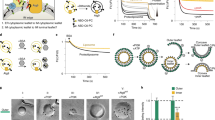
Abstract
De novo formation of the double-membrane compartment autophagosome is seeded by small vesicles carrying membrane protein autophagy-related 9 (ATG9), the function of which remains unknown. Here we find that ATG9A scrambles phospholipids of membranes in vitro. Cryo-EM structures of human ATG9A reveal a trimer with a solvated central pore, which is connected laterally to the cytosol through the cavity within each protomer. Similarities to ABC exporters suggest that ATG9A could be a transporter that uses the central pore to function. Moreover, molecular dynamics simulation suggests that the central pore opens laterally to accommodate lipid headgroups, thereby enabling lipids to flip. Mutations in the pore reduce scrambling activity and yield markedly smaller autophagosomes, indicating that lipid scrambling by ATG9A is essential for membrane expansion. We propose ATG9A acts as a membrane-embedded funnel to facilitate lipid flipping and to redistribute lipids added to the outer leaflet of ATG9 vesicles, thereby enabling growth into autophagosomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Cryo-EM density maps and atomic coordinates for human ATG9A have been deposited in the Electron Microscopy Data Bank and wwPDB, respectively, under accession codes EMD-22375 and PDB 7JLO (amphipol); EMD-22376 and PDB 7JLP (nanodisc); and EMD-22377 and PDB 7JLQ (LMNG). Source data are provided with this paper.
References
Levine, B. & Kroemer, G. Biological functions of autophagy genes: a disease perspective. Cell 176, 11–42 (2019).
Jiang, P. & Mizushima, N. Autophagy and human diseases. Cell Res. 24, 69–79 (2014).
Lamb, C. A., Yoshimori, T. & Tooze, S. A. The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 14, 759–774 (2013).
Yamamoto, H. et al. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J. Cell Biol. 198, 219–233 (2012).
De Tito, S., Hervas, J. H., van Vliet, A. R. & Tooze, S. A. The Golgi as an assembly line to the autophagosome. Trends Biochem. Sci. 45, 484–496 (2020).
Orsi, A. et al. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Mol. Biol. Cell 23, 1860–1873 (2012).
Ohashi, Y. & Munro, S. Membrane delivery to the yeast autophagosome from the Golgi-endosomal system. Mol. Biol. Cell 21, 3998–4008 (2010).
Mari, M. et al. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J. Cell Biol. 190, 1005–1022 (2010).
Zhuang, X. et al. ATG9 regulates autophagosome progression from the endoplasmic reticulum in Arabidopsis. Proc. Natl Acad. Sci. USA 114, E426–E435 (2017).
Karanasios, E. et al. Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles. Nat. Commun. 7, 12420 (2016).
Itakura, E., Kishi-Itakura, C., Koyama-Honda, I. & Mizushima, N. Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J. Cell Sci. 125, 1488–1499 (2012).
Koyama-Honda, I., Itakura, E., Fujiwara, T. K. & Mizushima, N. Temporal analysis of recruitment of mammalian ATG proteins to the autophagosome formation site. Autophagy 9, 1491–1499 (2013).
Kakuta, S. et al. Small GTPase Rab1B is associated with ATG9A vesicles and regulates autophagosome formation. FASEB J. 31, 3757–3773 (2017).
Imai, K. et al. Atg9A trafficking through the recycling endosomes is required for autophagosome formation. J. Cell Sci. 129, 3781–3791 (2016).
Suzuki, K., Akioka, M., Kondo-Kakuta, C., Yamamoto, H. & Ohsumi, Y. Fine mapping of autophagy-related proteins during autophagosome formation in Saccharomyces cerevisiae. J. Cell Sci. 126, 2534–2544 (2013).
Graef, M., Friedman, J. R., Graham, C., Babu, M. & Nunnari, J. ER exit sites are physical and functional core autophagosome biogenesis components. Mol. Biol. Cell 24, 2918–2931 (2013).
Gomez-Sanchez, R. et al. Atg9 establishes Atg2-dependent contact sites between the endoplasmic reticulum and phagophores. J. Cell Biol. 217, 2743–2763 (2018).
Mizushima, N. et al. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 152, 657–668 (2001).
Nishimura, T. et al. Autophagosome formation is initiated at phosphatidylinositol synthase-enriched ER subdomains. EMBO J. 36, 1719–1735 (2017).
Maeda, S., Otomo, C. & Otomo, T. The autophagic membrane tether ATG2A transfers lipids between membranes. Elife 8, e45777 (2019).
Valverde, D. P. et al. ATG2 transports lipids to promote autophagosome biogenesis. J. Cell Biol. 218, 1787–1798 (2019).
Osawa, T. et al. Atg2 mediates direct lipid transfer between membranes for autophagosome formation. Nat. Struct. Mol. Biol. 26, 281–288 (2019).
Young, A. R. et al. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J. Cell Sci. 119, 3888–3900 (2006).
He, C. et al. Recruitment of Atg9 to the preautophagosomal structure by Atg11 is essential for selective autophagy in budding yeast. J. Cell Biol. 175, 925–935 (2006).
Lai, L. T. F. et al. Subnanometer resolution cryo-EM structure of Arabidopsis thaliana ATG9. Autophagy 16, 575–583 (2020).
He, C., Baba, M., Cao, Y. & Klionsky, D. J. Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy. Mol. Biol. Cell 19, 5506–5516 (2008).
Aryal, P., Sansom, M. S. & Tucker, S. J. Hydrophobic gating in ion channels. J. Mol. Biol. 427, 121–130 (2015).
Entova, S., Billod, J. M., Swiecicki, J. M., Martin-Santamaria, S. & Imperiali, B. Insights into the key determinants of membrane protein topology enable the identification of new monotopic folds. Elife 7, e40889 (2018).
Schaeffer, R. D. et al. ECOD: identification of distant homology among multidomain and transmembrane domain proteins. BMC Mol. Cell Biol. 20, 18 (2019).
Ho, H. et al. Structural basis for dual-mode inhibition of the ABC transporter MsbA. Nature 557, 196–201 (2018).
Choudhury, H. G. et al. Structure of an antibacterial peptide ATP-binding cassette transporter in a novel outward occluded state. Proc. Natl Acad. Sci. USA 111, 9145–9150 (2014).
Wang, Z., Hu, W. & Zheng, H. Pathogenic siderophore ABC importer YbtPQ adopts a surprising fold of exporter. Sci. Adv. 6, eaay7997 (2020).
Johnson, Z. L. & Chen, J. Structural basis of substrate recognition by the multidrug resistance protein MRP1. Cell 168, 1075–1085.e9 (2017).
Malvezzi, M. et al. Ca2+-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nat. Commun. 4, 2367 (2013).
Menon, I. et al. Opsin is a phospholipid flippase. Curr. Biol. 21, 149–153 (2011).
Chang, Q. L., Gummadi, S. N. & Menon, A. K. Chemical modification identifies two populations of glycerophospholipid flippase in rat liver ER. Biochemistry 43, 10710–10718 (2004).
Kaizuka, T. et al. An autophagic flux probe that releases an internal control. Mol. Cell 64, 835–849 (2016).
Sun, D., Wu, R., Zheng, J., Li, P. & Yu, L. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 28, 405–415 (2018).
Brunner, J. D., Lim, N. K., Schenck, S., Duerst, A. & Dutzler, R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 516, 207–212 (2014).
Lee, B. C. et al. Gating mechanism of the extracellular entry to the lipid pathway in a TMEM16 scramblase. Nat. Commun. 9, 3251 (2018).
Guardia, C. M. et al. Structure of human ATG9A, the only transmembrane protein of the core autophagy machinery. Cell Reports 31, 107837 (2020).
Feng, S. et al. Cryo-EM studies of TMEM16F calcium-activated ion channel suggest features important for lipid scrambling. Cell Rep. 28, 1385 (2019).
Falzone, M. E. et al. Structural basis of Ca2+-dependent activation and lipid transport by a TMEM16 scramblase. Elife 8, e43229 (2019).
Herzik, M. A. Jr., Wu, M. & Lander, G. C. Achieving better-than-3-Å resolution by single-particle cryo-EM at 200 keV. Nat. Methods 14, 1075–1078 (2017).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Lander, G. C. et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J. Struct. Biol. 166, 95–102 (2009).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, e42166 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Lomize, M. A., Pogozheva, I. D., Joo, H., Mosberg, H. I. & Lomize, A. L. OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 40, D370–D376 (2012).
Baker, N. A., Sept, D., Joseph, S., Holst, M. J. & McCammon, J. A. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl Acad. Sci. USA 98, 10037–10041 (2001).
Klesse, G., Rao, S., Sansom, M. S. P. & Tucker, S. J. CHAP: a versatile tool for the structural and functional annotation of ion channel pores. J. Mol. Biol. 431, 3353–3365 (2019).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Holm, L. DALI and the persistence of protein shape. Protein Sci. 29, 128–140 (2020).
Li, Z., Jaroszewski, L., Iyer, M., Sedova, M. & Godzik, A. FATCAT 2.0: towards a better understanding of the structural diversity of proteins. Nucleic Acids Res. 48, W60–W64 (2020).
Zimmermann, L. et al. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 430, 2237–2243 (2018).
Dolinsky, T. J., Nielsen, J. E., McCammon, J. A. & Baker, N. A. PDB2PQR: an automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res. 32, W665–W667 (2004).
Lee, J. et al. CHARMM-GUI membrane builder for complex biological membrane simulations with glycolipids and lipoglycans. J. Chem. Theory Comput. 15, 775–786 (2019).
Lee, J. et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 12, 405–413 (2016).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Adelman, S. A. & Doll, J. D. Generalized Langevin equation approach for atom/solid-surface scattering: collinear atom/harmonic chain model. J. Chem. Phys. 61, 4242–4245 (1974).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N.Log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph 14, 33–38 (1996).
Falzone, M. E. & Accardi, A. Reconstitution of proteoliposomes for phospholipid scrambling and nonselective channel assays. Methods Mol. Biol. 2127, 207–225 (2020).
Brunner, J. D. & Schenck, S. Preparation of proteoliposomes with purified TMEM16 protein for accurate measures of lipid scramblase activity. Methods Mol. Biol. 1949, 181–199 (2019).
Tsuboyama, K. et al. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science 354, 1036–1041 (2016).
Saitoh, T. et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl Acad. Sci. USA 106, 20842–20846 (2009).
Acknowledgements
We thank K. Ohashi, S. Chowdhury and G. C. Lander for their contributions to preliminary studies of this project; S. Chowdhury and G. C. Lander for also providing cryo-EM training; M. Shirakawa, K. Igarashi, Y. Ishida, C. Saito, and I. Koyama-Honda for technical assistance with cellular studies; B. Anderson for technical assistance with cryo-EM operation; J-C. Ducom and the Scripps HPC Facility for computational support for cryo-EM data processing and MD simulations; and I. J. Macrae for critical reading of the manuscript. The MultiBac expression system, cDNA of BRIL, and pMSP2N2 (Addgene plasmid no. 29520), pMXs-puro, Atg9a KO MEFs, pCG-gag-pol and pCG-VSV-G retrovirus plasmids were gifts from I. Berger, R. Stevens, S. Sligar, T. Kitamura, T. Saitoh and T. Yasui, respectively. This work was supported by grants from the National Institute of Health (GM092740 to T.O., GM069832 to S.F. and GM127390 to N.V.G.), the Welch Foundation (I-1505 to N.V.G.), a Grant-in-Aid for Exploratory Research for Advanced Technology (ERATO) (JPMJER1702 to N.M.) from the Japan Science and Technology Agency (JST). Computational analyses of EM data were performed using shared instrumentation at The Scripps Research Institute funded by NIH S10OD021634.
Author information
Authors and Affiliations
Contributions
S.M. purified proteins, collected and processed cryo-EM data and performed lipid scrambling assays; H.Y. performed autophagy assays, fluorescence and 3D CLEM imaging, and analyses. L.N.K. performed bioinformatics analyses under supervision of N.V.G. C.M.G performed MD simulations under supervision of S.F. S.T. collected and analyzed 3D CLEM data. C.O. generated plasmids and expressed proteins. N.M. supervised cellular studies. T.O. conceived the project, assisted structural and biochemical analyses and wrote the initial draft of the manuscript. All authors discussed the results and edited or commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Anke Sparmann and Inês Chen were the primary editors on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Conformational variability of ATG9A.
a, Superposition of ATG9A structures in amphipols (gray) and nanodiscs (colored). b, Conservation mapping of ATG9A. c, The structure of ATG9A in LMNG micelles. d,e, Superpositions of ATG9A structures in nanodiscs (colored) and LMNG (gray and violet): overall superposition (d) and focused on a protomer (e). f,g, Top views of the nanodisc (f) and LMNG (g) structures visualizing the dilation of the central pore. h, CHAP analysis of the LMNG structure.
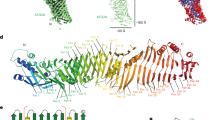
Extended Data Fig. 2 Similarities between the N- and C-terminal halves of ATG9A.
a,b, The bundles of α-helices flanking the TMH1–2 (a) and TMH3–4 (b) hairpins. c, Superposition of the bundles. d, Structural overlay of the N- and C-terminal halves generated by superposing TMH1–2 and TMH3–4. e, FATCAT flexible structural alignment of the N- and C-terminal halves. f, Sequence alignment between the N- and C-terminal halves of ATG9A.
Extended Data Fig. 3 Structural alignments of ATG9A with ABCexps.
a,b, Structures of YbtQ (a, PDB 6P6J) and MRP1 (b, PDB 5UJA) bound to their substrates. TMH1–3 are colored violet. c, A cutaway view of the central pore of ATG9A. d,e, Superpositions of ATG9A with YbtQ (d) and MRP1 (e). ATG9A’s TMH3–4 can be superimposed with TMH1–2 from the identified ABCexp structures using as a guide for the helix register the conserved Asp (D398 in ATG9 with D53 in MsbA, D56 in YbtQ, D355 in MRP1). In all cases, ABCexps’ substrates locate to the central pore of ATG9A.
Extended Data Fig. 4 MD simulation of the nanodisc structure.
a, The inter-residue distances measured are indicated on the nanodisc structure. b–d, Analyses of the simulation. The top panels are structure snapshots at t = 894 ns. The middle panels show time-dependent changes of the three pore openings between three selected pairs of residues at the A-C (b), B-A (c), and C-B (d) chain interfaces. The bottom panels show the trajectory of individual lipids that penetrated through the interface during the simulation. Each trace shows the Z position of the phosphate head of a lipid of interest, normalized by the average z coordinate of the luminal (0) and cytosolic (1) leaflets. The simulation shows lipid movements induced by the dynamic behavior of the protein. The luminal sides of the A-C and B-A interfaces (blue and orange lines in b and c) opened early (t = ~100–200 ns), followed by insertions of the headgroups of the lipids #59 and #42 from the luminal leaflet through the A-C and B-A interfaces, respectively (b, c, cyan lines, t = ~300–450 ns). As these headgroups moved toward the cytosolic side, the lipids #5 and #61 in the luminal leaflet inserted their headgroups into the pore through the A-C and B-A interfaces, respectively (b, c). Subsequently, the cytosolic side of the A-C and B-A interfaces opened (b, c, green lines), and at the A-C interface, the lipid #258 in the cytosolic leaflet entered the pore. Conversely, the C-B interface remained closed longer with respect to the A-C and B-A interfaces, but it eventually started to open after t = ~700 ns, allowing lipid #2 in the luminal leaflet to insert its headgroup into the luminal side of the pore (d). All the lipids mentioned here became laterally orientated following the placement of their headgroups into the pore. Source data for plots are available online.
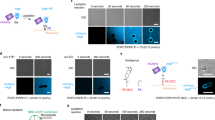
Extended Data Fig. 5 Dithionite permeation assays.
a,b, Top panels show the schemes of the experiments with no protein (a) and ATG9A (b). 26 µM NBD-phosphoethanolamine (PEA), which was synthesized by conjugating NBD-chloride and PEA and purified by agarose gel electrophoresis, was included in the (proteo)liposome reconstitution mixtures. After reconstitutions, NBD-PEA was removed by dialysis. Fluorescence was recorded as described for the dithionite lipid scramblase assays. 30 mM dithionite was added to the reaction mixtures at t = 100 s. The initial drops of fluorescence indicate that the residual amounts of NBD-PEA at the outside of the (proteo)liposomes were quenched. Between the two experiments, the addition of dithionite quenched the same amount of fluorescence and left the same amount of fluorescence, indicating that the same amount of NBD-PEA was included in the (proteo)liposomes. Fluorescence remained almost unchanged after reaching plateaus quickly. At 600 s, 0.1 % TritonX-100 was added to the reaction mixtures to dissolve the membranes for full quenching. The same amount of fluorescence was lost between the two experiments, confirming that the same amounts of NBD-PEA were included in liposomes and proteoliposomes. Thus, the ATG9A proteoliposomes were impermeable to dithionite. The experiments were repeated three times. All results are shown in different colors. Source data are available online.
Extended Data Fig. 6 BSA back extraction lipid scrambling assays.
a, Schematic for the BSA back extraction-based lipid scrambling assay. NBD-lipids are preferentially extracted by fatty acid-free BSA. Upon extraction, the fluorescence of BSA-bound NBD-lipids is reduced by ~ 50%. b, The results with reconstituted liposomes and ATG9A-containing proteoliposomes. 1.5 mg/ml BSA was added to each reaction at t = 100 s. BSA extracts NBD-lipids only from the outer leaflet of the membranes. Thus, in the absence of scrambling, ~50% of NBD-lipids on the outer leaflet lose their fluorescence by ~50%, reducing the total fluorescence by ~25%. In the presence of ATG9A, an additional ~15% of fluorescence was lost upon BSA addition, which indicates that NBD-lipids that were originally in the inner leaflet were exposed to BSA upon flipping. Source data are available online.
Extended Data Fig. 7 GFP-LC3-RFP autophagic flux assays with ATG9A mutants.
a, Locations of the mutations on the ATG9A nanodisc structure. Hydrophilic and hydrophobic amino acids were replaced with hydrophobic and hydrophilic ones, respectively. b,c, Flow cytometry autophagy flux assays. GFP/RFP fluorescence ratios of Torin 1-treated ATG9A KO HeLa cells stably expressing GFP-LC3-mRFP and ATG9A-FLAG mutants are plotted. Data shown in (b) and (c) are the results of the first and second rounds of mutant screenings, respectively. Data of cell populations (n = 4800 for b and n = 2500 for c) are presented as box plots. The box limit, the horizontal line, and the whisker show the first and third quartiles, the median, and the minimum and maximum values, respectively. No outliers are shown. The assay was repeated for the mutants shown in Fig. 4, and they were reproducible. The data shown in blue and green are of mutations in the lateral cavity and central pore, respectively. d,e, Western blotting of ATG9A KO HeLa cells stably expressing ATG9A-FLAG mutants. Source data for the box plots are available online.
Extended Data Fig. 8 Autophagosome formation in ATG9A mutant-expressing cells.
a,b, ATG9A KO HeLa cells (a) and Atg9a KO MEFs (b) stably expressing GFP-LC3-mRFP and ATG9A-FLAG mutants were observed by fluorescence microscopy. GFP fluorescence was monitored. In (a) additional mutants M26 (T412W) and M32 (M8 + M26) are shown. ATG9A KO HeLa ells and WT ATG9A-FLAG-expressing cells are the same image as shown in Fig. 4. Images were acquired after 3 h starvation. M26 yielded typical GFP-LC3 puncta while M32 generated smaller autophagosomes. The results are similar to those of M28 (T419W) and M33 (M8 + M28), which were selected for followed-up 3D CLEM and biochemical studies, as shown in Fig. 4. (b) MEFs were starved for 1 h, and GFP fluorescence was monitored. In Atg9a KO MEFs, GFP-LC3 forms larger puncta than typical autophagosomes. Expression of WT ATG9A-FLAG, as well as M26 and M28, generated ring-shaped autophagosomes, while M8-expressing, M32-expressing and M33-expressing cells yielded markedly smaller GFP-puncta. These observations are consistent with the results with HeLa cells, leading to the conclusion that the observed effects of the mutations in ATG9A are cell type-independent.
Supplementary information
Supplementary Information
Supplementary Note 1, Supplementary Figures 1–11 and Supplementary Tables 1 and 2.
Supplementary Data 1
HHpred hits with various ATG9 sequences and sequence alignments.
Supplementary Video 1
Movie of the MD simulation in a top cutaway view and three side views.
Supplementary Video 2
3D reconstruction of a cup-shaped phagophore observed in M33-expressing cells. A cup-shaped phagophore, a closed autophagosome and the ER are colored green, light green and red, respectively.
Source data
Source Data Fig. 4
Statistical source data
Source Data Extended Data Fig. 4
Statistical source data
Source Data Extended Data Fig. 5
Statistical source data
Source Data Extended Data Fig. 6
Statistical source data
Source Data Extended Data Fig. 7
Statistical source data
Rights and permissions
About this article
Cite this article
Maeda, S., Yamamoto, H., Kinch, L.N. et al. Structure, lipid scrambling activity and role in autophagosome formation of ATG9A. Nat Struct Mol Biol 27, 1194–1201 (2020). https://doi.org/10.1038/s41594-020-00520-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-020-00520-2