1573 (2005).
Shultz, L. D. et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγnull mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174, 6477–6489 (2005).
Anthony-Gonda, K. et al. Multispecific anti-HIV duoCAR-T cells display broad in vitro antiviral activity and potent in vivo elimination of HIV-infected cells in a humanized mouse model. Sci. Transl. Med. 11, eaav5685 (2019).
Kitchen, S. G. & Zack, J. A. Engineering HIV-specific immunity with chimeric antigen receptors. AIDS Patient Care STDs 30, 556–561 (2016).
Borsotti, C., Borroni, E. & Follenzi, A. Lentiviral vector interactions with the host cell. Curr. Opin. Virol. 21, 102–108 (2016).
Morizono, K. et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat. Med. 11, 346–352 (2005).
Liang, M. et al. Targeted transduction via CD4 by a lentiviral vector uses a clathrin-mediated entry pathway. J. Virol. 83, 13026–13031 (2009).
Yang, H., Joo, K.-I., Ziegler, L. & Wang, P. Cell type-specific targeting with surface-engineered lentiviral vectors co-displaying OKT3 antibody and fusogenic molecule. Pharm. Res. 26, 1432–1445 (2009).
Jamali, A. et al. Highly efficient and selective CAR-gene transfer using CD4- and CD8-targeted lentiviral vectors. Mol. Ther. Methods Clin. Dev. 13, 371–379 (2019).
Ruella, M. et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 24, 1499–1503 (2018).
Navaratnarajah, C. K., Generous, A. R., Yousaf, I. & Cattaneo, R. Receptor-mediated cell entry of paramyxoviruses: mechanisms, and consequences for tropism and pathogenesis. J. Biol. Chem. 295, 2771–2786 (2020).
Rasbach, A. et al. The receptor attachment function of measles virus hemagglutinin can be replaced with an autonomous protein that binds Her2/neu while maintaining its fusion-helper function. J. Virol. 87, 6246–6256 (2013).
Frank, A. M. et al. Combining T-cell specific activation and in vivo gene delivery through CD3-targeted lentiviral vectors. Blood Adv. 4, 5702–5715 (2020).
Smith, T. T. et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 12, 813–820 (2017).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 (2007).
Manz, M. G. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity 26, 537–541 (2007).
Yahata, T. et al. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor γ null mice. J. Immunol. 169, 204–209 (2002).
Mhaidly, R. & Verhoeyen, E. Humanized mice are precious tools for preclinical evaluation of CAR T and CAR NK cell therapies. Cancers 12, 1915 (2020).
Brendel, C., Rio, P. & Verhoeyen, E. Humanized mice are precious tools for evaluation of hematopoietic gene therapies and preclinical modeling to move towards a clinical trial. Biochem. Pharmacol. 174, 113711 (2020).
Tanaka, S. et al. Development of mature and functional human myeloid subsets in hematopoietic stem cell-engrafted NOD/SCID/IL2rγKO mice. J. Immunol. 188, 6145–6155 (2012).
Huntington, N. D. et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J. Exp. Med. 206, 25–34 (2009).
Cosgun, K. N. et al. Kit regulates HSC engraftment across the human-mouse species barrier. Cell Stem Cell 15, 227–238 (2014).
McIntosh, B. E. et al. Nonirradiated NOD,B6.SCID Il2rγ−/− KitW41/W41 (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Rep. 4, 171–180 (2015).
Yurino, A. et al. Enhanced reconstitution of human erythropoiesis and thrombopoiesis in an immunodeficient mouse model with KitWv mutations. Stem Cell Rep. 7, 425–438 (2016).
Miller, P. H. et al. Analysis of parameters that affect human hematopoietic cell outputs in mutant c-kit-immunodeficient mice. Exp. Hematol. 48, 41–49 (2017).
Rongvaux, A. et al. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu. Rev. Immunol. 31, 635–674 (2013).
Traggiai, E. et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304, 104–107 (2004).
Covassin, L. et al. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rγnull (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin. Exp. Immunol. 174, 372–388 (2013).
Smith, D. J. et al. Propagating humanized BLT mice for the study of human immunology and immunotherapy. Stem Cells Dev. 25, 1863–1873 (2016).
Münch, R. C. et al. DARPins: an efficient targeting domain for lentiviral vectors. Mol. Ther. 19, 686–693 (2011).
Goujon, C. et al. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology 4, 2 (2007).
Berger, G. et al. A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1–derived lentiviral vectors. Nat. Protoc. 6, 806–816 (2011).
Friedel, T. et al. Receptor-targeted lentiviral vectors are exceptionally sensitive toward the biophysical properties of the displayed single-chain Fv. Protein Eng. Des. Sel. 28, 93–106 (2015).
Zufferey, R., Nagy, D., Mandel, R. J., Naldini, L. & Trono, D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15, 871–875 (1997).
Folks, T. et al. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc. Natl Acad. Sci. USA 82, 4539–4543 (1985).
Hartmann, J. et al. A library-based screening strategy for the identification of DARPins as ligands for receptor-targeted AAV and lentiviral vectors. Mol. Ther. Methods Clin. Dev. 10, 128–143 (2018).
Acknowledgements
This work was supported by grants from the NIH (grant number 1 R01 AI145045-01) and Health Holland (LentiPace II) to C.J.B. The authors acknowledge continous support by the Federal Ministry of Health (03292364) to C.J.B. The authors thank the staff of the Plateau de Biologie Expérimentale de la Souris (PBES) animal care facility at the Ecole Normale Supérieure de Lyon and the flow cytometry platform (SFR BioSciences Lyon) (UMS3444/US8), Lyon, France.
Author information
Authors and Affiliations
Contributions
T.W. drafted the protocol on LV vector stock generation and designed figures. S.A. drafted the protocol on CAR T cell detection, and S.P. and F.F. drafted the protocol on mouse humanization. G.B. and J.H. contributed to the writing of the manuscript. C.J.B. and E.V. supervised work, drafted the general parts and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
E.V. and C.J.B are listed as inventors on patents on receptor-targeted LVs that have been licensed out. All other authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Bryan Strauss and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Additional initial assessment was performed by informal referee Stephen Gottschalk.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Pfeiffer, A. et al. EMBO Mol. Med. 10, e9158 (2018): https://doi.org/10.15252/emmm.201809158
Agarwal, S., Weidner, T., Thalheimer, F. B. & Buchholz, C. J. Oncoimmunology 8, e1671761 (2019): https://doi.org/10.1080/2162402X.2019.1671761
Agarwal, S. et al. Mol. Ther. 28, 1783–1794 (2020): https://doi.org/10.1016/j.ymthe.2020.05.005
Supplementary information
Supplementary Data 1
These scripts can be copied into the script panel of the NTA software. There are two options. The continuous flow script should be used when a syringe pump is in place (‘NTA_Script_Continous_Flow’), whereas the stop flow script (‘NTA_Script_Stop_Flow’) should be used in its absence.
Source data
Source Data Fig. 4
Data provided in Fig. 4D displayed as individual data points, showing titers (t.u./ml) of each LV stock obtained by incubation with target cells.
Source Data Fig. 5
Data shown in Fig. 5, B and C displayed as individual data points, showing the percentage of hCD45+ cells (B) and the percentage of CD19+ or CD3+ of hCD45+ cells (C) for each mouse and time point, respectively.
Rights and permissions
About this article
Cite this article
Weidner, T., Agarwal, S., Perian, S. et al. Genetic in vivo engineering of human T lymphocytes in mouse models. Nat Protoc 16, 3210–3240 (2021). https://doi.org/10.1038/s41596-021-00510-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00510-8