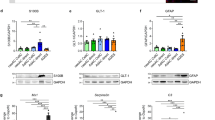
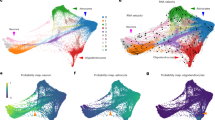
Abstract
Extrinsic signaling between diverse cell types is crucial for nervous system development. Ligand binding is a key driver of developmental processes. Nevertheless, it remains a significant challenge to disentangle which and how extrinsic signals act cooperatively to affect changes in recipient cells. In the developing human brain, cortical progenitors transition from neurogenesis to gliogenesis in a stereotyped sequence that is in part influenced by extrinsic ligands. Here we used published transcriptomic data to identify and functionally test five ligand–receptor pairs that synergistically drive human astrogenesis. We validate the synergistic contributions of TGFβ2, NLGN1, TSLP, DKK1 and BMP4 ligands on astrocyte development in both hCOs and primary fetal tissue. We confirm that the cooperative capabilities of these five ligands are greater than their individual capacities. Additionally, we discovered that their combinatorial effects converge in part on the mTORC1 signaling pathway, resulting in transcriptomic and morphological features of astrocyte development. Our data-driven framework can leverage single-cell and bulk genomic data to generate and test functional hypotheses surrounding cell–cell communication regulating neurodevelopmental processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data generated in this work are available through GEO accession number (GSE213245). All scripts generated are available without restrictions upon request and at GitHub site below. Source data are provided with this paper.
Code availability
References
Holguera, I. & Desplan, C. Neuronal specification in space and time. Science 362, 176–180 (2018).
Kohwi, M. & Doe, C. Q. Temporal fate specification and neural progenitor competence during development. Nat. Rev. Neurosci. 14, 823–838 (2013).
Cooper, J. A. Mechanisms of cell migration in the nervous system. J. Cell Biol. 202, 725–734 (2013).
Eroglu, C. & Barres, B. A. Regulation of synaptic connectivity glia. Nature 468, 223–231 (2010).
Chung, W.-S., Allen, N. J. & Eroglu, C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb. Perspect. Biol. 7, a020370 (2015).
Sancho, L., Contreras, M. & Allen, N. J. Glia as sculptors of synaptic plasticity. Neurosci. Res. 167, 17–29 (2021).
Allen, N. J. & Barres, B. A. Neuroscience: glia—more than just brain glue. Nature 457, 675–677 (2009).
Freeman, M. R. & Rowitch, D. H. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron 80, 613–623 (2013).
Lui, J. H., Hansen, D. V. & Kriegstein, A. R. Development and evolution of the human neocortex. Cell 146, 18–36 (2011).
Miller, F. D. & Gauthier, A. S. Timing is everything: making neurons versus glia in the developing cortex. Neuron 54, 357–369 (2007).
Howard, B. M. et al. Radial glia cells in the developing human brain. Neuroscientist 14, 459–473 (2008).
Nakashima, K. et al. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J. Neurosci. 19, 5429–5434 (1999).
Namihira, M. & Nakashima, K. Mechanisms of astrocytogenesis in the mammalian brain.Curr. Opin. Neurobiol. 23, 921–927 (2013).
Namihira, M. et al. Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev. Cell 16, 245–255 (2009).
Nakashima, K. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284, 479–482 (1999).
Urayama, S. et al. Chromatin accessibility at a STAT3 target site is altered prior to astrocyte differentiation. Cell Struct. Funct. 38, 55–66 (2013).
Deneen, B. et al. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron 52, 953–968 (2006).
Kang, P. et al. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron 74, 79–94 (2012).
Freeman, M. R. Specification and morphogenesis of astrocytes. Science 330, 774–778 (2010).
Tchieu, J. et al. NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells. Nat. Biotechnol. 37, 267–275 (2019).
Neyrinck, K. et al. SOX9-induced generation of functional astrocytes supporting neuronal maturation in an all-human system. Stem Cell Rev. Rep. 17, 1855–1873 (2021).
Li, X. et al. Fast generation of functional subtype astrocytes from human pluripotent stem cells. Stem Cell Rep. 11, 998–1008 (2018).
Tiwari, N. et al. Stage-specific transcription factors drive astrogliogenesis by remodeling gene regulatory landscapes. Cell Stem Cell 23, 557–571 (2018).
Hong, S. & Song, M.-R. STAT3 but not STAT1 is required for astrocyte differentiation. PLoS ONE 9, e86851 (2014).
Lattke, M. et al. Extensive transcriptional and chromatin changes underlie astrocyte maturation in vivo and in culture. Nat. Commun. 12, 4335 (2021).
Kwon, I.-S. et al. Expression of disabled 1 suppresses astroglial differentiation in neural stem cells. Mol. Cell. Neurosci. 40, 50–61 (2009).
Sun, Y. et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 104, 365–376 (2001).
Morrow, T., Song, M. R. & Ghosh, A. Sequential specification of neurons and glia by developmentally regulated extracellular factors. Development 128, 3585–3594 (2001).
Molofsky, A. V. et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 26, 891–907 (2012).
Barnabé-Heider, F. et al. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron 48, 253–265 (2005).
Pierfelice, T., Alberi, L. & Gaiano, N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 69, 840–855 (2011).
Caja, L. et al. Snail regulates BMP and TGFβ pathways to control the differentiation status of glioma-initiating cells. Oncogene 37, 2515–2531 (2018).
Gomes, W. A., Mehler, M. F. & Kessler, J. A. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev. Biol. 255, 164–177 (2003).
Nakashima, K., Yanagisawa, M., Arakawa, H. & Taga, T. Astrocyte differentiation mediated by LIF in cooperation with BMP2. FEBS Lett. 457, 43–46 (1999).
Gross, R. E. et al. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 17, 595–606 (1996).
Bonaguidi, M. A. et al. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development 132, 5503–5514 (2005).
Stipursky, J. & Gomes, F. C. A. TGF-β1/SMAD signaling induces astrocyte fate commitment in vitro: implications for radial glia development. Glia 55, 1023–1033 (2007).
Takizawa, T. et al. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev. Cell 1, 749–758 (2001).
Derouet, D. et al. Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proc. Natl Acad. Sci. USA 101, 4827–4832 (2004).
Fukuda, S. et al. Potentiation of astrogliogenesis by STAT3-mediated activation of bone morphogenetic protein-Smad signaling in neural stem cells. Mol. Cell. Biol. 27, 4931–4937 (2007).
Sloan, S. A. & Barres, B. A. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol. 27, 75–81 (2014).
Eze, U. C., Bhaduri, A., Haeussler, M., Nowakowski, T. J. & Kriegstein, A. R. Single-cell atlas of early human brain development highlights heterogeneity of human neuroepithelial cells and early radial glia. Nat. Neurosci. 24, 584–594 (2021).
Polioudakis, D. et al. A single-cell transcriptomic atlas of human neocortical development during mid-gestation. Neuron 103, 785–801 (2019).
Fan, X. et al. Spatial transcriptomic survey of human embryonic cerebral cortex by single-cell RNA-seq analysis. Cell Res. 28, 730–745 (2018).
Browaeys, R., Saelens, W. & Saeys, Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods, https://doi.org/10.1038/s41592-019-0667-5 (2019).
Li, J. et al. Astrocyte-to-astrocyte contact and a positive feedback loop of growth factor signaling regulate astrocyte maturation. Glia 67, 1571–1597 (2019).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53 (2015).
Camp, J. G. et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl Acad. Sci. USA 112, 15672–15677 (2015).
Barbar, L. et al. CD49f is a novel marker of functional and reactive human iPSC-derived astrocytes. Neuron 107, 436–453 (2020).
Zhou, B., Zuo, Y. & Jiang, R. Astrocyte morphology: diversity, plasticity, and role in neurological diseases. CNS Neurosci. Ther. 25, 665–673 (2019).
Kriegstein, A. & Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184 (2009).
Pruszak, J., Ludwig, W., Blak, A., Alavian, K. & Isacson, O. CD15, CD24, and CD29 define a surface biomarker code for neural lineage differentiation of stem cells. Stem Cells 27, 2928–2940 (2009).
Blair, J. D., Hockemeyer, D. & Bateup, H. S. Genetically engineered human cortical spheroid models of tuberous sclerosis. Nat. Med. 24, 1568–1578 (2018).
Banerjee, S., Crouse, N. R., Emnett, R. J., Gianino, S. M. & Gutmann, D. H. Neurofibromatosis-1 regulates mTOR-mediated astrocyte growth and glioma formation in a TSC/Rheb-independent manner. Proc. Natl Acad. Sci. USA 108, 15996–16001 (2011).
Methot, L. et al. Nuclear factor-kappaB regulates multiple steps of gliogenesis in the developing murine cerebral cortex. Glia 66, 2659–2672 (2018).
Magri, L. et al. Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex-associated lesions. Cell Stem Cell 9, 447–462 (2011).
Grabole, N. et al. Genomic analysis of the molecular neuropathology of tuberous sclerosis using a human stem cell model. Genome Med. 8, 94 (2016).
Weiss, A. & Attisano, L. The TGFβ superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2, 47–63 (2013).
Guo, X. & Wang, X.-F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res 19, 71–88 (2009).
Niida, A. et al. DKK1, a negative regulator of Wnt signaling, is a target of the β-catenin/TCF pathway. Oncogene 23, 8520–8526 (2004).
Yu, X. et al. The autoimmune encephalitis-related cytokine TSLP in the brain primes neuroinflammation by activating the JAK2-NLRP3 axis. Clin. Exp. Immunol. 207, 113–122 (2021).
Südhof, T. C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008).
Gabriel, S. S. et al. Transforming growth factor-β-regulated mTOR activity preserves cellular metabolism to maintain long-term T cell responses in chronic infection. Immunity 54, 1698–1714 (2021).
Peng, Q. et al. Bone morphogenetic protein 4 (BMP4) alleviates hepatic steatosis by increasing hepatic lipid turnover and inhibiting the mTORC1 signaling axis in hepatocytes. Aging (Albany NY) 11, 11520–11540 (2019).
Kim, B.-R. et al. Dickkopf-1 (DKK-1) interrupts FAK/PI3K/mTOR pathway by interaction of carbonic anhydrase IX (CA9) in tumorigenesis. Cell. Signal. 24, 1406–1413 (2012).
Xing, X. et al. Suppression of Akt-mTOR pathway rescued the social behavior in Cntnap2-deficient mice. Sci. Rep. 9, 3041 (2019).
Khoutorsky, A. et al. Translational control of nociception via 4E-binding protein 1. eLife 4, e12002 (2015).
Pasca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Arlotta, P. et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45, 207–221 (2005).
Trevino, A. E. et al. Chromatin accessibility dynamics in a model of human forebrain development. Science 367, eaay1645 (2020).
Acknowledgements
We would like to thank the Sloan Laboratory for helpful discussion and A. Erwood (Emory Department of Neurosurgery) for his contributions to early ligand identification. We would also like to thank J. Lee for his support with AWS management and usage. This study was supported by the National Institute of Health under grants R01MH125956 and R01NS123562 (to S.A.S.), and Brain and Behavior NARSAD Young Investigator Award. A.V. is supported by the Barry Goldwater Scholarship and the Emory IMSD program.
Author information
Authors and Affiliations
Contributions
A.J.V. and S.A.S. designed all experiments and performed NicheNet analysis. A.J.V. and S.N.L. performed ligand exposures. A.J.V., S.N.L., A.K. and A.S. performed hiPSC cultures, organoid formation and maintenance. A.J.V., S.N.L., M.M.S., E.H. and C.S. assisted with fetal tissue preparations and experimental procedures. S.N.L. designed and performed immunoblotting assays with input from T.N.B. and J.M.S. Bioinformatic processing was performed by A.J.V. and S.N.L. A.J.V., S.N.L. and S.A.S. wrote the manuscript with input from all contributing authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Omer Ali Bayraktar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8 and Supplementary Note.
Supplementary Tables
Supplementary Table 1: List of human astrocyte genes. Supplementary Table 2: List of candidate ligands. Supplementary Table 3: Concentration of candidate ligands. Supplementary Table 4: Targeted RNA-seq gene panel. Supplementary Table 5: List of neuronal and astrocyte signature genes.
Supplementary Data 1
Statistical supporting data for Supplementary Fig. 5.
Supplementary Data 2
Statistical supporting data for Supplementary Fig. 6.
Supplementary Data 3
Statistical supporting data for Supplementary Fig. 7.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Voss, A.J., Lanjewar, S.N., Sampson, M.M. et al. Identification of ligand–receptor pairs that drive human astrocyte development. Nat Neurosci 26, 1339–1351 (2023). https://doi.org/10.1038/s41593-023-01375-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01375-8